Abstract
This study investigated the effects of Artemisia argyi extract (AAE) on broilers challenged with lipopolysaccharide (LPS). A total of 96 Arbour Acres broilers (1-d-old) were assigned to a 2 × 2 factorial arrangement with two dietary treatments (AAE at 0 or 1000 mg/kg) and two immunological challenge treatments (saline or LPS). On d 14, 16, 18 and 20, the broilers were injected intra-abdominally with LPS solution at 500 μg/kg of body weight or an equivalent amount of sterile saline. Blood, liver, spleen and small intestine were collected on day 21. The increased relative weights of the thymus and spleen induced by LPS were significantly decreased by AAE supplementation. The level of serum nitric oxide (NO) and the activity of inducible NO synthase (iNOS) were significantly compromised by AAE inclusion. Dietary AAE significantly inhibited the mRNA expression of toll-like receptor 4 (TLR4), myeloid differentiation factor 88, nuclear factor-kappa B (NF-κB) and iNOS in the different tissues of LPS-challenged broilers. The AAE supplementation tended to increase the levels of serum GSH-Px and CAT, but significantly reduced the level of serum malondialdehyde (MDA). Collectively, feeding AAE to LPS-challenged broilers could decrease serum NO level, maintain the relative weight of internal organs, enhance the antioxidant capacity, and inhibit TLR4/NF-κB signalling pathway at the transcriptional level.
AAE inclusion significantly inhibited the serum NO level, and the activity of iNOS induced by LPS.
AAE supplementation tended to increase the levels of serum GSH-Px and CAT, but significantly reduced the level of serum MDA.
Feeding AAE to the LPS-challenged broilers reduced the expression of TLR4, MyD88 and NF-κB in the liver, spleen and small intestine.
Highlights
Introduction
Broilers are unavoidably confronted with the stress related to bacteria or their products even in normal situations, such as lipopolysaccharide (LPS), which can lead to an intensive systemic inflammatory reaction (Wang et al. Citation2004; Marketon and Glaser Citation2008; Nishanth et al. Citation2011) and oxidative stress (Suliman et al. Citation2004). LPS can be recognised by toll-like receptor 4 (TLR4), activate the downstream nuclear factor-kappa B (NF-κB) signalling pathway and ultimately lead to the release of nitric oxide (NO), tumour necrosis factor-alpha (TNF-α) and reactive oxygen species (Miller et al. Citation2005; Morgan and Liu Citation2011). Although these mediators play important roles in the regulation of immunological and antioxidant systems, the over-accumulation of them can compromise immune defence and antioxidant system, finally inhibiting the growth performance of animals (Johnson Citation1997; Jacobi et al. Citation2006). It is, therefore, important to minimise the negative impact of immune stress, and diet supplemented with natural plant extract could represent a better solution (Li et al. Citation2015a; Liu et al. Citation2015).
A previous study demonstrated that dietary Artemisia argyi extract (AAE) could relieve the growth inhibition caused by LPS, partly through inhibiting the production of stress hormone (adernocorticotropic hormone and corticosterone), inflammatory cytokines (interleukin-1, 2, 6) and immunoglobulin G (Zhang et al. Citation2017). In addition, the antioxidant properties of A. argyi water extracts and the inhibition of NO and TNF-α production induced by LPS challenge has also been proved in vitro (Diaz et al. Citation2012). These studies provided the preliminary understandings of the anti-stress effect of AAE, but the regulatory mechanisms of AAE stayed unclear. Therefore, the aim of this study was to further investigate the protective effects of AAE on the inflammatory mediators and antioxidant function of broilers challenged with LPS. To further investigate the molecular mechanism of AAE, the gene expression of NO, antioxidant enzymes and TLR4/NF-κB signalling pathway was examined. This may provide a basis for the use of AAE as a potential natural feed additive.
Materials and methods
Preparation of A. argyi extract
For this study, A. argyi was collected at a local farm (Hohhot, Inner Mongolia, China), and the water extract powders were prepared based on a previous study (Zhang et al. Citation2017). Before mixing with basal diet, the extraction rate, total polysaccharide (Saha and Brewer Citation1994), total flavonoid and total phenol (Wan et al. Citation2016) of AAE were determined as previously described.
Animal handling and diets
All experimental procedures performed in this study were approved by the Animal Care and Use Committee of Inner Mongolia Agricultural University (Hohhot, China).
A total of 96 one-d-old mixed-sex Arbour Acres broilers were distributed in a 2 × 2 factorial arrangement, the main factors were AAE-supplemented diet and immunological challenge. All broilers were randomly divided into two treatment groups with two subgroups each. Each subgroup consisted of six replicates (one replicate per cage) with four broilers per replicate. The broilers in both groups were fed the basal diet (control), or the basal diet supplemented with AAE at 1000 mg/kg for 21 d. On day 14, 16, 18 and 20 of age, broilers from each subgroup of the two groups were injected intra-abdominally either with LPS solution (Escherichia coli, serotype O55:B5, L2880; Sigma-Aldrich, St. Louis, MO) at the dose of 5 mL/kg of body weight (LPS was dissolved in sterile saline at a concentration of 100 µg/mL) or with an equal dose of 0.9% sterile saline. During the 21 d’ trial, the broilers were fed a maize- and soybean-based basal diet (Table ) that was formulated to meet the nutritional requirements of broilers according to the National Research Council (Citation1994) and Agricultural Industry Standards of P.R. China (Feed Standard of Chicken, NY/T 33-2004). Feed and water were provided ad libitum throughout the experiment.
Table 1. Composition and nutrient levels of the basal diet (calculated on an air-dry basis).
Sampling and measurements
Blood analysis
At 21 d of age, one broiler from each replicate was randomly selected and a 5 mL blood sample was obtained from the wing vein. Serum samples were obtained by centrifugation at 1 790×g for 10 min at room temperature and later stored in microtubes at −20 °C. The levels of NO, inducible NO synthase (iNOS), malondialdehyde (MDA), total antioxidant capacity (T-AOC), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) and catalase (CAT) were measured using a commercial ELISA kit with quantitative sandwich enzyme immunoassay technique (R & D Systems China, Shanghai, China).
Relative weights of internal organs
At 21 d of age, broilers were euthanised by cervical dislocation and dissected. The thymus, spleen and bursa of Fabricius were collected and the weight of each organ was immediately measured. The results were expressed as relative weight to body weight (g/kg of body weight).
Collection of liver, spleen and small intestine
At 21 d of age, the liver and spleen were collected, washed with ice-cold sterile saline solution to remove blood contamination, then placed in cryogenic vials. The small intestine was dissected, and 7cm intestinal segments were collected from the distal duodenum, mid-jejunum and mid-ileum. The intestinal segments were opened longitudinally and the contents were rinsed gently with RNase-free water and then placed in cryogenic vials. All samples were stored at −80 °C until analysis of relative gene expression.
Real-time quantitative PCR
The mRNA levels of TLR4, myeloid differentiation factor 88 (MyD88), NF-κB p65, iNOS, SOD, CAT and GPx7 in the liver, spleen and small intestine were determined by the SYBR Green II chimeric fluorescence method (Zhang et al. Citation2014). Total RNA was extracted from tissue samples using an RNAiso Plus kit (TaKaRa Biotechnology, Dalian, China). The integrity of the RNA was checked using agarose gel electrophoresis with ethidium bromide staining. The concentration and purity of the RNA were determined using an automatic microplate reader (Synergy H4; BioTek, Tokyo, Japan) at an optical density of 260 and 280 nm. The OD260/280 ratio value of total RNA between 1.8 and 2.1 is assumed as suitable for gene expression measurements. After this, 1 µg of total RNA was reverse-transcribed into cDNA using a PrimeScript RT Reagent Kit with gDNA Eraser (TaKaRa Biotechnology). Real-time PCR was performed on an ABI StepOnePlus Real-Time PCR System (Applied Biosystems, Grand Island, NY) using a SYBR Premix Ex Taq II Reagent Kit (TaKaRa Biotechnology) according to the manufacturer’s instruction. Briefly, the total reaction volume was 20 µL, which consisted of 10 µL of SYBR Premix Ex Taq II, 0.4 µL of PCR forward primer (10 µM), 0.4 µL of PCR reverse primer (10 µM), 2 µL of cDNA and 7.2 µL of RNase-free water. Primer sequences of the target genes (TLR4, MyD88, NF-κB p65, iNOS, SOD, CAT and GSH-Px) and the reference gene (β-actin) were obtained from GenBank and are shown in Table . The relative levels of mRNA expression were calculated using the 2−ΔΔCt method after normalisation against the reference gene β-actin (Livak and Schmittgen Citation2001).
Table 2. Genes and their primer sequences.
Statistical analysis
All data were analysed using the GLM procedure of SAS version 9.0 software (SAS Institute, Inc., Cary, NC; SAS Citation2003) according to a 2 × 2 factorial arrangement with dietary treatments of AAE and LPS challenge as the main effects, and the broiler in each replicate as the experimental unit. If a significant interactive effect was observed, the significance level of the differences between treatments was identified using Duncan’s multiple comparisons test. A probability level of p < .05 was chosen as the limit for statistical significance, whereas probability level of .05 < p < 0.1 was considered to be a tendency. Results were presented as the mean and standard error of the mean (SEM).
Results and discussion
Main content of AAE
The extraction rate of AAE (Table ) was 73.9 ± 0.20 mg per g of A. argyi plant powder. The contents of total polysaccharide, flavonoid and phenol in AAE were 93.8 ± 0.20 mg glucose equivalents per g of AAE, 109.7 ± 0.92 mg rutin equivalents per g of AAE and 51.0 ± 0.13 mg gallic acid equivalents per g of AAE, respectively. The above bio-chemicals have been found to possess immune-modulating and antioxidative effect (Cai et al. Citation2004; Lan et al. Citation2010; Bao et al. Citation2013; Lv et al. Citation2013).
Table 3. The extraction rate, total polysaccharide, total flavonoid and total phenol, of Artemisia argyi extract (AAE).
Table 4. Effects of Artemisia argyi extract (AAE) on the relative weights of internal organs in broilers challenged with lipopolysaccharide (LPS; g/kg of body weight).
Relative weights of immune organs
In comparison with the unchallenged groups, LPS injection (Table ) increased the relative weights of the thymus (p < .01) and spleen (p < .01) of the broilers, which were likely due to the urgent need for the synthesis of antibodies and cytokines during the inflammatory reaction (Li et al. Citation2015b). However, the increased relative weights of the thymus (p= .03) and spleen (p= .05) induced by LPS were counteracted by AAE supplementation. The possible reason for this may be the inhibitory effect of AAE on the production of inflammatory cytokines (interleukins-1 and -6) and immunoglobulins (immunoglobulins A and G), which would lower the inflammatory response and weaken the over-activation of the immune system (Zhang et al. Citation2017).
Table 5. Effects of Artemisia argyi extract (AAE) on the immune response of broilers challenged with lipopolysaccharide (LPS).
Serum NO content, iNOS activity and gene expression related to TLR4/NF-κB signalling pathway
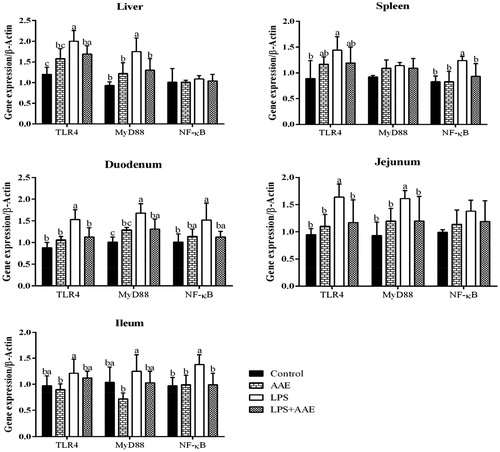
Injection of LPS (Table ) increased the serum NO contents (p= .05) and the activity of iNOS (p= .04). Several in vitro cellular tests have shown that A. argyi and its water extract possess anti-inflammatory properties in LPS-challenged macrophages through reduction of the levels of NO, TNF-α and iNOS (Diaz et al. Citation2012; Wang et al. Citation2013; Zeng et al. Citation2014). In this study, the increased serum contents of NO (p= .04) and the increased activity of iNOS (p= .03) induced by LPS injection were alleviated by AAE supplementation (), which implies that AAE also has anti-inflammatory activity in vivo. Furthermore, the gene expression of iNOS in different tissues of broilers was investigated (). The data obtained in this study showed that feeding AAE to the LPS-challenged groups reduced the expression of iNOS in the duodenum, jejunum and ileum (p < .05). This finding suggested that AAE inhibited LPS-induced serum NO production through suppressing gene expression of iNOS in small intestine at the transcriptional level.
Table 6. Effects of Artemisia argyi extract (AAE) on the antioxidative status of broilers challenged with lipopolysaccharide (LPS).
The gene expression of iNOS is regulated by several signalling pathways associated with inflammation, particularly the TLR4/NF-κB pathway. In our study, challenging with LPS increased the expression of TLR4 and MyD88 in the liver (p < .05), of TLR4 and NF-κB in the spleen (p < .05), of TLR4, MyD88 and NF-κB in the duodenum (p < .05), of TLR4 and MyD88 in the jejunum (p < .05), of NF-κB in the ileum (p < .05). In contrast, Feeding AAE to the LPS-challenged groups reduced the gene expression of MyD88 in the liver (p < .05), of NF-κB in the spleen (p < .05), of TLR4 in the duodenum (p < .05), of TLR4, MyD88 in the jejunum (p < .05), and of NF-κB in the ileum (p < .05). The above results indicated that the anti-inflammatory mechanism of AAE is partly through inhibiting TLR4/NF-κB pathway at the transcriptional level ().
Serum antioxidant level and gene expression related to antioxidant enzymes
The stress induced by LPS is also associated with the alteration of antioxidant defence mechanism through the generation of ROS (Kim and Ha Citation2010). Similar to the previous study, challenge with LPS reduced serum T-AOC activity (p= .04) and the levels of serum GSH-Px (p= .04) and CAT (p= .03). Concerning gene expression (Figure ), LPS decreased the expression of SOD in the liver and duodenum (p < .05), of GPx7 in the liver, duodenum, jejunum and ileum (p < .05). However, AAE supplementation tended to increase the levels of serum GSH-Px (p= .09) and CAT (p= .06), and feeding AAE to the LPS-challenged groups increased the expression of jejunal CAT and GPx7, as well as the gene expression of ileac GPx7 (p < .05). In addition, AAE, as a main effect, reduced serum MDA content (p= .02), which may be the result of free-radical-scavenging capacity of AAE reported by Zhao et al. (Citation2016), or the improvement of serum CAT and GSH-Px content observed in this study. Therefore, combining the experiments in vivo and in vitro, it could reveal the remarkable suppressive effect of AAE on oxidative stress induced by LPS.
Figure 1. Effects of Artemisia argyi extract (AAE) on the gene expression of inducible NO synthase (iNOS) in broilers challenged with lipopolysaccharide (LPS). a,bWithin the same row, means with different superscripts differ (p<.05).
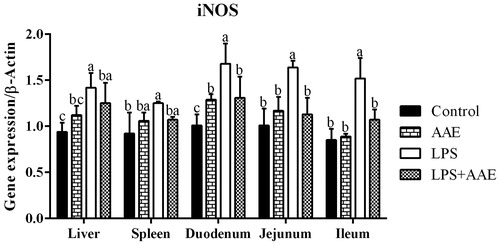
Figure 2. Effect of Artemisia argyi extract (AAE) on the expression of genes regulating antioxidant capacity in broilers challenged with lipopolysaccharide (LPS). a,bWithin the same row, means with different superscripts differ (p<.05).
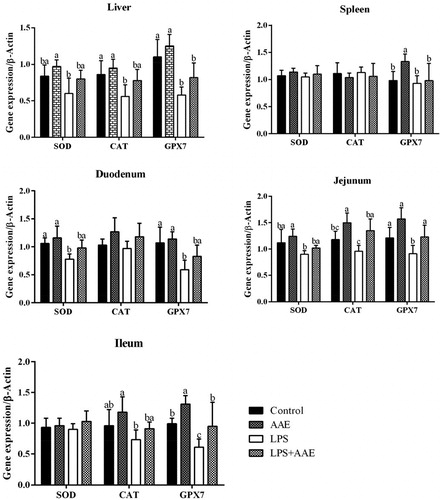
Figure 3. Effect of Artemisia argyi extract (AAE) on the expression of Toll-like receptor 4 (TLR4), myeloid differentiation factor 88 (MyD88) and nuclear factor-Kappa B (NF-κB) in broilers challenged with lipopolysaccharide (LPS). a,bWithin the same row, means with different superscripts differ (p<.05).
Conclusions
In conclusion, dietary AAE could inhibit the production of NO, maintain the relative weight of internal organs, and enhance the antioxidant capacity of LPS-challenged broilers. The mechanism of AAE may be related to the negative regulation of the TLR4/NF-κB signalling pathway at the transcriptional level.
Ethical approval
The experiment was conducted in the experimental farm of Inner Mongolia Agricultural University (Hohhot, China). All animal procedures were performed under the national standard Guideline for Ethical Review of Animal Welfare (GB/T 35892-2018).
Acknowledgements
The authors express gratitude to laboratory colleagues from laboratory of Animal Production, College of Animal Science for their assistance in sample collection, data and laboratory analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bao X, Yuan H, Wang C, Liu J, Lan M. 2013. Antitumor and immunomodulatory activities of a polysaccharide from Artemisia argyi. Carbohydr Polym. 98(1):1236–1243.
- Cai Y, Luo Q, Sun M, Corke H. 2004. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 74(17):2157–2184.
- Diaz P, Jeong SC, Lee S, Khoo C, Koyyalamudi SR. 2012. Antioxidant and anti-inflammatory activities of selected medicinal plants and fungi containing phenolic and flavonoid compounds. Chin Med. 7(1):26–34.
- Jacobi SK, Gabler NK, Ajuwon KM, Davis JE, Spurlock ME. 2006. Adipocytes, myofibers, and cytokine biology: new horizons in the regulation of growth and body composition. J Anim Sci. 84(13):E140–E149.
- Johnson RW. 1997. Inhibition of growth by pro-inflammatory cytokines: an integrated view. J Anim Sci. 75(5):1244–1255.
- Kim ID, Ha BJ. 2010. The effects of paeoniflorin on LPS-induced liver inflammatory reactions. Arch Pharm Res. 33(6):959–966.
- Lan MB, Zhang YH, Zheng Y, Yuan HH, Zhao HL, Gao F. 2010. Antioxidant and immunomodulatory activities of polysaccharides from moxa (Artemisia argyi) leaf. Food Sci Biotechnol. 19(6):1463–1469.
- Li Y, Zhang H, Chen YP, Yang MX, Zhang LL, Lu ZX, Zhou YM, Wang T. 2015a. Bacillus amyloliquefaciens supplementation alleviates immunological stress and intestinal damage in lipopolysaccharide-challenged broilers. Anim Feed Sci Tech. 208:119–131.
- Li Y, Zhang H, Chen YP, Yang MX, Zhang LL, Lu ZX, Zhou YM, Wang T. 2015b. Bacillus amyloliquefaciens supplementation alleviates immunological stress in lipopolysaccharide-challenged broilers at early age. Poult Sci. 94(7):1504–1511.
- Liu L, Shen J, Zhao C, Wang X, Yao J, Gong Y, Yang X. 2015. Dietary Astragalus polysaccharide alleviated immunological stress in broilers exposed to lipopolysaccharide. Int J Biol Macromol. 72:624–632.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 25(4):402–408.
- Lv JI, Duan JA, Shen B, Yin YY. 2013. Caffeic acid esters from Artemisia argyi and their antioxidant activities. Chem Nat Compd. 49(1):8–11.
- Marketon JIW, Glaser R. 2008. Stress hormones and immune function. Cell Immunol. 252(1–2):16–26.
- Miller SI, Ernst RK, Bader MW. 2005. LPS, TLR4 and infectious disease diversity. Nat Rev Microbiol. 3(1):36–46.
- Morgan MJ, Liu ZG. 2011. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 21(1):103–115.
- National Research Council. 1994. Nutrient requirements of poultry. 9th ed. Washington (DC): National Academic Press.
- Nishanth RP, Jyotsna RG, Schlager JJ, Hussain SM, Reddanna P. 2011. Inflammatory responses of RAW 264.7 macrophages upon exposure to nanoparticles: role of ROS-NFκB signaling pathway. Nanotoxicology. 5(4):502–516.
- Saha SK, Brewer CF. 1994. Determination of the concentrations of oligo-saccharides, complex type carbohydrates, and glycoproteins using the phenol-sulfuric acid method. Carbohyd Res. 254:157–167.
- SAS. 2003. User’s guide: statistics version 9.0. Cary (NC): SAS Institute, Inc.
- Suliman HB, Welty-Wolf KE, Carraway M, Tatro L, Piantadosi CA. 2004. Lipopolysaccharide induces oxidative cardiac mitochondrial damage and biogenesis. Cardiovasc Res. 64(2):279–288.
- Wan XL, Niu Y, Zheng XC, Huang Q, Su WP, Zhang JF, Zhang LL, Wang T. 2016. Antioxidant capacities of artemisia annua L. leaves and enzymatically treated artemisia annua L. in vitro and in broilers. Anim Feed Sci Tech. 221:27–34.
- Wang C, Levis GBS, Lee EB, Levis WR, Lee DW, Kim BS, Park SY, Park E. 2004. Platycodin D and D3 isolated from the root of Platycodon grandiflorum modulate the production of nitric oxide and secretion of TNF-alpha in activated RAW 264.7 cells. Int Immunopharmacol. 4(8):1039–1049.
- Wang S, Li J, Sun J, Zeng KW, Cui JR, Jiang Y, Tu PF. 2013. NO inhibitory guaianolide-derived terpenoids from Artemisia argyi. Fitoterapia. 85:169–175.
- Zeng KW, Wang S, Dong X, Jiang Y, Tu PF. 2014. Sesquiterpene dimer (DSF-52) from Artemisia argyi inhibits microglia-mediated neuroinflammation via suppression of NF-κB, JNK/p38 MAPKs and Jak2/Stat3 signaling pathways. Phytomedicine. 21(3):298–306.
- Zhang H, Chen Y, Li Y, Yang L, Wang J, Wang T. 2014. Medium-chain TAG attenuate hepatic oxidative damage in intra-uterine growth-retarded weanling piglets by improving the metabolic efficiency of the glutathione redox cycle. Br J Nutr. 112(6):876–885.
- Zhang PF, Shi BL, Su JL, Yue YX, Cao ZX, Chu WB, Li K, Yan SM. 2017. Relieving effect of Artemisia argyi aqueous extract on immune stress in broilers. J Anim Physiol Anim Nutr. 101(2):251–258.
- Zhao F, Shi B, Sun D, Chen H, Tong M, Zhang P, Guo X, Yan S. 2016. Effects of dietary supplementation of Artemisia argyi aqueous extract on antioxidant indexes of small intestine in broilers. Anim Nutr. 2(3):198–203.
