 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Diacylglycerol acyltransferase-1 (DGAT1) has been recognised as one of the functional genes during fat deposition and closely related to meat quality. It is well known that yak meat has low intramuscular fat (IMF) and poor tenderness, influencing the purchase decision for consumers. In this study, the variations of DGAT1 gene were detected using polymerase chain reaction single-stranded conformational polymorphism (PCR-SSCP) analysis so as to ascertain the association of DGAT1 variation with yak carcase and meat quality traits. Two variants (A1 and B1) in intron 1 – exon 2 and another three variants (A2 to C2) in intron 15 – exon 17 were identified in the yaks investigated. Three sequence variations (c.192-117 G > A, c.1252-23 C > T and c.1339 C > T) were detected among these variants, with c.192-117 G > A located in intron 1, c.1252-23 C > T located in intron 15 and c.1339 C > T located in exon 17. The mutation c.1339 C > T resulted in an amino acid change (p. Arg 447 Cys). Variants A1 and B2 were associated with a decrease and increase in Warner–Bratzler shear force (WBSF) (p = 0.027 and p < 0.001), respectively. Variant C2 was associated with a decrease in WBSF and hot carcase weights (HCW) (p = 0.001 and p = 0.037, respectively). The presence of haplotype H3 and absence of H5 were highly associated with a decrease in WBSF (p < 0.05). These finding suggested that the variations in DGAT1 could be potential targets for gene-assisted selection to improve meat tenderness in Gannan yak.
Three SNPs were identified in the DGAT1 gene in Gannan yak.
Variants (A1, B2 and C2) and haplotypes (H3 and H5) have a significant influence on Warner–Bratzler shear force (WBSF) in Gannan yak.
DGAT1 variations may have potential in selection for improving meat tenderness in Gannan yaks.
Highlights
Keywords:
Introduction
Carcase and meat quality traits play an economically important role in the value assessment of the beef cattle industry. Among these traits considered, tenderness is a key factor in determining meat quality in the beef industry (Fiems et al. Citation2000). Gannan yak is a unique indigenous Bos grunniens breed well adapted to high altitudes and hypoxic conditions, which is located in the Southwest of the Tibetan Plateau in China. Yak meat has a high protein and minerals content that is beneficial for human health and would be a new resource for meat industry (Tian et al. Citation2013). However, yak breeds are known to produce meat with low intramuscular fat (IMF) and poor tenderness.
Meat tenderness is positively correlated with fat content and composition (Hocquette et al. Citation2010; Nishimura Citation2015). Fat deposition is a complex biological process that is regulated by controlling the activity of enzymes in lipid synthesis and metabolism. Triglycerides (triacylglycerols, TGs) are the major storage form of lipid droplets in adipocytes (Chitraju et al. Citation2019). Diacylglycerol acyltransferases (DGATs), including DGAT1 and DGAT2, are key enzymes for catalysing the terminal step in the formation of TGs through the acylation of diacylglycerol (DAG) with a fatty acyl-CoA and thus regulating lipid digestion, absorption, and glycerol lipid metabolism pathways (Bhatt-Wessel et al. Citation2018). DGAT1 is nearly ubiquitous in eukaryotes and widely expressed in tissues (Turchetto-Zolet et al. Citation2011). Studies have shown that DGAT1 is highly expressed in tissues with TGs synthesis and metabolism, such as subcutaneous fat, longissimus dorsi muscle, breast and small intestine (Cases et al. Citation1998; Chen Citation2006). It is well documented that mice lacking the DGAT1 enzyme may have a 50% reduction in TGs storage in adipose tissue (Smith et al. Citation2000) and a slower rate of fat absorption in the intestines (Hung and Buhman Citation2019). Thus, DGAT1 plays a pivotal role in fat deposition.
DGAT1 is located within a quantitative trait locus (QTL) for fat deposition mapped to bovine chromosome 14 (Casas et al. Citation2003). In previous studies, the expression of DGAT1 was positively correlated to IMF content in Angus cattle and pigs (Anton et al. Citation2011; Cui et al. Citation2019). Variation in DGAT1 has been reported to closely relate to mutton tenderness (Xu et al. Citation2009) and level of beef marbling (Li et al. Citation2013), as well as carcase weight in sheep (Armstrong et al. Citation2018).
Currently, relatively little is known about the effect of DGAT1 on fat-related traits of muscles in Gannan yak. Therefore, the objective of this study was to identify genetic variations of the DGAT1 gene and to evaluate any associations with carcase and meat quality traits to assist with breeding and selection to improve meat quality in Gannan yak.
Materials and methods
Experimental animals and genome DNA extraction
A total of 616 Gannan yaks, obtained from twelve populations that grazed on the plateau pasture under the same feeding and management conditions all year round in Gannan Tibetan Autonomous Prefecture (Gansu, China), were investigated in this study. All yaks ranged in age from three to seven years. When yaks investigated have been slaughtered in slaughterhouse in October, gender and age were recorded, and blood samples were collected from each yak onto Flinders Technology Associates (FTA) cards (Whatman BioScience, Middlesex, UK). Genomic DNA was .............................................................................................. .......................................................................................................................... ............................................................................................... obtained using a two-step procedure (Zhou et al. Citation2006) from FTA cards.
All yaks were humanely sacrificed to alleviate suffering and handled in accordance with an animal use protocol approved by the animal use committee of the Chinese Ministry of Agriculture (Approval number 2006-398).
Carcase and meat quality traits measurements
Hot carcase weights (HCW; kg) from 301 yaks were measured immediately after slaughter. A cross-section of the longissimus dorsi muscle between the twelfth and thirteenth rib of the right carcase side was used to measure the ribeye area (REA; cm2), and meat samples were taken from longissimus dorsi muscle to assess the meat quality, at 48 h post-mortem. Warner-Bratzler shear force (WBSF) was determined according to Fortes et al. (Citation2009) as follows. Meat samples were cooked until reaching an internal temperature of 71 °C, and then removed from the water bath and cooled for 24 h to 5 °C-6 °C. A total of six cores (1.27 cm diameter) were obtained from each sample parallel to the muscle fibre orientation using a sampler. The analysis using the Digital Muscle Tenderometer (Model C-LM3, Northeast Agricultural University, Harbin, China) that had crosshead speed force set at 200 mm/min to measure the WBSF (kg). The 6 core values were used to calculate mean WBSF value for each sample. Meat samples used for drip loss rate (DLR; %) measurement were cut from the longissimus dorsi muscle and immediately weighed (initial weight). Then, samples were suspended in an inflated bag and were again weighted after a storage period at chill temperature. At the time of cooked meat rate (CMR; %) measurement, the initial weight of samples were firstly weighted. Then, samples were heated in a water-bath. When the endpoint temperature has been attained, the samples were removed and cooled in an ice slurry and used to weight ( Honikel.Citation1998).
Polymerase chain reaction (PCR) amplification and Single-Stranded conformational polymorphism (SSCP) analysis
To rapidly detecting sequence variation in DGAT1 gene, genomic DNA from 50 yak individuals were randomly selected and used as a template for polymerase chain reaction (PCR) amplification. Eight sets of primers (Table ) for PCR were designed based on the bovine sequence (GenBank no. AY065621.1) to amplify the entire CDS region and some introns of DGAT1. All primers were designed by Primer-BLAST (NCBI https://www.ncbi.nlm.nih.gccov) and synthesised by Huada Biological Engineering Co., Ltd. PCR amplification was performed in a 20 μL reaction mixture, containing 100 ng of genomic DNA (one 1.2-mm punch of blood spot on FTA card), 0.25 μΜ of each primer, 150 μΜ of each dNTP (Takara, Japan), 2.5 mM Mg2+, 0.5 U of Taq DNA polymerase (Takara, Japan), 2.0 μL of 10 × PCR buffer and ddH2O to make up the volume. The cycling protocol was initial denaturation at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 57–64 °C (Table ) for 30 s, and extension at 72 °C for 30 s and a final extension at 72 °C for 5 min. PCR products were checked using 1% agarose gel electrophoresis and then were sequenced in both directions at Sangon Biotech (Shanghai, China).
Table 1. PCR primers for sequencing in yak DGAT1.
According to the sequencing results, variations were detected in intron 1-exon 2 and intron 15-exon 17 of DGAT1. Therefore, these two fragments were selected for the following SSCP analysis. The mixture of 2.0 μL PCR products with 8 μL denaturing solution (98% formamide, 10 mM ethylenediaminetetraacetic acid (EDTA), 0.025% bromophenol blue, 0.025% xylene-cyanol) was denatured at 95 °C for 5 min. Subsequently, this mixture was rapidly chilled on ice and then loaded on 16 cm × 18 cm 12% acrylamide: bisacrylamide (37.5:1) gels containing 3.5% v/v glycerol. Samples were electrophoresed in 0.5 × Tris-boric-acid-EDTA (TBE) buffer under electrophoretic conditions of 15 °C, 240 V, 11 h (intron 1 – exon 2) or 23 °C, 250 V, 15 h (intron 15 – exon 17). Gels were stained with silver nitrate using the method of Byun et al. (Citation2009).
Variant sequencing and analysis
PCR amplicons that were confirmed to be homozygous by SSCP were directly sequenced in both directions at Sangon Biotech (Shanghai, China). Variants that were only found in heterozygous yaks were sequenced using an approach described by Hu et al. (Citation2010). Briefly, a band corresponding to the variant was cut as a gel slice from the polyacrylamide gel, macerated, and then used as a template for re-amplification with the original primers. This second amplicon was then sequenced directly as described above for the homozygous patterns. Nucleotide sequence alignments were analysed by MEGA 5.0 software (CEMI, Tempe, AZ, USA) to determine the single-nucleotide polymorphisms (SNPs) location.
Haplotype determination
Haplotypes were constructed for intron 1 – exon 2 and intron 15 – exon 17 of DGAT1. Progeny that typed as homozygous in either of the regions could directly infer their haplotypes. If progeny typed as heterozygous in both regions, the haplotypes could not be analysed. This reduced the number of yaks studied in the haplotype association analyses.
Statistical analysis
Association analyses of DGAT1 variants and haplotypes with carcase and meat quality traits were performed using the General Linear Mixed Models (GLMMs) of IBM SPSS 22.0 software (IBM Corp., Armonk, NY, USA). The model was calculated as follows:
where Yijk is the trait of interest, μ is the overall mean, Gi (Hi) is the fixed effect of the ith variant (i = 0 or 1) or the fixed effect of the ith haplotype (i = 0 or 1) or the fixed effect of the ith number of copies of the haplotype ((i = 0, 1, 2), Pj is the fixed effect of the jth population (j = 1,…,12), Sk is the fixed effect of the sex (k = 1(male), 2 (female)), Al is the covariate effect of age (l = 3,…,7), and eijkl is the random error.
Each variant/haplotype was coded as either presence (1) or absence (0) for each yak investigated. Any variant and haplotype that had associations in the single-variant/haplotype models with a p-value of less than 0.20, and thus could potentially impact on the carcase and meat quality traits, were factored into the subsequent multi-variant/haplotype models. For these haplotypes with sufficiently common homozygous forms (>1% of all genotypes), a second set of analyses was performed with the number of haplotype copies present included (in place of presence or absence), followed by planned orthogonal contrasts to ascertain whether additive, dominant or recessive effects were present. These models were conducted in an identical manner to the GLMMs used for testing the presence/absence of each haplotype. Unless otherwise indicated, all p-value less than 0.05 were considered to be significantly different.
Results
Identification of variation in yak DGAT1
In 616 Gannan yaks investigated, three distinct genotypes A1A1, A1B1 and B1B1 were detected in intron 1 – exon 2 and a further five distinct genotypes A2A2, A2B2, A2C2, B2B2 and C2C2 were detected in intron 15 – exon 17 by PCR-SSCP (Figure ). Three SNPs were detected among those sequences, with c.192-117 G > A in intron 1, c.1252-23 C > T in intron 15 and c.1339 C > T in exon 17 (Figure ). Moreover, the c.1339 C > T was a non-synony mous SNP and would result in an arginine to cysteine substitution (p. Arg 447 Cys). All sequences were submitted to GenBank (accession number MN012932 to MN012936).
Figure 1. PCR-SSCP analysis of the DGAT1 gene in Gannan yaks. (A) SSCP banding patterns for two regions of yak DGAT1 gene. (B) Single-nucleotide polymorphism (SNP) detected in intron 1 – exon 2 and intron 15 – exon 17 of DGAT1 gene. (C) Confirmation of single nucleotide variations in two regions using sequence.
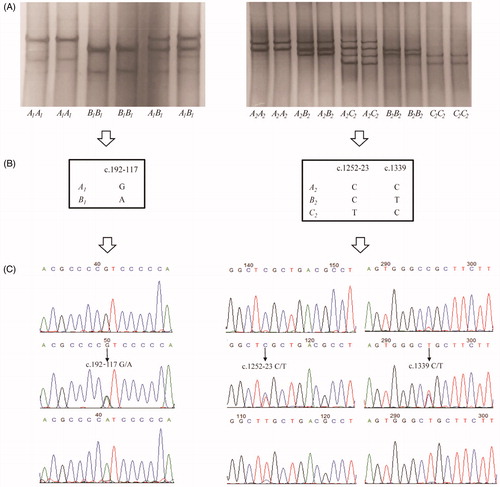
Polymorphisms in yak DGAT1
The genotype and variant frequencies of yak DGAT1 are shown in Table . In intron 1 – exon 2, A1A1 and A1 were the most common genotype and variant, with a frequency of 50.65% and 70.13%, respectively. In intron 15 – exon 17, A2A2 and A2 were the most common genotype and variant, with a frequency of 37.34% and 57.55%, respectively.
Table 2. Genotype and variant frequencies of DGAT1 gene in Gannan yak.
Construction and frequencies of haplotype in yak DGAT1 gene
Six haplotypes were constructed for intron 1 – exon 2 and intron 15 – exon 17 of DGAT1 in a subset of 519 individuals out of 616 Gannan yak (Table ). Of these haplotypes, H1, H2, H3 and H4 were the most common, occurring at frequencies of 42.20%, 14.45%, 17.24% and 16.28%, respectively. H5 and H6 haplotypes were rare, with frequencies of 5.68% and 4.14%, respectively.
Table 3. Haplotype construction and frequencies of the DGAT1 gene in Gannan yak.
Association between DGAT1 variants and carcase and meat quality traits
In the single-variant (presence/absence) model (Table ), the presence of variant A1 (in intron 1) was found to be associated with decrease WBSF (p = 0.027). The presence of variant B2 (in exon 17) was associated with an increase in WBSF (p < 0.001), whereas the presence of C2 (in intron 15) was related to decrease WBSF and HCW (p = 0.001 and p = 0.037, respectively). These associations remained significant when the other variants (p < 0.20) were factored into the models. No other significant associations were detected for any variant in both regions of yak DGAT1 (p > 0.05).
Table 4. Association of DGAT1 variants with carcase and meat quality traits in Gannan yak.
Association between haplotypes in DGAT1 and carcase and meat quality traits
Haplotype frequencies lower than 5% were excluded from association analyses. In the single-haplotype (presence/absence) model (Table ), the presence of haplotype H3 and the absence of haplotype H5 were associated with a decrease in WBSF (p = 0.002, p < 0.001).These associations persisted in the multi-haplotype models. No associations were found between the other haplotypes and DLR, CMR, REA and HCW (p > 0.05). A second set of analyses was performed with the number of haplotype copies present (in place of presence/absence). For WBSF traits, the presence of two copies of H3 and the absence of H5 were associated with decrease WBSF in both single-haplotype and multi-haplotype models (Table ).
Table 5. Association of DGAT1 haplotypes with carcase and meat quality traits in Gannan yak.
Table 6. Association of DGAT1 haplotypes copy number with carcase and meat quality traits in Gannan yak.
Discussion
Most of the meat quality traits, especially meat tenderness, have been presenting genetic differences among cattle populations (Xie et al. Citation2012; Boudon et al. Citation2020). Numer ous studies have been shown that calpain and calpastatin genotypes were associated with meat tenderness in different cattle populations (Casas et al. Citation2006; Curi et al. Citation2009; Allais et al. Citation2011; Tait et al. Citation2014) because of their key role in proteolysis. However, meat tenderness is under polygenic control. Identifying more gene markers related to meat quality traits would improve meat tenderness prediction. DGAT1 is an important functional enzyme involved in TGs biosynthesis and metabolism, and the gene encoding this protein has been considered to be a genetic factor influencing IMF deposition and meat tenderness in cattle (Thaller et al. Citation2003; Harris et al. Citation2011). This is the first study to report the associations between DGAT1 variants or haplotypes and carcase or meat quality traits in yaks and the findings suggest that these genetic variations may play an important role in yak meat tenderness trait.
Studies have shown that 17 and 5 SNPs in DGAT1 were detected in bovine and sheep, respectively (Scatà et al. Citation2009; Yuan et al. Citation2013). Also, Yuan et al. (Citation2007) reported that 7 SNPs were observed in the buffalo DGAT1 gene, and the c. 1481 C > T (originally g.11,785 C > T) was located in exon 17, potentially resulting in an alanine to valine substitution (p. Ala 484 Val). In this study, a total of 3 SNPs c.192-117 G > A, c.1252-23 C > T and c.1339 C > T were detected in intron 1, intron 15 and exon 17 of DGAT1 in Gannan yak, respectively. Substitution c.1339 C > T would potentially result in the 447th amino acid in the mature peptide from Arg to Cys.
DGAT1 is the key enzyme to catalyse TGs synthesis and the DGAT1 variations have been reported to be associated with meat quality traits (Thaller et al. Citation2003; Pannier et al. Citation2010). The K232A substitution at exon 8 of the DGAT1 was associated with IMF content and beef marbling level (Li et al. Citation2013). Yuan et al. (Citation2013) investigated c.1416 T > G (p. Gly 468 Val) in exon 17 of bovine DGAT1 gene and reported that the sequence variation had an effect on fatness deposited, WBSF and marbling score. Xu et al. (Citation2009) also reported that the c.1461 T > C in exon 17 of DGAT1 in sheep was positively correlated with WBSF, DLR, and negative with IMF and muscle marbling score. Similarly, Mohammadi et al. (Citation2013) found that the variations in exon 16 − 17 of DGAT1 were significantly associated with back fat thickness and other fat-related traits in Iranian sheep. In this study, the presence of variant B2 in exon 17 was associated with an increase in WBSF, and then had a negative effect on meat tenderness in Gannan yak. The significance of the substitution of Arg to Cys is unknown, but given that cysteine is essential for the formation of disulphide bonds (Wang et al. Citation2017), it is conceivable that this amino acid substitution may affect the DGAT1 protein folding. It is reported that the p. Arg 92 Cys substitution in colipase impaired its function and secretion by increasing protein misfolding (Xiao et al. Citation2013). Therefore, we speculate that the c.1339 C > T SNP may affect the structure and function of the DGAT1 protein, nevertheless further investigation is required.The association between intron variations of DGAT1 and the fat-related traits has been reported in numerous studies (Angiolillo et al. Citation2007; Armstrong et al. Citation2018). For instance, the SNP in intron 16 of DGAT1 may influence milk fat content and other economical traits in goat (Angiolillo et al. Citation2007). Armstrong et al. (Citation2018) showed that c. 512 + 411 C > T (originally rs411875883) in intron 6 of DGAT1 in sheep was highly associated with REA, HCW, and fat thickness. Consistent with these previous studies, the presence of variant A1 (in intron 1) and C2 (in intron 15) were associated with a decrease in WBSF and thus positively correlated with yak meat tenderness. In addition, variant C2 was also associated with HCW in Gannan yak. Although these variations were not located in the coding regions of DGAT1, they may impact gene function in other ways. There is some evidence that variations in introns may not directly affect the structure of gene, but could influence the transcription efficiency by affecting regulatory elements, such as enhancers, silencers, or other DNA structures (Chorev and Carmel Citation2012). Moreover, variation in introns may be linked to other variation in coding regions of the gene or regions that may affect the processing of the primary transcript or its stability (An et al. Citation2018).
Scheike et al. (Citation2010) reported that molecular markers could be accurately identified when integrating the SNPs and haplotypes into association analysis. Remarkably, haplotypes are particular combinations of variant sequences on a single chromosome (Hu et al. Citation2019) and may be considerably more powerful than single SNP analysis in association studies (Hagenblad et al. Citation2004). Aslan et al. (Citation2010) reported that the HAP2 was association with increased IMF and juiciness and HAP4 was correlated with tenderness in bovine, and these two haplotype in Ankyrin 1 gene could be used to select individuals with improved IMF, juiciness or tenderness. In current investigation, haplotype were constructed with 3 SNPs, and the presence of haplotype H3 and absence of H5 were highly related to a decrease in WBSF, suggesting that H3 and H5 may have potential in selection for yak meat tenderness. Meat tenderness is a complex quantitative trait regulated by multiple genes. Therefore, it is necessary to increase the sample size or study the effects of other related genes on tenderness to determine the common effect of DGAT1 gene and other related genes on meat tenderness.
Conclusions
In summary, three SNPs were identified in the DGAT1 gene in Gannan yak. Variants and haplotypes of intron 1, intron 15 and exon 17 in DGAT1 were significantly associated with WBSF of the longissimus dorsi muscle and had an important effect on yak meat tenderness. These results suggest that the DGAT1 variations may have potential in selection for improving meat tenderness in Gannan yaks.
Acknowledgements
The authors thank the co-workers of the Gansu Key Laboratory of Herbivorous Animal Biotechnology for the sample and data collection as well as analyses.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Allais S, Journaux L, Levéziel H, Payet-Duprat N, Raynaud P, Hocquette JF, Lepetit J, Rousset S, Denoyelle C, Bernard-Capel C, et al. 2011. Effects of polymorphisms in the calpastatin and µ-calpain genes on meat tenderness in 3 French beef breeds. J Anim Sci. 89(1):1–11.
- An QM, Zhou HT, Hu J, Luo YZ, Hickford JGH. 2018. Sequence and haplotypes variation of the ovine uncoupling protein-1 gene (UCP1) and their association with growth and carcass traits in new zealand romney lambs. Genes. 9(4):189.
- Angiolillo A, Amills M, Urrutia B, Domenech A, Sastre Y, Badaoui B, Jordana J. 2007. Identification of a single nucleotide polymorphism at intron 16 of the caprine Acyl-Coenzyme A: diacylglycerol acyltransferase 1 (DGAT1) gene. J Dairy Res. 74(1):47–51.
- Anton I, Kovács K, Holló G, Farkas V, Lehel L, Hajd Z, Zsolnai A. 2011. Effect of leptin, DGAT1 and TG gene polymorphisms on the intramuscular fat of Angus cattle in Hungary. Livestock Sci. 135(2-3):300–303.
- Armstrong E, Ciappesoni G, Iriarte W, Da Silva C, Macedo F, Navajas EA, Brito G, San Julián R, Gimeno D, Postiglioni A. 2018. Novel genetic polymorphisms associated with carcass traits in grazing Texel sheep. Meat Sci. 145:202–208.
- Aslan O, Sweeney T, Mullen AM, Hamill RM. 2010. Regulatory polymorphisms in the bovine Ankyrin 1 gene promoter are associated with tenderness and intramuscular fat content. BMC Genet. 11(1):111.
- Bhatt-Wessel B, Jordan TW, Miller JH, Peng L. 2018. Role of DGAT enzymes in triacylglycerol metabolism. Arch Biochem Biophys. 655(1):1–11.
- Boudon S, Henry-Berger J, Cassar-Malek I. 2020. Aggregation of omic data and secretome prediction enable the discovery of candidate plasma biomarkers for beef tenderness. IJMS. 21(2):664.
- Byun SO, Fang Q, Zhou H, Hickford JGH. 2009. An effective method for silver-staining DNA in large numbers of polyacrylamide gels. Anal Biochem. 385(1):174–175.
- Casas E, Shackelford SD, Keele JW, Koohmaraie M, Smith TP, Stone RT. 2003. Detection of quantitative trait loci for growth and carcass composition in cattle. J Anim Sci. 81(12):2976–2983.
- Casas E, White SN, Wheeler TL, Shackelford SD, Koohmaraie M, Riley DG, Chase CC, Johnson DD, Smith TPL. 2006. Effects of calpastatin and Micro-calpain markers in beef cattle on tenderness traits. J Anim Sci. 84(3):520–525.
- Cases S, Smith SJ, Zheng YW, Myers HM, Lear SR, Sande E, Novak S, Collins C, Welch CB, Lusis AJ, et al. 1998. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc Natl Acad Sci USA. 95(22):13018–13023.
- Chen HC. 2006. Enhancing energy and glucose metabolism by disrupting triglyceride synthesis: lessons from mice lacking DGAT1. Nutr Metab (Lond). 3:10.
- Chitraju C, Walther TC, Farese RV. 2019. The triglyceride synthesis enzymes DGAT1 and DGAT2 have distinct and overlapping functions in adipocytes. J Lipid Res. 60(6):1112–1120.
- Chorev M, Carmel L. 2012. The function of introns. Front Genet. 3:55.
- Cui JX, Zeng QF, Chen W, Zhang H, Zeng YQ. 2019. Analysis and preliminary validation of the molecular mechanism of fat deposition in fatty and lean pigs by high-throughput sequencing. Mamm Genome. 30(3-4):71–80.
- Curi RA, Chardulo LA, Mason MC, Arrigoni MD, Silveira AC, de Oliveira HN. 2009. Effect of singlenucleotide polymorphisms of CAPN1 and CAST genes on meat traits in Nellore beef cattle (Bos indicus) and in their crosses with Bos taurus. Anim Genet. 40(4):456–462.
- Fiems LO, Campeneere SD, De Smet S, Van de Voorde G, Vanacker JM, Boucqué CV. 2000. Relationship between fat depots in carcasses of beef bulls and effect on meat colour and tenderness. Meat Sci. 56(1):41–47.
- Fortes MRS, Curi RA, Chardulo LAL, Silveira AC, Assumpção MEOD, Visintin JA, de Oliveira HN. 2009. Bovine gene polymorphisms related to fat deposition and meat tenderness. Genet Mol Biol. 32(1):75–82.
- Hagenblad J, Tang C, Molitor J, Werner J, Zhao K, Zheng H, Marjoram P, Weigel D, Nordborg M. 2004. Haplotype structure and phenotypic associations in the chromosomal regions surrounding two Arabidopsis thaliana flowering time loci. Genetics. 168(3):1627–1638.
- Harris CA, Haas JT, Streeper RS, Stone SJ, Kumari M, Yang K, Han X, Brownell N, Gross RW, Zechner R, et al. 2011. DGAT enzymes are required for triacylglycerol synthesis and lipid droplets in adipocytes. J Lipid Res. 52(4):657–667.
- Hocquette JF, Gondret F, Baéza E, Médale F, Jurie C, Pethick DW. 2010. Intramuscular fat content in meat-producing animals: Development, genetic and nutritional control, and identification of putative markers. Animal. 4(2):303–319.
- Honikel KO. 1998. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 49(4):457–477.
- Hu J, Shi BG, Xie JP, Zhou HT, Wang JQ, Liu X, Li SB, Zhao ZD, Luo YZ. 2019. Tissue expression and variation of the DGAT2 gene and its effect on carcass and meat quality traits in yak. Animals. 9(2):61.
- Hu J, Zhou H, Smyth A, Luo Y, Hickford JGH. 2010. Polymorphism of the bovine ADRB3 gene. Mol Biol Rep. 37(7):3389–3392.
- Hung YH, Buhman KK. 2019. DGAT1 deficiency disrupts lysosome function in enterocytes during dietary fat absorption. Biochim Biophys Acta Mol Cell Biol Lipids. 1864(4):587–595.
- Li X, Ekerljung M, Lundström K, Lundén A. 2013. Association of polymorphisms at DGAT1, leptin, SCD1, CAPN1 and CAST genes with color, marbling and water holding capacity in meat from beef cattle populations in Sweden. Meat Sci. 94(2):153–158.
- Mohammadi H, Shahrebabak MM, Sadeghi M. 2013. Association between single nucleotide polymorphism in the ovine DGAT1 gene and carcass traits in two Iranian sheep breeds. Anim Biotechnol. 24(3):159–167.
- Nishimura T. 2015. Role of extracellular matrix in development of skeletal muscle and postmortem aging of meat. Meat Sci. 109:48–55.
- Pannier L, Mullen AM, Hamill RM, Stapleton PC, Sweeney T. 2010. Association analysis of single nucleotide polymorphisms in DGAT1, TG and FABP4 genes and intramuscular fat in crossbred Bos taurus cattle. Meat Sci. 85(3):515–518.
- Scatà MC, Napolitano F, Casu S, Carta A, De Matteis G, Signorelli F, Annicchiarico G, Catillo G, Moioli B. 2009. Ovine acyl CoA:diacylglycerol acyltransferase 1- molecular characterization, polymorphisms and association with milk traits. Anim Genet. 40(5):737–742.
- Scheike TH, Martinusse T, Silver JD. 2010. Estimating haplotype effects for survival data. Biometrics. 66(3):705–715.
- Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, Sanan DA, Raber J, Eckel RH, Farese RV. 2000. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet. 25(1):87–90.
- Tait RG Jr, Shackelford SD, Wheeler TL, King DA, Keele JW, Casas E, Smith TPL, Bennett GL. 2014. CAPN1, CAST, and DGAT1 genetic effects on preweaning performance, carcass quality traits, and residual variance of tenderness in a beef cattle population selected for haplotype and allele equalization. J Anim Sci. 92(12):5382–5393.
- Thaller G, Kühn C, Winter A, Ewald G, Bellmann O, Wegner J, Zühlke H, Fries R. 2003. DGAT1, a new positional and functional candidate gene for intramuscular fat deposition in cattle. Anim Genet. 34(5):354–357.
- Tian J, Han L, Yu Q, Shi X, Wang W. 2013. Changes in tenderness and cathepsins activity during post mortem ageing of yak meat. Can J Anim Sci. 93(3):321–328.
- Turchetto-Zolet AC, Maraschin FS, de Morais GL, Cagliari A, Andrade CM, Margis-Pinheiro M, Margis R. 2011. Evolutionary view of acyl-CoA diacylglycerol acyltransferase (DGAT), a key enzyme in neutral lipid biosynthesis. BMC Evol Biol. 11:263.
- Wang JJ, Liu G, Huang YB, Zeng QH, Hou Y, Li L, Ou S, Zhang M, Hu SQ. 2017. Dissecting the disulfide linkage of the N-terminal domain of HMW 1Dx5 and its contributions to dough functionality. J Agric Food Chem. 65(30):6264–6273.
- Xiao XJ, Ferguson MR, Magee KE, Hale PD, Wang Y, Lowe ME. 2013. The Arg92Cys colipase polymorphism impairs function and secretion by increasing protein misfolding. J Lipid Res. 54(2):514–521.
- Xie X, Meng Q, Cui Z, Ren L. 2012. Effect of cattle breed on meat quality, muscle fiber characteristics, lipid oxidation and fatty acids in china. Asian-Australas J Anim Sci. 25(6):824–831.
- Xu QL, Chen YL, Ma RX, Xue P. 2009. Polymorphism of DGAT1 associated with intramuscular fat-mediated tenderness in sheep. J Sci Food Agric. 89(2):232–237.
- Yuan J, Zhou J, Deng X, Hu X, Li N. 2007. Molecular cloning and single nucleotide polymorphism detection of buffalo DGAT1 gene. Biochem Genet. 45(7-8):611–621.
- Yuan ZG, Li JY, Li J, Gao X, Gao HJ, Xu SZ. 2013. Effects of DGAT1 gene on meat and carcass fatness quality in Chinese commercial cattle. Mol Biol Rep. 40(2):1947–1954.
- Zhou H, Hickford JGH, Fang Q. 2006. A two-step procedure for extracting genomic DNA from dried blood spots on filter paper for polymerase chain reaction amplification. Anal Biochem. 354(1):159–161.
