?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The interest of ensiling total mixed ration for ruminants re-emerged in the last decades. In many situations, ensiled total mixed ration (ETMR) has been a sustainable alternative to efficiently handle wet by-products in ruminant diets. The purpose of this study was to assess the effect of replacing common vetch with wet brewers’ grains (WBG) at different proportions on the fermentation quality, nutritive value, aerobic stability, in vitro gas production kinetics and digestibility of ETMR. Four ETMR formulations were designed according to the varied ratios on fresh weight basis: (i) 45% common vetch (Control); (ii) 35% common vetch +10% WBG; (iii) 25% common vetch +20% WBG; (iv) 15% common vetch +30% WBG. The laboratory silos (10 L) were opened after 70 days of ensiling, then the 70-day silages were subjected to aerobic stability for 14 days and changes in ETMR temperature were recorded. Replacing common vetch with WBG linearly increased the ratio of lactic acid to acetic acid, ethanol, water soluble carbohydrate contents and dry matter recovery, and decreased pH, volatile fatty acids and ammonia nitrogen contents. When exposed to air, no ETMR deteriorated and their temperature appeared stable during the 14 days test. With the WBG levels increasing, the organic matter digestibility, metabolisable energy, 72-h net gas production and the average gas production rate were linearly decreased, whereas no obvious differences were observed among the control, 10% and 20% WBG silages. Therefore, 20% WBG can be used effectively in ETMR, reducing the need for common vetch without negative effects.
Ensiling total mixed ration is a good way to use wet brewers’ grains
Reducing the need for legume and feed cost
20% wet brewers’ grains replacement had no adverse effect on in vitro gas production kinetics and digestibility
Highlights
Introduction
Traditionally, quality forages have been the major feedstuffs used to support milk production in dairy cows. In recent years, the price of some forages has risen to record high levels in China (Shi et al. Citation2015). The high feed costs have already been a financial challenge for dairy farmers. Furthermore, the seasonal shortage of forage production and environmental concerns should be worried. To solve these problems, replacing commercial forages with by-products is an effective method.
Nowadays, rapid expansion of the beer industry has increased availability of distillery by-products. Wet brewers’ grains (WBG) are the by-products from brewer industry. It is an extracted residue of barley malt with other cereal grains or grain products resulting from production of wort or beer. They are rich in crude protein (CP), crude fibre (CF), ether extract (EE), vitamins and minerals (Chiou et al. Citation1998), making it a potential source for feed production. Wet brewers’ grains are lower cost because more than 10 million tons of WBG are produced annually in China. Moreover, feeding WBG could avoid the cost of drying the product. Considering these advantages, it is conceivable that WBG could serve as a replacement for some forages in diets. However, the disadvantages of using WBG as a fresh feed are the relatively higher moisture and easy deterioration between 3 to 5 days. Some attempts to ensile brewers’ grains resulted in poor quality silage with characteristic offensive odours and high dry matter losses (Allen and Stevenson 1975). Hence, claims are made for some silage technology to improve the storage of WBG.
Recently, ensiled total mixed ration (ETMR) has become gradually dominant in many countries as an effective use of a high-moisture by-product in animal feed. In Tibet region of China, ETMR has been widely used to feed dairy cows. ETMR application can make up for the inadequacy of feeding roughage and concentrate separately (Chen et al. Citation2015). If high-moisture by-product was ensiled with dry feeds as an ETMR, the risk of effluent production would be minimised and the time for mixing prior to feeding could be omitted (Nishino et al. Citation2003). Ensiling WBG with concentrate could also provide year-round nutrition balance feed, and enhance the palatability by improving odours and flavours from by-products through fermentation (Cao et al. Citation2009). However, plentiful WBG in ETMR could be an asset or a liability.
The economics of ration formulation indicates that it is most profitable to feed as much WBG as possible. Admittedly, no strong nutritional advantages occur with feeding more WBG, but the possibility of feeding excess protein or phosphorus may occur, which probably mean excessive amounts of nitrogen and phosphorus to dispose of in manure. If improperly handled, they can create severe environmental problems with possible air- and water-quality impact (Schingoethe et al. Citation2009). More importantly, higher proportions of WBG may reduce the energy of feed. Given this, a laboratory test for ETMR including varied ratios of WBG should be conducted to verify potential problems before feeding.
The objective of this study was to determine the effects of ETMR including different proportions of WBG on fermentation quality, nutritive value, aerobic stability, in vitro gas production kinetics and digestibility.
Materials and methods
ETMR preparation
Total mixed ration (TMR) consisted of hulless barley straw, common vetch, whole-crop oat, wet brewers’ grains and mixed concentrate. Hulless barley, common vetch and oat were cultivated in the experimental field of the Shigatse Grassland Station (Tibet, China: N 29°16′, E 88°51′, elevation 3836 metres, annual mean temperature 6.5 °C and average annual precipitation 400 mm). All of these crops were harvested by hand on 24 September 2014. Hulless barley straw was the residue remaining after grain harvest (excluding the grains); common vetch was harvested at the pod bearing stage; whole-crop oat was harvested at the milk ripe stage. Wet brewers’ grain (WBG) was obtained from a private brewery at Shigatse of Tibet in China and subjected to ensiling within 10 h of production. After brewery processing, WBG was in a powdery form with high moisture. Mixed concentrate contained 7.5% cracked corn, 20% rapeseed cake meal, 20% cotton seed, 27.5% distillers dried grains with solubles, 20% wheat bran and 5% vitamin-mineral. After harvest, all the forages were collected and chopped into approximately 2 ∼ 3 cm lengths by a manual forage chopper and immediately transported to the laboratory. The chemical compositions of ingredients for ETMR is shown in Table .
Table 1. Chemical compositions of ingredients used in total mixed ration.
The ingredient proportions, chemical and microbial compositions of ETMR before ensiling are shown in Table . The partial replacement of common vetch to WBG was aimed to maximise WBG utilisation based on a regular ration formulation in the control. Four ETMR formulations were designed according to the varied ratios on fresh weight (FW) basis: (i) 45% common vetch (Control); (ii) 35% common vetch +10% WBG; (iii) 25% common vetch +20% WBG; (iv) 15% common vetch +30% WBG. After a thorough mixing, about 7.6 kg raw material for each formulation was packed tightly into a laboratory silo (polyethylene bottle, 10 L capacity), ensuring expulsion of the air and to obtain a density of 760 g L−1. The silos were sealed with a screw top and plastic tapes, and then placed at ambient temperature (∼20 °C). A total of 24 silos (4 ETMR formulations ×6 replicates = 24) was prepared, and the silos for each treatment were opened at 70 days after ensiling, and then subjected to an aerobic stability test for 14 days.
Table 2. Ingredient proportions, chemical and microbial compositions of total mixed ration substrates.
Chemical and microbiological analyses
At sampling, fresh forage, pre-ensiled TMR and ETMR were put into an ethanol-disinfected plastic container and mixed uniformly. Each sample was split into three subsamples. The first subsample was analysed immediately for the dry matter (DM) content in a forced-draft oven to a constant weight drying at 65 °C for at least 48 h, and then ground to pass a 1 mm screen for further analysis. Dry matter, CP, EE and ash were determined according to Association of Official Analytical Chemists guidelines (AOAC Citation1990). Dry matter recovery of the ETMR was estimated by measuring the differences of DM in silo before and after ensiling. The water-soluble carbohydrate (WSC) content was analysed by colorimetric after-reaction with anthrone reagent (Thomas Citation1977). Total nitrogen (TN) was determined by Kjeldahl procedure (Krishnamoorthy et al. Citation1982) and CP content was calculated by multiplying TN by 6.25. Amylase-treated neutral detergent fibre (aNDFom) and acid detergent fibre (ADFom) contents were determined according to the procedures as described by Van Soest et al. (Citation1991), heat stable amylase and sodium sulphite were used in the aNDF procedure and the results of aNDFom and ADFom were expressed on DM basis exclusive of residual ash. Non-fibrous carbohydrate (NFC) was calculated by the formula: NFC =1000 -CP -NDF -EE -Ash (NRC Citation2000).
To determine ensiling traits of pre-ensiled TMR and fermentation end products of ETMR, 35 g of each TMR fresh material or ETMR sample was blended with 70 g deionised water extracted at 4 °C for 24 h (Chen et al. Citation2015). Then, the extracts were filtered through two layers of cheesecloth and a filter paper. The filtrates were used for measuring pH, buffering capacity (BC), ammonia nitrogen (NH3-N) and organic acid contents. The pH of the ETMR was measured immediately with a glass electrode pH metre (HANNA pH 211, Hanna Instruments Italia Srl, Padova, Italy). Buffering capacity was determined using the method of Wang et al. (Citation2018). The NH3-N content was determined by the phenol-hypochlorite procedure (Wang et al. Citation2016). Organic acid (lactic acid, acetic acid, propionic acid and butyric acid) and ethanol contents of the silage were analysed using the Agilent HPLC 1260 (Agilent Technologies, Inc., Berlin, Germany; column: Carbomix H-NP5, Sepax Technologies, Inc., Santa Clara, CA, USA; detector: refractive index detector, Agilent Technologies, Inc., Germany; eluent: 2.5 mmol L−1 H2SO4, 0.5 mL min−1; temperature: 55 °C). Total volatile fatty acids (VFAs) were calculated as the sum of acetic acid, propionic acid and butyric acid, expressed on a DM basis.
Total digestible nutrients (TDN) were estimated as follows (Harlan et al. Citation1991):
where TDN and ADF contents are based on DM percent (%).
For enumeration of the microorganisms, 10 g pre-ensiled TMR sample or ETMR was sampled and blended with 90 mL of sterilised saline solution (8.50 g L−1 NaCl), and shaken for 10 minutes; serial dilutions (10−1 through 10−6) were prepared in sterile saline solution (Liu et al. Citation2016). The colonies of lactic acid bacteria (LAB) were counted on MRS agar medium after incubation in an anaerobic incubator (N2: H2: CO2 = 85:5:10, YQX-II; CIMO Medical Instrument Manufacturing Co., Ltd., Shanghai, China) at 37 °C for 3 days. Aerobic bacteria were cultured and counted on nutrient agar medium (Nissui-seiyaku Ltd., Tokyo, Japan). Yeasts were counted on potato dextrose agar (Nissui-seiyaku Ltd., Tokyo, Japan) and acidified with sterilised tartaric acid solution to pH 3.5. These agar plates were incubated at 37 °C for 3 days. All microbial data were transformed to log10 and are presented on a fresh weight basis.
Aerobic stability test
A total of 24 silos were opened on 70 day and then subjected to aerobic deterioration test for 14 days. During the aerobic exposure stage, sextuplicate silages of each formulation were taken out from each silo, fully mixed and placed into 15 L open-top and sterile polyethylene bottle without compaction. The bottles were stored at room temperature (∼15 °C) wrapping with double layer of gauze to prevent impurities and drying, but allow penetration of air. The probes of multi-channel temperature recorder (MDL-1048A high-precision temperature recorder, Shanghai Tianhe Automation Instrument Co., Ltd.) were placed in the centre of the bottle for measuring temperature variation. And six probes were placed in the environment as blank. Temperature recording was conducted every 30 minutes for 14 days. The aerobic stability was denoted as the time (hours) that silage remained stable before rising at least 2 °C above the ambient temperature (Wilkinson and Davies Citation2013). Samples were analysed for the dynamic changes of organic acids, NH3-N, WSC and microbial numbers after aerobic exposure at 0, 6, 9 and 14 days.
In vitro gas production and digestibility measurements
The following procedures were approved by the Ethics Committee of Nanjing Agricultural University. 70-day silage samples were used to measure the in vitro gas production and digestibility. Samples (1 g dry weight) were put into filter bags (F57; ANKOM Technology, Macedon, NY, USA) that were previously washed with acetone, dried at 55 °C for 24 h and weighed. Bags were heat-sealed, and a single bag was placed in a 120-mL capacity serum bottles. three repetitions were prepared for each silo. Rumen fluid was obtained before morning feeding from four rumen-cannulated Boer male goats fed with a diet that contained 50% roughage and 50% concentrates plus supplemental vitamins and minerals. Rumen fluid was filtered through four layers of cheesecloth under CO2 and kept at 39 °C in a water bath while continuously flushed with CO2. Then, the rumen fluid was mixed with the buffer solution (1:2, v/v) (Menke and Steingass Citation1988) and 60 mL of the mixture were transferred into each serum bottle. The bottles were sealed with a rubber stopper and aluminium crimp and then were incubated at 39 °C. According to the description of Chen et al. (Citation2017), gas production (GP) was recorded at 4, 8, 12, 24, 48 and 72 h of incubation by the pressure transducer technique, and corrected by subtracting GP from blank bottles (rumen fluid + buffer solution). After the in vitro incubation process, the bags containing the samples were gently rinsed with cold tap water and dried at 65 °C for 48 hours. The difference between initially incubated DM and residual DM, corrected by the blanks after the incubation, was calculated to determine in vitro dry matter digestibility (IVDMD). The in vitro NDF digestibility (IVNDFD) was determined and calculated using the methods described by Liu et al. (Citation2016).
The organic matter digestibility (OMD) was calculated according to Kamalak et al. (Citation2011):
where GP24 is 24 h net gas production (mL 200 mg−1 DM), CP is crude protein (%DM), EE is ether extract (%DM) and ash is based on DM percent (%).
The metabolisable energy (ME) and net energy for lactation (NEL) were calculated according to Menke and Steingass (Citation1988):
where GP24 is 24 h net gas production (mL 200 mg−1 DM), CP is crude protein (%DM), EE is ether extract (%DM) and ME is metabolisable energy (Mcal kg−1).
Short-chain fatty acid contents (SCFA) were calculated according to Getachew et al. (Citation2002) and microbial crude protein (MCP) biomass production was calculated according to Blümmel et al. (Citation1997) as:
where GP24 is 24 h net gas production (mL 200 mg−1 DM), 2.2 mg mL−1 is a stoichiometric factor, which expresses mg of C, H and O required for the production of SCFA gas associated with the production of 1 mL of gas.
The kinetic parameters of gas production were determined by fitting gas production data to the nonlinear equation:
where Y is the volume of gas produced at time t, b is the asymptotic value of gas production (mL), c is the gas production rate constant. Parameters b and c were estimated by an iterative least square method using a non-linear regression (NLIN) option of the Statistical Analysis Systems (SAS Citation2001).
The average GP rate (AGPR, mL h−1) was defined as the AGPR between the start of the incubation and the time at which the GP was half of its asymptotic value according to the following equation (Zhang et al. Citation2017):
where b represents the asymptotic GP generated at a constant fractional rate (c) per unit time; t is the gas recording time, and Lag represents a lag time phase before GP commenced.
Statistical analysis
All statistical procedures were performed with Statistical Packages for the Social Sciences (SPSS 13.0 for Windows; SPSS Inc., Chicago, IL). Data on chemical compositions of pre-ensiled TMR, fermentation quality and in vitro characteristics of ETMR were subjected to one-way analysis of variance (ANOVA):
where Yi is the dependent variable; μ is the least square mean; Ti is the effect of WBG proportions; and eij is the residual error term. The effects of the increasing levels of WBG were also partitioned into linear and quadratic components by orthogonal polynomials.
Data on aerobic stability parameters of ETMR was analysed by two-way analysis of variance according to the model for a factorial treatment design as follows:
where Yij is the dependent variable; µ is overall mean; Ti is the effect of WBG proportions; Dj is the effect of exposure days; (T × D)ij is the effect of interaction between WBG proportions and exposure days; and eij is the residual error term.
Statistical differences among means were determined using Tukey’s multiple comparison. Differences were considered significant at p < .05.
Results
TMR ingredient and TMR formula prior to ensiling
As presented in Table , WBG showed relatively higher CP, aADFom and EE contents, and lower DM, ash, WSC, NFC contents and buffering capacity than common vetch. Other chemical compositions of TMR ingredient were described in Table .
As seen in Table , the effect of WBG proportions on CP content and buffering capacity were pronounced (p < .01). With the WBG proportion increasing, the CP contents were linearly (p < .01) increased, while DM contents and buffering capacity were linearly (p < .01) decreased.
Fermentation characteristics and chemical compositions of ETMR after 70 days of ensiling
As shown in Table , the effect of WBG proportions on pH, lactic acid (LA), ratio of LA to acetic acid (AA) (LA/AA), propionic acid (PA), butyric acid (BA), ethanol, WSC, NH3-N, DM recovery and EE contents were significant (p < .05). With the increase of WBG ratios, the LA/AA, ethanol, WSC, DM recovery and EE contents were linearly (p < .05) increased, while pH, PA, VFAs and NH3-N contents were linearly (p < .05) decreased. The pH, PA, BA and EE were quadratically (p < .01) affected by the increased levels of WBG.
Table 3. Chemical and microbial composition of ensiled total mixed ration after 70 days of ensiling.
Compared to the control ETMR (without WBG), all the ETMR inclusion of WBG had markedly (p < .05) lower pH, PA, BA and NH3-N contents, and higher LA contents, LA/AA and DM recovery. Compared to the ETMR containing 10% WBG, the ETMR including 20% and 30% WBG showed evidently (p < .05) lower pH, BA and NH3-N contents, and higher BA, ethanol, WSC and EE contents. Compared to the ETMR including 20% WBG, the ETMR including 30% WBG had pronouncedly (p < .05) higher WSC and EE contents, whereas no notable (p > 0.05) differences were observed on pH, LA, ethanol and NH3-N contents. Among all the ETMR, no obvious (p > 0.05) differences were found in the AA, DM, CP, ash, aNDFom, aADFom, NFC, TDN and microbial populations.
Aerobic stability
As illustrated in Tables and , the effect of WBG levels in silages or the silages exposure days on their chemical and microbial compositions was significant (p < .01), except effect of WBG levels on WSC, NH3-N and VFAs contents, aerobic bacteria, yeasts (p < .05) and LAB counts (p > 0.05), as well as the effect of exposure days on LA (p < .05), NH3-N and VFAs contents and aerobic bacteria counts (p > 0.05). The interaction between WBG levels and exposure days markedly influenced the WSC (p < .01) and ethanol contents, LAB and aerobic bacteria counts (p < .05), whereas it failed to affect the pH, LA, NH3-N, VFAs contents and yeasts counts (p > 0.05).
Table 4. Chemical compositions of four ensiled total mixed ration (ETMR) after exposure to air.
Table 5. Microbial compositions of four ensiled total mixed ration (ETMR) after exposure to air.
With the increase of WBG ratios, the ethanol contents were linearly (p < .01) increased, while pH, LA, WSC, NH3-N contents, LAB and aerobic bacteria counts were linearly (p < .05) decreased. The pH, LA, WSC and VFAs contents and yeasts counts were quadratically (p < .05) affected by the increased levels of WBG.
Irrespective of the effect of aerobic exposure days, with the WBG levels increasing during aerobic exposure, pH, NH3-N contents and aerobic bacteria counts gradually decrease, while WSC, ethanol and VFAs contents gradually increase. Irrespective of the effect of the WBG levels, with the increase of exposure days, WSC, ethanol contents and yeast counts gradually decrease.
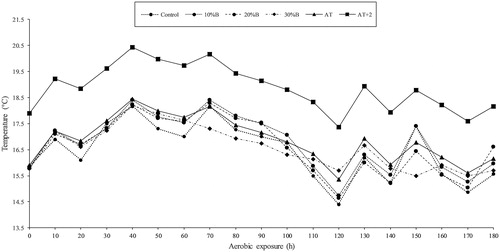
As described in Figure , the temperature of all ETMR were no more than 2 °C above the ambient temperature during the whole aerobic exposure phase
In vitro gas production kinetics and digestibility
As presented in Table , the effect of WBG proportions on OMD, ME, NEL, SCFA, GP72, gas production rate constant, half time and AGRP contents were significant (p < .05). With the increase of WBG levels, the OMD, ME, NEL, SCFA, GP72, gas production rate constant, half time and AGRP contents were linearly (p < .05) decreased. No evident (p > 0.05) differences were observed in the OMD, ME, NEL, SCFA, GP72, gas production rate constant, half time and AGRP contents among the control, 10% and 20% ETMR. The ETMR containing 30% WBG had markedly (p < .05) lower OMD, ME, NEL, SCFA, GP72, gas production rate constant, half time and AGRP contents compared to the control ETMR. Among all the ETMR, no obvious (p > 0.05) differences were found on IVDMD, IVNDFD, MCP and potential gas production.
Table 6. In vitro gas production kinetics and digestibility, and available energy of ensiled total mixed ration after 70 days of ensiling.
Discussion
Common vetch is a popular forage in China, but owing to its rising price, dairy farmers have taken interest in replacing it with other lower-cost feedstuffs. In the present study, the WBG had higher CP (286 vs. 185 g kg−1 DM) and lower buffering capacity (148 vs. 336 g kg−1 DM) contents than common vetch, thus motivating the use of WBG as common vetch replacer in ETMR. The buffering capacity of plants would resist pH change during ensiling, whereas a lower pH range (3.7 ∼ 4.2) is usually accepted as beneficial for forage preservation. Thus, lower buffering capacity is considered as a positive characteristic (McDonald et al. 1991).
Silage is a very complex fermentation matrix that exhibits variability in natural microbiota, chemical compositions and nutrients. The occurrence of desirable silage fermentation is guided by the amount and type of epiphytic microflora, moisture, buffering capacity and WSC of the material (McDonald et al. Citation1991). In this study, all ETMR containing WBG had markedly higher LA contents and lower pH than control. There are two possible explanations for this finding. One possibility is that all mixed TMR substrates including WBG had higher moisture contents than control. The moisture content of silage material plays a decisive role in influencing silage fermentation, because moisture is required by LAB for metabolic reactions and has a significant effect on the initial level and transport of oxygen during ensilage process (McDonald et al. Citation1991). A second possibility may be the lower buffering capacity in mixed TMR substrates including WBG, making it easier to change in pH values. pH is one of the main factors that influence the extent of fermentation and silage quality of ensiled forage. Commonly, the drop in pH values is mainly caused by LA production during fermentation; and a lower pH is favourable, since the silages would be better preserved in a more stable form.
Inclusion of WBG in ETMR could significantly promote the ratio of LA/AA, and significantly or linearly reduce the PA, BA and total VFAs contents. These results indicate that the fermentation in ETMR including WBG was more efficient than control. Chamberlain and Wilkinson (Citation1996) stated that the VFAs comprise AA, PA, BA and other acids. The production of these acids is a reflection of an inefficient fermentation or of secondary fermentation of lactic acid to butyric acid and degradation of amino acids to ammonia.
Excellent fermentation process should have a highly efficient conversion of WSC to LA. In this study, the WSC contents in all mixed TMR substrates have no obvious difference, but the ETMR including WBG had significantly higher residual WSC and LA contents than control. Moreover, the residual WSC content was lineally increased with increasing WBG levels in ETMR. It was suggested that inclusion of WBG in ETMR could enhance the conversion efficiency of WSC to LA. The formation of NH3-N reflects part of the proteolysis occurring during ensiling and is explained by plant enzymatic, clostridial and/or enterobacterial activity (McDonald et al. Citation1991). In our study, all ETMR containing WBG had markedly less NH3-N contents than control, which indicates that inclusion of WBG could suppress extensive protein proteolysis. The protein in WBG are probably more resistant to the degradation in silo due to the heat treatment during production and this could partly explain the lower NH3-N contents in ETMR including WBG. In the present experiment, significantly higher DM recovery could be observed in ETMR including WBG, indicating inclusion of WBG could inhibit the DM loss during fermentation. These superior fermentation characteristics in ETMR including WBG might be resulted from the faster decline in pH values during the early stage of ensiling, thus inhibiting protein breakdown by plant enzymes and the growth and activity of undesirable microorganisms (McDonald et al. Citation1991). In addition, inclusion of WBG tended to enhance the feeding value including CP and EE contents in ETMR. The TDN of all ETMR slightly or no changed, indicating small losses of nutrients during silage fermentation.
When silage is exposed to air on the opening silo, or after its removal from the silo, fermentation acids and other substrates would be oxidised by undesirable microorganisms. Among of them, yeasts are regarded as the initiator of aerobic deterioration of silage, and a silage with 105 cfu g−1 FW yeasts is susceptible to aerobic deterioration upon air exposure (McDonald et al. Citation1991). And lactate serves as a substrate for the growth and multiplication of yeasts and its consumption is accompanied by heat generation. Intriguingly, results of our aerobic stability experiment showed that ETMR with or without WBG both had considerable resistance to aerobic deterioration, even with high mean values of yeast population (>105 cfu g−1 FW) and LA content (87.9 g kg−1 DM) after the 14-day test. Also, the temperature of all ETMR were no more than 2 °C above the ambient temperature during the whole aerobic exposure phase. This could be ascribed to the relatively high VFAs contents in ETMR. As the basis of VFAs, acetic acid can enter the cell of microorganisms in undissociated form under a low pH (<4.73) environment, and then the undissociated acetic acid would break the acid-base balance in the cells of microorganisms, which delays the growth and potentially results in the death of the cells. Propionic acid and butyric acid also proved to be effective inhibitor of aerobic deterioration (Wilkinson and Davies 2013). Furthermore, certain antifungal compounds were reported to be produced when legume herbage was ensiled (Muck and O’Kiely 1992), suggesting that ingredients other than WBG may also improve the aerobic stability of ETMR.
The contents of ethanol were <3.29 g kg−1 DM during aerobic exposure, suggesting that little inhibitory action was shown. Although alcoholic fermentation may sometimes occur in ETMR, ethanol may have little influence on the stability in the presence of air. This is in agreement with the report of Wang and Nishino (Citation2008), who found that after silo opening, much ethanol would disappear rapidly before the silage is fed to animals. The decrease in ethanol was thus assumed to be due to volatilisation rather than microbial metabolism, indicating that excessive ethanol production should be avoided in order to minimise energy loss between silo opening and animal feeding.
It is worth noting that inclusion of WBG in ETMR improved the aerobic stability and fermentation quality at the same time during aerobic exposure, indicated by significantly higher LA production and lower pH values and NH3-N contents. According to the fact that silages of higher LA contents are less stable after exposure to air (McDonald et al. Citation1991), it was expected that ETMR including WBG would deteriorate faster than control. However, the result obtained was opposite to that assumption. It is thus difficult to explain but may be related to the microbial compositions.
In vitro techniques such as in vitro rumen culture and enzymatic digestion have been developed as common methods to evaluate the nutritional value of ruminant feeds. Measurement of in vitro DM digestibility has been used widely to evaluate the nutritional quality of feeds. Generally, it was expected that replacing common vetch with by-product like WBG may decrease the nutrient digestibility of ETMR. In the present study, no evident decrease was observed in IVDMD, IVNDFD, MCP and potential gas production for the WBG groups compared with the control. The stable digestibility for the replacement ETMR might be in part as a result of the sufficient amounts of physically effective fibre (peNDF) supplied by WBG. Non-forage fibre sources such as WBG can supply energy needed for lactation or growth without the ruminal acid load that often occurs when rapidly fermented starchy feeds are consumed (Schingoethe et al. Citation2009). As one of the most widespread techniques, in vitro gas production is a simple way to evaluate feed quality that reflects the extent of feed fermentation and digestibility. Inclusion of WBG linearly (p < .05) decreased the OMD, ME, NEL, SCFA, GP72, gas production rate constant, half time and AGRP contents, whereas no obvious differences were observed among the control, 10% and 20% WBG silages. This may be because the 10% and 20% WBG silages could still provide enough energy for microbial growth, and therefore maintained nutrient degradation (Kozelov et al. Citation2008; Elghandour et al. Citation2013).
Conclusions
With continuing rise of feed cost, it is significant that continued research should be conducted to investigate the feasibility of feeding the ETMR including WBG to ruminants to enhance the profitability on dairy farms. Results from this study indicated that replacing common vetch in ETMR with 20% WBG had no negative effects on the fermentation quality, nutritive value, aerobic stability, in vitro gas production kinetics and digestibility.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Allen WR, Stevenson KR. 1975. Influence of additives on the ensiling process of wet brewers’ grains. Can J Anim Sci. 55(3):391–402.
- AOAC (Association of Official Analytical Chemists) 1990. Official Methods of Analysis. 15th ed. Association of Official Analytical Chemists, Arlington, VA, USA.
- Blümmel M, Steingaβ H, Becker K. 1997. The relationship between in vitro gas production, in vitro microbial biomass yield and 15N incorporation and its implications for the prediction of voluntary feed intake of roughages. Br J Nutr. 77(6):911–921.
- Cao Y, Takahashi T, Horiguchi K. 2009. Effects of addition of food by-products on the fermentation quality of a total mixed ration with whole crop rice and its digestibility, preference, and rumen fermentation in sheep. Anim Feed Sci Technol. 151(1-2):1–11.
- Chamberlain AT, Wilkinson JM. 1996. Feeding the dairy cow. Lincoln, UK: Chalcombe Publications.
- Chen L, Guo G, Yu C, Zhang J, Shimojo M, Shao T. 2015. The effects of replacement of whole-plant corn with oat and common vetch on the fermentation quality, chemical composition and aerobic stability of total mixed ration silage in Tibet. Anim Sci J. 86(1):69–76.
- Chen L, Yuan X, Li J, Wang S, Dong Z, Shao T. 2017. Effect of lactic acid bacteria and propionic acid on conservation characteristics, aerobic stability and in vitro gas production kinetics and digestibility of whole-crop corn based total mixed ration silage. J Integrative Agric. 16(7):1592–1600.
- Chiou WS, Chen CR, Chen KJ, Yu B. 1998. Wet brewers’ grains or bean curd pomance as partial replacement of soybean meal for lactating cows. Anim Feed Sci Technol. 74(2):123–134.
- Elghandour MMY, Salem AZM, Gonzalez-Ronquillo M, Bórquez JL, Gado HM, Odongo NE, Peñuelas CG. 2013. Effects of exogenous enzymes on in vitro gas production kinetics and ruminal fermentation of four fibrous feeds. Anim Feed Sci Technol. 179(1-4):46–53.
- Getachew G, Makkar HPS, Becker K. 2002. Tropical browses: Contents of phenolic compounds, in vitro gas production and stoichiometric relationship between short chain fatty acid and in vitro gas production. J Agric Sci. 139(3):341–352.
- Harlan DW, Holter JB, Hayes HH. 1991. Detergent fiber traits to predict productive energy of forages fed free choice to nonlactating dairy cattle1. J Dairy Sci. 74(4):1337–1353.
- Kamalak A, Canbolat O, Gurbuz Y, Ozay O. 2011. Comparison of in vitro gas production technique with in situ nylon bag technique to estimate dry matter degradation. Czech J Anim Sci. 50(No. 2):60–67.
- Kozelov LK, Iliev F, Hristov AN, Zaman S, McAllister TA. 2008. Effect of fibrolytic enzyme and an inoculation in vitro degradability and gas production of low-dry matter alfalfa silage. J Sci Food Agric. 88(14):2568–2575.
- Krishnamoorthy U, Muscato TV, Sniffen CJ, Van Soest PJ. 1982. Nitrogen fractions in selected feedstuffs. J Dairy Sci. 65(2):217–225.
- Liu QH, Li XY, Desta ST, Zhang JG, Shao T. 2016. Effects of lactobacillus plantarum and fibrolytic enzyme on the fermentation quality and in vitro digestibility of total mixed rations silage including rape straw. J Integr Agric. 15(9):2087–2096.
- McDonald P, Henderson N, Heron S. 1991. The biochemistry of Silage. 2nd ed. Marlow, UK: Chalcombe Publ.
- Menke KH, Steingass H. 1988. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim Res Dev. 8:7–55.
- Muck RE, O’kiely P. 1992. Aerobic deterioration of Lucerne (Medicago sativa) and maize (Zea mais) silages-effects of fermentation products. J Sci Food Agric. 59(2):145–149.
- Nishino N, Harada H, Sakaguchi E. 2003. Evaluation of fermentation and aerobic stability of wet brewers' grains ensiled alone or in combination with various feeds as a total mixed ration. J Sci Food Agric. 83(6):557–563.
- NRC 2000. Nutrient requirements of beef cattle: update 2000. Washington, DC, USA: National Academy Press.
- SAS (Statistical Analysis System). 2001. Inc: SAS® User’s Guide: Statistics. ver. 8.2 ed. Cary, NC, USA: SAS Institution.
- Schingoethe DJ, Kalscheur KF, Hippen AR, Garcia AD. 2009. Invited review: the use of distillers products in dairy cattle diets. J Dairy Sci. 92(12):5802–5813.
- Shi HT, Li SL, Cao ZJ, Wang YJ, Alugongo GM, Doane PH. 2015. Effects of replacing wild rye, corn silage, or corn grain with CaO-treated corn stover and dried distillers grains with solubles in lactating cow diets on performance, digestibility, and profitability. J Dairy Sci. 98(10):7183–7193.
- Thomas TA. 1977. An automated procedure for the determination of soluble carbohydrates in herbage. J Sci Food Agric. 28:639–642.
- Van Soest PJ, Robertson JB, Lewis BA. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 74(10):3583–3597.
- Wang F, Nishino N. 2008. Resistance to aerobic deterioration of total mixed ration silage: Effect of ration formulation, air infiltration and storage period on fermentation characteristics and aerobic stability. J Sci Food Agric. 88(1):133–140.
- Wang S, Yuan X, Dong Z, Li J, Guo G, Bai Y, Zhang J, Shao T. 2016. Characteristics of isolated lactic acid bacteria and their effects on the silage quality. Asian-Australas J Anim Sci. 30(6):819–827.
- Wang S, Yuan X, Dong Z, Li J, Shao T. 2018. Characteristics of lactic acid bacteria isolated from different sources and their effects on the silage quality of oat (Avena sativa L.) straw on the Tibetan Plateau. Grassl Sci. 64(2):128–136.
- Wilkinson JM, Davies DR. 2013. The aerobic stability of silage: key findings and recent developments. Grass Forage Sci. 68(1):1–19.
- Zhang Q, Zhao M, Wang X, Yu Z, Na R. 2017. Ensiling alfalfa with whole crop corn improves the silage quality and in vitro digestibility of the silage mixtures. Grassl Sci. 63(4):211–217.