Abstract
The aims of this study were to determine: (i) whether sexually inexperienced photo-stimulated males housed individually in pens display less intense sexual behaviour than sexually inexperienced photo-stimulated males housed in a group, and (ii) whether these treatments vary the sexual response in anoestrous goats. Five males were housed either individually in pens (2 × 2 m) or together in a pen (5 × 6 m) and received artificially long days (16 h/light/day/2.5 months). Males were exposed to females (n = 50/group) for 15 days. Plasma testosterone did not differ between males. Male sexual behaviour was recorded on days 0-2 after introduction to females. On days 0-2, nudging was higher in males in group than those individual. On days 0 and 2 anogenital sniffing were higher in males in group, on day 1 was similar between males. Mounting attempts were similar between groups. On day 1 mounts with intromission did not differ but on day 2 were more in males in group. On day 0 self-urination were more in males in group, but on days 1 and 2 were similar between groups. On day 0 flehmen were more in males individually but on days 1-2 were similar between groups. Interval increases the words to first oestrus differ significantly between females, whereas ovulatory responses were similar. Thus, sexually inexperienced photo-stimulated males housed individually showed less sexual behaviour that those photo-stimulated housed in group but did not show reduced ability to induce ovulatory responses in anoestrous females; these responses resembled those induced by group-housed males.
Plasma testosterone concentration is high in sexually inexperienced photo-stimulated males housed in group or individually during the rest sexual.
Sexually inexperienced photo-stimulated males housed in group or individually display sexual behaviour in spring.
Sexually inexperienced photo-stimulated males housed in group or individually induce sexual response in anoestrous female goats.
Highlights
Introduction
In temperate and subtropical climates, small ruminants such as goats (Capra hircus) and sheep (Ovis aries), display reproductive seasonality (Duarte et al. Citation2008; Chanvallon et al. Citation2010). Groups of males held together sexually experienced subjected to a treatment of artificially long days for a duration of 2.5 months in a pen stimulated testosterone secretion, sexual behaviour, and odour during the natural sexual rest period (March-April; Delgadillo et al. Citation2002). Likewise, testosterone secretion increased during the natural sexual rest period in groups of sexually experienced males previously subjected to artificially long days, i.e., 75 days, and then returned to the natural photoperiod. Similarly, sexually inexperienced photo-stimulated males, housed as a group, display high level sexual behaviour when exposed to anoestrous females for the first time (Fernández et al. Citation2018). Whereas sexually inexperienced rams, housed as a group, show lower sexual behaviour during the first contact with ewes in the breeding season (Katz et al. Citation1988; Price et al. Citation1991). In fact, sexually inexperienced rams are incompetent to detect ewes displaying oestrous behaviour (Orgeur Citation1991).
In mammalian species such as boars (Sus scrofa, Hemsworth et al. Citation1977), beef bull (Bos taurus, Price and Wallach Citation1990), male guinea pigs (Cavia porcellus, Gerall Citation1963), rams (Illius et al. Citation1976a; Price Citation1985), and male goats (Lacuesta et al. Citation2018) socially isolated sexual behaviour and mating are lower. Likewise, stressors as social isolation and stress affect the reproductive system which is under the control of the hypothalamic-pituitary-gonadal (HPG) axis (Ferin Citation2006). During early social isolation the animals do not have the opportunity to establish social bonds, limiting socio-sexual learning, thus sexual behaviour is negatively affected in adulthood (Price Citation1985; Woodson Citation2002). It is known that sexual learning occurs in the limbic system and in the pathways that controls reproductive behaviour (Woodson Citation2002; Ferin Citation2006).
The hypothesis of this study was that sexually inexperienced photo-stimulated males housed individually in a pen, could display sexual behaviour as those sexually inexperienced photo-stimulated males housed as a group. The aims of this study were to determine: i) whether sexually inexperienced photo-stimulated males housed individually in pens display less intense sexual behaviour than sexually inexperienced photo-stimulated males housed as a group, and ii) whether these treatments vary the sexual response in anoestrous goats.
Material and methods
Study area
This study was carried out in the Comarca Lagunera in the State of Coahuila, Mexico, situated in the Chihuahuan Desert (26°23′N, 104°47′W, 1200 m above sea level), the climate is semi-arid (Köppen: BS0 hw). This area is characterised by an average annual rainfall of 227 mm (range: 163-504 mm) between June and September. Mean annual maximum and minimum temperature and humidity relative is from 36 °C and 8 °C, and 53% and 28%, respectively. The natural photoperiod varies from 13:36 h of light at the summer solstice to 10:24 h of light at the winter solstice (Delgadillo et al. Citation1999; Duarte et al. Citation2008).
Experimental procedures and management of goats
Creole goats were used, the phenotypic characteristics were coat colour with dark tones, white and from brown to black. The nose is with straight or convex profile. Female shows wattles and male shows beards, and both show horns with lengths from 10 to 20 cm (Escareño Sánchez et al. Citation2011).
In this area, males display sexual rest from December-January to May-June (Delgadillo et al. Citation1999), and females isolated from males the seasonal anoestrous is from February-March to August-September (Duarte et al. Citation2008). Goat kids were born in December (20 ± 1 day; mean value ± standard error of the mean (SEM)). Goat kids (n = 10) were weaned at 40 days of age and their body weight (BW) was 7.2 ± 0.2 kg. Males were housed in a single group in a pen (5 × 6 m) and were totally isolated from females. From 41 days of age and for the remainder of the study, the males received alfalfa hay ad libitum (21% crude protein, 2.0 Mcal/kg of ME). In addition, each male received 300 g of commercial concentrate per day. Drinking water and mineral salts (12% phosphorus and 11% calcium) were supplied ad libitum.
Photoperiodic treatment of males
When males were 10 months old (October 31), they were divided into two groups (n = 5 per group) according to their BW (26.0 ± 1.7 kg and 26.1 ± 1.2 kg) and body condition score (BCS, 2.5 ± 0.0 and 2.4 ± 0.1); BCS was measured using a scoring system of 1-4, where 1 = emaciated and 4 = fat (Walkden-Brown et al. Citation1997). Five males remained as a group in the same pen (5 × 6 m), as mentioned above, and there was full visual, auditory, olfactory and tactile contact among the members of the group. Five different males were separated and housed individually in pens (2 × 2 m each). These pens were separated by wooden trellises, thereby restricting visual, auditory, olfactory and tactile contact. The two groups of animals were separated by a distance of more than 200 m to avoid any interference between treatments. Under these conditions, the photoperiod treatment of artificially long days was applied to each group of males to induce an increase in testosterone secretion, sexual behaviour, odour, vocalizations and sperm production during the natural sexual rest (March-April; Delgadillo et al. Citation2002). During the photoperiodic treatment, lamps of 65 W with a light intensity of 300 lx were used at the lateral level of the eyes. The treatment was carried out for 2.5 months, and consisted of 16 h light and 8 h darkness per day, starting on November 1 and ending January 15. From January 16, males perceived the natural photoperiod. Males remained in their pens from the beginning of the photoperiodic treatment until the end of the study (November 1 to May 29), except when the males were exposed to females from March 26 to April 10. In this study, a group of males without photoperiodic treatment of artificially long days was not included, because previous studies have shown that untreated males do not exhibit sexual behaviour during the sexual rest to stimulate anoestrous females (Delgadillo et al. Citation2002; Muñoz et al. Citation2016).
Preparation and assessment of anoestrous females
Dewormed multiparous females (n = 100) which gave birth between mid-November and mid-December were used and their kids were weaned at about 25 days of age. Prior to the start of the experiment the females were kept under natural grazing from 1000 to 1700 h without supplementary feeding. The natural vegetation consisted of scrubs (Prosopis glandulosa, Acacia farneciana, Atiplex acantocarpa, Agave scabra, Mimosa biuncifera), herbaceous plants (Heliantus ciliaris, Salsola kali, Solanum elaeagnilolium) and grasses (Sorghum halepense, Chloris virgata, Sectaria verticillata, Eragrostis pectinacea, Bouteloua curtipendula, Boluteloua barbata and Aristida purpurea) (Duarte et al. Citation2008). At night the females were housed in open pens.
To determine the anovulatory status, each female was submitted to two studies of transrectal ultrasonography, the first on March 6 and the second on March 16. For this purpose, an Aloka SSD-500 machine connected to a transrectal 7.5 MHz linear probe was used. No female was detected with any corpora lutea. On March 23, anovulatory females were divided in two groups of 50 females according to their BW (33.3 ± 0.7 kg and 34.2 ± 0.9 kg) and BCS (1.8 ± 0.03 and 1.8 ± 0.04). Afterwards, each group was subdivided into five subgroups of 10 females. The females were placed in open pens (5 × 6 m) with artificial shade. During the study, each female received 2 kg of alfalfa hay (21% crude protein, 2.0 Mcal/kg of EM) and 200 g of commercial concentrate (12% crude protein, 1.95 Mcal/kg of EM) per day. Drinking water was supplied ad libitum. During the study, females were milked manually in the morning once a day.
The male effect
The male effect was carried out when the males were 15 months old. A day before the beginning of the study, BW and BCS were recorded for photo-stimulated males housed in group and for those photo-stimulated housed individually (42.6 ± 3.7 kg and 37.8 ± 1.5 kg and 2.7 ± 0.12 and 2.8 ± 0.12). On March 26 at 0800 h (day 0), each male housed in group and each male housed individually were placed in pens containing females (one male per 10 females). The two groups of females were separated by a distance of more than 250 m to avoid any contact among males. During 15 consecutive days, the males remained with females at all times. Males housed in group and those housed individually were interchanged twice by day into subgroups of females at 0800 h and 1800 h.
Measurements
Blood sampling and testosterone assay
To determine plasma testosterone concentrations, the males were subjected to a blood sampling by jugular venipuncture. Each blood sample was put in a 5 mL tube containing 30 µL of sodium heparin as anticoagulant. Blood samples were taken at 0800 h every Monday from January 16, when the photoperiodic treatment ended, until May 29 when the experiment ended. Afterwards blood samples were centrifuged at 3500 × g 30 min, and blood plasma was stored in 1.5 mL centrifuge tubes at −15 °C until determination of hormone concentrations. Testosterone was determined by Enzyme-Linked Immunosorbent Assay (ELISA, EIA-1559, DRG International, Inc., USA) according to the technique described and validated for goats by Gholib et al. (Citation2016). The sensitivity of the assay was 0.53 ng/mL. The intra- and inter-assay coefficients of variation were 2.45 and 6.5%, respectively.
Malé sexual behaviour was recorded from 0800 to 0900 h on days 0, 1 and 2 after introduction into the female groups. Each male was observed individually by recording the following components of sexual behaviour: anogenital sniffing, nudging, mounting attempts, mounts with intromission, self-urination and flehmen (Bedos et al. Citation2016).
Oestrous behaviour and ovulatory activity
During the study, oestrous behaviour was checked twice daily, from 0800 to 0900 h and from 1800 to 1900 h. A female was considered to be in oestrus when she was receptive and accepted being mounted by the male (Gelez et al. Citation2004; Fatet et al. Citation2011). In addition, ovulatory response and ovulation rates were measured by the presence and number of corpora lutea. For this purpose, a transrectal ultrasonography was made on days 6 and 15 after introduction of males into female groups, as mention above. Pregnancy rates (pregnant females divided by total of females exposed to males) were determined by transrectal ultrasonography 40 days after introduction of males into female groups.
Ethical note
All procedures performed in this study were in accordance with the protocol of the Official Mexican Rule NOM-062-ZOO-1999 (SAGARPA Citation2001), that provides technical specifications for the production, care, and use of laboratory animals.
Statistical analyses
The two groups of males were analysed by a model of repeated measures with a level significance of α = 0.05, the correlation between measurements was 0.7, and the non-sphericity correction ε = 0.9, the statistical power was 1- β = 0.75 (Faul et al. Citation2007). Based on the results of the Shapiro-Wilk and Levenés tests to evaluate the homogeneity of variances and normality of plasma testosterone concentrations, male sexual behaviour (except mounts with intromission) were analysed using generalised equation estimation procedures that included the effect of measure within subject, the subject was the male, the fixed effect was the group and the interaction group × measure. For parameter estimation the Fisher method was used. To counteract the effects of the model the X2 of Wald was used. The selection of the structure of covariances and distribution, an extension of the Akaike information criterion called corrected quasi-likelihood under independence model (QICC) was used. The Gamma distribution for plasma testosterone concentrations, interval to the first oestrus, and ovulation rate; the Poisson distribution for mounting attempts, self-urination, and flehmen; and the Tweedie-type (1.5) distribution for nudging and anogenital sniffing were used; in all cases the Log link function and covariance matrix was of first-order autoregressive [AR(1)]. The interval to the first oestrus and ovulation rate were analysed by a generalised linear model that included the fixed effect of group. The proportion of females displaying oestrous behaviour, ovulations, pregnancy rates, and mounts with intromission the exact Fisher’s exact test was used. Data are presented as the mean ± SEM. Statistical analyses were conducted using the statistical package SPSS 20 (IBM Corp. Citation2011).
Results
Testosterone profile
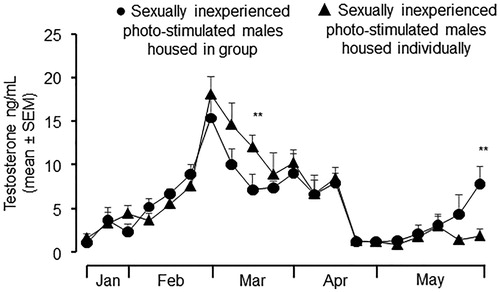
Plasma testosterone concentrations did not differ between the two groups of males (X2 de Wald = 0.365; gl = 1; p > .05; Figure ). However, the interaction treatment × measure differ significantly between groups of males (X2 Wald = 0.365; gl = 1; p < .001; Figure ). On March 13 photo-stimulated males housed individually, exhibited higher plasma testosterone concentrations than photo-stimulated males housed in a group (p < .01; Figure ). Whereas on May 29, when the study end, photo-stimulated males housed in a group exhibited higher plasma testosterone concentrations than those housed individually (p < .01; Figure ). Both groups of males, plasma testosterone concentrations decreased at the same time when the females were withdrawn.
Figure 1. Plasma testosterone profiles (mean ± SEM) in sexually inexperienced photo-stimulated males housed as a group in a pen (●) and sexually inexperienced photo-stimulated males housed individually in pens (▲) (p >.05). Males were submitted to a treatment of artificially long days (16 h of light/8 h of darkness) from November 1 to January 15. Afterwards, males received the natural photoperiod. Interaction treatment × measure differ significantly between groups of males **(p <.001).
Male sexual behaviour
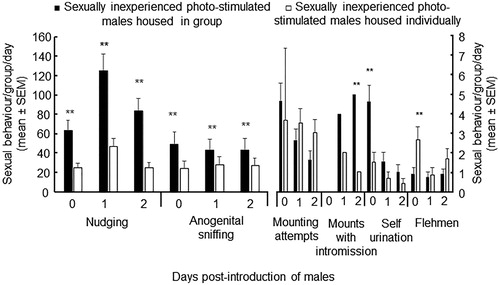
On days 0, 1 and 2, nudging were higher in photo-stimulated males housed in a group than those housed individually (p < .0001; Figure ). Likewise, on days 0 and 2 ano-genital sniffing were higher in photo-stimulated males housed in a group (p < .0001), but on day 1 did not differ between males (p > .05; Figure ). Mounting attempts did not differ between the groups of males (p >.05). On days 1 mounts with intromission did not differ between groups of males, but on day 2 were more in photo-stimulated males housed in a group than those individually (p < .001; Figure ). On day 0 self-urination was expressed more in photo-stimulated males housed in a group (p < .001), whereas on days 1 and 2 did not differ between males (p > .05; Figure ). On day 0 flehmen were observed more in photo-stimulated males housed individually (p < .001), whereas on days 1 and 2 did not differ between males (p >.05; Figure ).
Figure 2. Sexual behaviour (mean ± SEM) in sexually inexperienced photo-stimulated males housed as group in a pen (■), and sexually inexperienced photo-stimulated males housed individually in pens (□). Male goats were rendered sexually active during the non-breeding season by exposure to 16 h of light/8 h of darkness from November 1 to January 15. Afterwards, males received the natural photoperiod. Differences between groups of males were detected *(p <.05), **(p <.0001).
Response of females to sexually inexperienced males
The interval from males introduction to first oestrus was significantly longer in females exposed to photo-stimulated males housed in a group than those housed individually (p < .001; Table ). The proportion of females exhibiting oestrous behaviour during the 15 days of exposure to males did not differ between the two groups of females (p > .05; Table ). Likewise, the proportion of females that ovulated on days 6 and 15 after introduction of males, as well as, ovulation rates, and pregnancy rates did not differ between the two groups of females (p > .05; Table ).
Table 1. Oestrus, ovulatory and reproductive responses in anoestrous female goats in contact with sexually inexperienced photo-stimulated males housed in a group and sexually inexperienced photo-stimulated males housed individually in pens.
Discussion
The results of the present study show that sexually inexperienced photo-stimulated males housed individually exhibited a sexual behaviour less intense compared to those photo-stimulated housed in a group. The present study agreed with previous studies where social isolation diminished male sexual behaviour (Illius et al. Citation1976a; Hemsworth et al. Citation1977; Price and Wallach Citation1990). However, the sexually inexperienced and individually photo-stimulated males were able to stimulate sexual response in anoestrous female goats similar to sexually inexperienced and group-housed males. Evidence for this were the high percentages of oestrous behaviour and ovulation recorded in both groups of females. Our results showed for the first time that sexually inexperienced males isolated and housed individually from the beginning of photoperiodic treatment until the first contact with anoestrous females exhibited anyway their sexual activity.
Goats are a gregarious species, and in this case, sexually inexperienced males were negatively affected by social separation from their original group (Miranda-de la Lama and Mattiello Citation2010; Siebert et al. Citation2011). In our sexually inexperienced photo-stimulated males housed individually, social separation affected their locomotion diminishing nudging and anogenital sniffing, activities that imply high motor skills. In fact, this effect was observed in females’ sexual response, because the interval from males introduction to first oestrus was shorter in females exposed to sexually inexperienced photo-stimulated males housed in a group. For example, in the study by Fernández et al. (Citation2018), sexually inexperienced photo-stimulated males housed in a group only varied the frequency of nudging during the first three days of contact with anoestrous females. In this study, photo-stimulated males housed individually or in a group displayed similar mounting attempts; therefore, the two groups of males exhibited appetite behaviour, this result agrees with a previous study in which sexually inexperienced males displayed the same behaviour and frequency as did sexually experienced males (Fernández et al. Citation2018). In this regard, our results differ of a previous study which indicated that Saanen males isolated from females during rearing displayed significantly fewer mounting attempts compared to male goats previously exposed to females (Lacuesta et al. Citation2018). Interestingly on day 1 sexually inexperienced photo-stimulated males housed individually as those in group displayed similar mounts with intromission. This fact show the males were able to stimulate females´ sexual response. However, on day 2 inexperienced photo-stimulated males housed in group displayed more mounts with intromission. Perhaps this fact is due to the normal variation of sexual behaviour exhibited by males (Bedos et al. Citation2016). In fact, the proportion of females that ovulated on day 6 after introduction of males into female groups was high and similar among the two groups of females. In addition, inexperienced photo-stimulated males housed individually expressed with more frequency flehmen, whereas males isolated from females from weaning, and exposed to females in adulthood was observed with less frequency (Lacuesta et al. Citation2018). Our results show that photo-stimulated males housed individually or in a group attracted interest from anoestrous females since first contact with them, and were able to re-activate the hypothalamus-pituitary-ovarian axis. Therefore, the photoperiodic treatment applied to sexually inexperienced males exerts an effect so strong that the lack of sexual experience and the effect of social separation does not influence their ability to stimulate a sexual response in anoestrous females. In fact, oestrous behaviour and pregnancies were similar between all females as was reported previously in sexually experienced photo-stimulated males exposed to anoestrous females (Muñoz et al. Citation2016; Fernández et al. Citation2018).
Throughout the study, plasma testosterone concentrations did not differ between the two groups of males. Similar results were reported in rams that experienced different social environments (Illius et al. Citation1976b). In this study, experimental males perceived artificially long days during autumn-winter, which promoted the increase of testosterone secretion in spring, which is out of breeding season. In male goats, the perception of long days is the main signal inducing the onset of the breeding season (Ponce et al. Citation2014). Our results showed that plasma testosterone concentrations increased from February to mid-April in both groups of males, although on March 13 was higher in males housed individually. This pattern is similar to that reported previously in males exposed to artificially long photoperiod over 75 days where testosterone secretion was stimulated during the natural sexual rest period (Ponce et al. Citation2014).
Lack of sexual experience is observed in adult males kept isolated from females, this effect can be due to that during isolation these animals cannot express all sensory cues. For example, into a group of males held together, they have the opportunity to look, sniffing, hear or touch one another, and display some components of sexual behaviour among them improving their sexual performance during exposure with the opposite sex. Therefore, previous socio-sexual experience makes them sexually competent in adulthood.
Conclusions
Sexually inexperienced photo-stimulated males housed individually, sexual behaviour is less intense from those photo-stimulated housed in a group but does not decrease their ability to induce sexual and ovulatory responses in seasonally anoestrous female goats.
Ethical approval
In addition, the management of experimental animals in this study was carried out in compliance with the ARRIVE Guidelines for Reporting Animal Research (Kilkenny et al. Citation2010) specific for the care and use of animals in research.
Acknowledgments
The authors are grateful to all members of the Centro de Investigación en Reproducción Caprina of the Universidad Autónoma Agraria Antonio Narro. They thank Jesús Palomo for taking care of the animals. The authors express our thanks to Susana Rojas of the Laboratorio de Reproducción of the Facultad de Medicina Veterinaria y Zootecnia, Universidad Nacional Autónoma de México for carrying out the testosterone assays.
Disclosure statement
The authors declare that they have no conflict of interest.
References
- Bedos M, Muñoz AL, Orihuela A, Delgadillo JA. 2016. The sexual behavior of male goats exposed to long days is as intense as during their breeding season. Appl Anim Behav Sci. 184:35–40.
- Chanvallon A, Blache D, Chadwick A, Esmaili T, Hawken PAR, Martin GB, Viñoles C, Fabre-Nys C. 2010. Sexual experience and temperament affect the response of Merino ewes to the ram effect during the anoestrous season. Anim Reprod Sci. 119(3-4):205–211.
- Delgadillo JA, Canedo GA, Chemineau P, Guillaume D, Malpaux B. 1999. Evidence for an annual reproductive rhythm independent of food availability in male goats in subtropical Northern Mexico. Theriogenology. 52(4):727–737.
- Delgadillo JA, Flores JA, Véliz FG, Hernández HF, Duarte G, Vielma J, Poindron P, Chemineau P, Malpaux B, 2002. Induction of sexual activity in lactating anovulatory female goats using male goats treated only with artificially long days. J Anim Sci. 80(11):2780–2786.
- Duarte G, Flores JA, Malpaux B, Delgadillo JA. 2008. Reproductive seasonality in female goats adapted to a subtropical environment persists independently of food availability. Domest Anim Endocrinol. 35(4):362–370.
- Escareño Sánchez LM, Wurzinger M, Pastor López F, Salinas H, Sölkner J, Iniguez L. 2011. La cabra y los sistemas de producción caprina de los pequeños productores de la Comarca Lagunera, en el norte de México. RCHSCFA. XVII(Especial):235–246.
- Fatet A, Pellicer-Rubio MT, Leboeuf B. 2011. Reproductive cycle of goats. Anim Reprod Sci. 124(3-4):211–219.
- Faul F, Erdfelder E, Lang AG, Buchner A. 2007. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 39(2):175–191.
- Ferin M. 2006. Stress and the reproductive system. In: Editor Neill, JD, editor. Knobil and Neill´s physiology of reproduction, 3th ed. USA: Elsevier Academic Press, p. 2627–2640.
- Fernández IG, Flores Medina E, Flores JA, Hernández H, Vielma J, Fitz-Rodriguez G, Duarte G. 2018. Absence of previous sexual experience did not modify the response of anoestrous goats to photo-stimulated bucks in Spring. Ital J Anim Sci. 17(2):306–311.
- Gelez H, Archer E, Chesneau D, Campan R, Fabre-Nys C. 2004. Importance of learning in the response of ewes to male odor. Chem Senses. 29(7):555–563.
- Gerall AA. 1963. An exploratory study of the effect of social isolation variable on the sexual behaviour of male guinea pigs. Anim Behav. 11(2-3):274–282.
- Gholib G, Wahyuni S, Kadar OH, Adam M, Lubis TM, Azhar A, Akmal M, Siregar TN, Armansyah T, Nugraha TP. 2016. Measurement of serum testosterone in Kacang goat by using Enzime-Linked Immunosorbent Assay (ELISA) technique: The importance of kit validation. J Ked Hewan. 10(1):32–36.
- Hemsworth PH, Beilharz RG, Galloway DB. 1977. Influence of social conditions during rearing on the sexual behavior of the domestic boar. Anim Sci. 24(2):245–251.
- IBM Corp. 2011. Released IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.
- Illius AW, Haynes NB, Purvis K, Lamming GE. 1976b. Plasma concentrations of testosterone in the developing ram in different social environments. J Reprod Fertil. 48(1):17–24.
- Illius AW, Haynes NB, Lamming GE. 1976a. Effects of ewe proximity on peripheral plasma testosterone levels and behaviour in the ram. J Reprod Fertil. 48(1):25–32.
- Katz LS, Price EO, Wallach SJR, Zenchak JJ. 1988. Sexual performance of rams reared with or without females after weaning. J Anim Sci. 66(5):1166–1173.
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DC. 2010. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 8, e1000412.
- Lacuesta L, Giriboni J, Orihuela A, Ungerfeld R. 2018. Rearing bucks isolated from females affects their sexual behavior when adults. Anim Reprod. 15(2):114–117.
- Miranda-de la Lama GC, Mattiello S. 2010. The importance of social behavior for goat welfare in livestock farming. Small Rumin Res. 90(1-3):1–10.
- Muñoz AL, Bedos M, Aroña RM, Flores JA, Hernández H, Moussu C, Briefer EF, Chemineau P, Keller M, Delgadillo JA. 2016. Efficiency of the male effect with photostimulated bucks does not depend on their familiarity with goats. Physiol Behav. 158:137–142.
- Orgeur P. 1991. Identification of sexual receptivity in ewes by young sexually inexperienced rams. Appl Anim Behav Sci. 31(1-2):83–90.
- Ponce JL, Velázquez H, Duarte G, Bedos M, Hernández H, Keller M, Chemineau P, Delgadillo JA. 2014. Reducing exposure to long days from 75 to 30 days of extra-light treatment does not decrease the capacity of the male goats to stimulate ovulatory activity in seasonally anovulatory females. Domest Anim Endocrinol. 48:119–125.
- Price EO, Estep DQ, Wallach SJR, Dally MR. 1991. Sexual performance of rams as determined by maturation and sexual experience. J Anim Sci. 69(3):1047–1052.
- Price EO, Wallach SJR. 1990. Short-term individual housing temporarily reduces the libido of bulls. J Anim Sci. 68(11):3572–3577.
- Price EO. 1985. Sexual behavior of large domestic farm animals: an overview. J Anim Sci. 61(suppl_3):62–74.
- SAGARPA. 2001. Norma Oficial Mexicana NOM-062-ZOO. Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. Secretary of Agriculture. Livestock, Rural Development, Fishing and Food. Diario Oficial de la Federación, México DF, 22 Agosto 2001.
- Siebert K, Langbein J, Schön P-C, Tuchscherer A, Puppe B. 2011. Degree of social isolation affects behavioral and vocal response patterns in dwarf goats (Capra hircus). Appl Anim Behav Sci. 131(1-2):53–62.
- Walkden-Brown SW, Restall BJ, Scaramuzzi RJ, Martin GB, Blackberry MA. 1997. Seasonality in male Australian cashmere goats: long term effects of castration and testosterone or oestradiol treatment on changes in LH, FSH and prolactin concentrations, and body growth. Small Rumin Res. 26(3):239–252.
- Woodson JC. 2002. Including 'learned sexuality' in the organization of sexual behavior. Neurosci Biobehav Rev. 26(1):69–80.
