 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This work evaluated the reliability of the multivariate statistical analysis to discriminate the feeding system and the species of ruminants using their intramuscular fatty acids (FA) profile. FA composition of 53 meat samples (longissimus dorsi muscle) from animals of different species (sheep and cattle) raised with different feeding systems (pasture and stall-fed) (4 groups overall) was determined and expressed as % fatty acid methyl ester (FAME). A stepwise discriminant analysis (SDA) was applied to the full set of FA to select the variables that best discriminated between feeding systems and animal species. The selected variables were then submitted to a canonical discriminant analysis (CDA) to test the ability of those variables in discriminating against the four groups. Discriminant analysis (DA) was then exploited to classify meat samples. From the 62 initial variables detected in the FA profile, 24 were retained in the SDA. The subsequent CDA developed by using the selected variables, significantly discriminated the four groups (Hotelling's test p < 0.0001) by extracting three canonical functions. Heptadecenoic acid C17:1 c10, seemed to play a pivotal role both in discriminating species and feeding system while some 18:1 isomers (C18:1 c12, C18:1 c13 C18:1 t13/t14) together with CLA c9, t11 and ω-3 were important in discriminating feeding systems. Multivariate statistical analysis of FA was able to track both the species and the feeding system of source animals with good accuracy.
The increasing interest in the ‘green image’ of meat obtained from grass-based systems guides the search for methods to trace the animal feeding system.
Extracting more information from the large amounts of meat data provided by laboratory equipment is of utmost importance.
Multivariate statistical analysis is able to trace with good accuracy meat samples back to their animal species and feeding system origin.
Highlights
Introduction
The traceability of food is an essential element in ensuring safety and high-quality food. For meat customers, the awareness on authenticity and traceability of meat are of utmost importance and the increasing interest in the ‘green image’ of meat obtained from grass-based systems guides the search for methods to trace animal feeding system (Piasentier et al. Citation2003; Osorio et al. Citation2013; Huang et al. Citation2015; Moloney et al. Citation2018; Monahan et al. Citation2018; Prache et al. Citation2020). Thus, there is a pressing need for accurate standardised food authentication techniques and, to extract more information from the large amounts of data provided by the instrumental laboratory equipment. The chemical complexity of meat implies that the traditional methods for discrimination suffer some disadvantage, i.e. a single or a few components could not sufficiently characterise its quality, or the fact that they are very expensive (Xu et al. Citation2011). The synergistic use of instrumental analytical techniques and chemometrics represents a promising way in the development of authenticity and traceability models (Osorio et al. Citation2013; Danezis et al. Citation2016; Granato et al. Citation2018; Arsalane et al. Citation2019; Rohman Citation2019; López-Pedrouso et al. Citation2020). The objective of this work was to evaluate the reliability of the multivariate statistical analysis of intramuscular FA profile of bovine and ovine meat samples to discriminate the feeding system and the animal species they originate from.
Materials and methods
The animal protocol described below was fully in compliance with the European Union (EU) and Italian regulations on animal welfare and experimentation, and it was approved by the veterinarians responsible for the ethic and welfare control in animal experimentation of AGRIS and the University of Sassari. All measurements were taken by personnel previously trained and authorised by the institutional authorities on ethical issues both from AGRIS and the University of Sassari. Fifty-three meat samples collected in different experiments were used. Twenty were obtained from grazing animals (BP group: 13 bulls fed natural pasture; OP group: 7 male lambs grazing on Lolium multiflorum Lam. pasture). The other 33 came from animals stall-fed with hay and commercial concentrate (BS group: 26 bulls; OS group: 7 male lambs). All animals (both sheep and cattle) belonged to the Sarda breeds. Grazing period lasted 60 days in spring for young bulls and lambs, and 16 months for bulls. The length of the stall-feeding period mirrored that of the grazing one for the young bulls and lambs while for the bulls lasted 20 months. The animals were slaughtered in a commercial abattoir according to standard procedures at different ages: 416 ± 25 days (lsmeans ± s.d.) the young bulls (27 animals in total), 94 ± 9 days the lambs (14 animals), 22.6 ± 4.7 months the bulls (12 animals). Twenty-four hours post-mortem samples of Longissimus dorsi were removed between sixth and tenth thoracic vertebrae from each left half-carcass, ground, homogenised, vacuum-packaged and stored frozen at −20 °C until analysis. Intramuscular fat and fatty acid (FA) composition in intra-muscular fat, expressed as % FAME, was determined according to Addis et al. (Citation2013).
Statistical analysis
A stepwise discriminant analysis (SDA) was applied to the full set of FA to select the variables that best discriminated between feeding systems and animal species. The selected variables were then submitted to a canonical discriminant analysis (CDA) to test the ability of those variables in discriminating the four groups (BP, OP, BS, OS), (Mardia et al. Citation2000). In general, if k is the number of involved groups, CDA derives k-1 linear equations, called canonical functions (CAN), whose structure is:
where Xi is the original variables and d the canonical coefficients (CC) that indicate the partial contribution of each original variable in composing the CAN. In consequence, the higher the absolute value of a CC, the higher the weight of the corresponding variable in the CAN.
The distance between groups was evaluated by using the Mahalanobis’ distance, whereas the effective groups’ separation was tested by using the corresponding Hotelling's T-square test (De Maesschalck et al. Citation2000). Discriminant analysis (DA) was then exploited to classify animals. Practically, CANs obtained in the CDA were applied to each individual thus producing a discriminant score. An animal is assigned to a particular group (BP, OP, BS, OS) if its discriminant score is lower than the cut-off value obtained by calculating the weighted mean distance among group centroids (Mardia et al. Citation2000).
All multivariate analyses were run using SAS (SAS Institute, Inc., 2014).
Results and discussion
Table shows the age and body weight of experimental animals and intramuscular fat content (IMF) of Longissimus dorsi samples.
Table 1. Means and standard error of age, body weight, and IMF content of Longissimus dorsi meat samples of experimental animals.
From the 62 initial variables detected in the FA profile of meat, 24 were retained in the SDA (Table ).
Table 2. Fatty acids selected by stepwise discriminant analysis and their standardised canonical coefficients.
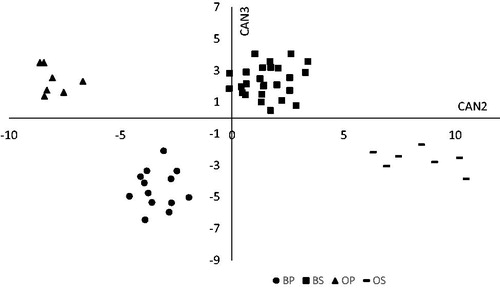
The CDA based on the selected variables, significantly discriminated against the four groups (Hotelling's test p < 0.0001) by extracting three canonical functions whose CCs are reported in Table . The variance explained by CAN1, CAN2 and CAN3 were 0.89. 0.08 and 0.03, respectively. The CAN1 versus CAN2 scatter plot (Figure ) displays a clear separation between the two species (sheep and cattle) and the two feeding systems (stall and pasture). In particular, CAN1, which accounted for 89% of the total variation, separated species, whereas CAN2, which explained 8% of variation, separated stall from the pasture sample group. The plot of CAN2 vs. CAN3 (Figure ) further highlights the ability of CAN2 in separating groups. In particular, when projected on the horizontal axis (CAN2), the four groups are clearly separated, without the aid of CAN3. Being most of the total variation accounted by CAN1, which is able to separate the species, CAN2 allows to distinguish the feeding regimen by animal species.
Figure 1. Plot of canonical variables (CAN 1, CAN 2) generated from the discriminant analysis. BP: bulls fed natural pasture; OP: male lambs grazing on Lolium multiflorum Lam. pasture; BS: bulls stall-fed with hay and commercial concentrate; OS: lambs stall-fed with hay and commercial concentrate.
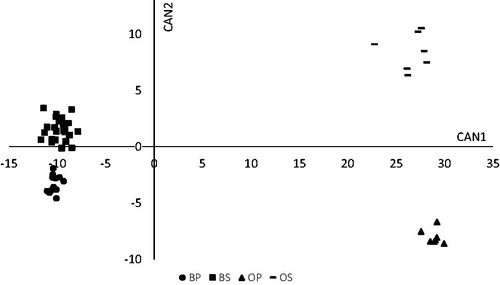
Figure 2. Plot of canonical variables (CAN 2, CAN 3) generated from the discriminant analysis. BP: bulls fed natural pasture; OP: male lambs grazing on Lolium multiflorum Lam. pasture; BS: bulls stall-fed with hay and commercial concentrate; OS: lambs stall-fed with hay and commercial concentrate.
In CAN1, the variables with the highest absolute CC values were C17:1 c10; C18:2 c9,t12 and AI (Table ). This result indicates that the separation of the two species is mainly determined by those variables. In CAN2, C17:1 c10; C18:1 isomers (C18:1 c12; C18:1 c13; C18:1 t13/t14), CLA c9,t11 and ω3 showed the highest CC values thus indicating that these FA are responsible in differentiating the feeding systems.
At this point it is necessary, to keep in mind that additional and targeted research is required to give an exhaustive explanation and interpretation regarding the role of the FA identified by the DA. Indeed, the rumen is a complex anaerobic microbial ecosystem where various microorganisms interact to convert plant biomass into fermentation end products as volatile fatty acids (VFA), organic acids, microbial proteins, vitamins and other organic compounds then utilised by the ruminant host. The ruminal microbiota hydrogenates UFA (unsatured fatty acids), a detoxifying process (Maia et al. Citation2010), and this biohydrogenation (BH) process depends on UFA, diet and ruminal environment (Enjalbert et al. Citation2017). Despite a high functional stability, the rumen microbiota exhibits large interindividual variations (Jami and Mizrahi Citation2012) being affected by ruminant species, diet, feed additives, season, and geographic region (Zhou et al. Citation2009; Kong et al. Citation2010; Moon et al. Citation2010; Lee et al. Citation2012). Toral et al. (Citation2016) provides evidence of interspecies differences in rumen BH, likely linked to differences in microbial composition, in intestinal FA digestibility or tissue lipid metabolism. Duncan and Poppi (Citation2008) explained differences in rumen FA concentrations through interspecies differences in eating behaviour, ruminal passage rates, and digestion kinetics. Plant secondary compounds (essential oils, tannins, saponins) could also modulate the BH activity of bacteria (Lourenco et al. Citation2008; Vasta et al. Citation2011; Cabiddu et al. Citation2014; Enjalbert et al. Citation2017).
From this brief review emerges that several aspects, not explored in this work, can affect the meat FAs composition. On the other hand, the main aim of this work was to evaluate the capacity of a multivariate statistical analysis to rapidly discriminate meat samples only relying on meat FA composition. Nevertheless, this statistic approach in our case provided a beneficial side-effect: it helped to identify the best biomarkers of our beef and sheep meat supply chain. With regard to the variables indicated as the most important in discriminating between animal species, we cannot exclude a difference of genetic nature in their pathways but, in the absence of specific research data, we can also hypothesise a relevant species-by-diet interactions due to differences in rumen FA metabolism and/or rumen bacterial community, in line with Toral et al. (Citation2016). Heptadecenoic acid C17:1 c10 plays a pivotal role both in discriminating species and feeding system (Table ). It is a minor constituent of ruminant fats (Alves et al. Citation2006) but Vlaeminck et al. (Citation2005) consider it, along with other odd-chain FAs, as a potential marker of microbial biomass, assuming to be mainly of rumen microbial origin. Genetic differences in FA composition between sheep and cattle probably exist both in the case of C17:1 content (Lisitsyn et al. Citation2013) and for other FA. Doreau et al. Citation2011, reported mean concentrations of CLA c9,t11 between 0.5 and 1.1% of total FA in bovine muscle and up to 1.4% in lamb muscle. Species differences in FA formation are also reported by Smith and Prior Citation1986 and Sinanoglou et al Citation2013 (though in this case differences concern sheep and goats) though, according to Esteves et al (Citation2019), the molecular mechanisms that control their synthesis in different species are not yet fully understood. Moreover, in line with Vlaeminck et al. (Citation2005), variations in rumen odd-chain FAs composition (including C17:1 c10) could be induced by diet changes, thus explaining its role in discriminating feeding systems. The C18:2 c9,t12 is a transient intermediate in the process of conversion of trans-11 octadecenoic acid to CLA by Δ9-desaturase (Griinari and Bauman Citation1999) and, though present in small quantities, plays a role in discriminating ovine and bovine species. This role could be linked to a genetic difference in Δ-9 desaturase activity between species, but we cannot rule out species-by-diet interactions. In particular, cattle diet was probably higher in concentrate levels respect to ovine-diet as indirectly evidenced by the greater IMF content in bovine than ovine meat (2.79 ± 0.23 and 1.29 ± 0.38% DM, lsmeans ± se, in cattle and sheep, respectively, p = 0.001), and this could have affected the rumen biohydrogenation activity (Troegeler-Meynadier et al. Citation2003; Santos-Silva et al. Citation2019). With regard to AI, previous research showed significant differences in most lipid quality indices among species (Sinanoglou et al. Citation2013, in lambs and kids meat). Furthermore, being AI an index resulting from the combination of some FAs (Table ), genetic and species-by-diet interactions induced differences may also apply to it. Beyond C17:1 c10, some 18:1 isomers (C18:1 c12; C18:1 c13; C18:1 t13/t14) were important in discriminating feeding systems. In ruminants, cis and trans C18:1 isomers are intermediates of dietary FA rumen BH and isomerisation. The cis-9-octadecenoic acid (oleic acid C18:1 c9), a FA often present in ruminant diets, although usually described as hydrogenated directly to stearic acid without the formation of intermediates, is not completely saturated to stearic acid, but isomerised to other acids of the C18:1 family (Wu et al. Citation2016). Many researcher papers point out that diet composition, in particular the forage:concentrate ratio, influences the rumen biohydrogenation and isomeration. Laverroux et al. (Citation2011) stated that some vaccenic acid (C18:1 t11) is isomerised to all cis and trans C18:1 isomers, with probably more isomerisation in sheep fed a forage diet. Fruet et al. (Citation2018) stated the biohydrogenation of polyunsaturated FAs in the rumen of cows is reduced with high-concentrate diets causing accumulation of trans-18:1 isomers. Buccioni et al. (Citation2012) reported that many dietary factors affects lipolysis of dietary fat and biohydrogenation in the rumen, among others the maturity of forage and the plant secondary metabolites (PSM), which includes polyphenol oxidase (PPO), and tannins. Nudda et al. (Citation2008), with goats fed with whole cottonseed or extruded linseed, reported that the meat FA profile of their suckling kids was effectively modified by manipulating the diet of the dams. The role of conjugated linoleic acid (CLA) c9,t11 and ω-3 as marker of feeding system is in agreement with several authors (Scollan et al. Citation2006; Lourenco et al. Citation2008; Daley et al. Citation2010).
Conclusions
In conclusion, this study demonstrates the reliability of multivariate analysis of meat FA to trace with good accuracy meat samples back to their animal species and feeding system origin. Moreover, this study highlights that multivariate DA can cast light on the most relevant FA for discriminating meat samples, allowing to target research on microbiota, animal and feed factors most probably involved in the metabolism of these FA.
Acknowledgments
Mr. Stefano Picconi and Salvatore Pintus for their excellent cooperation as well as the staff of Foresta Burgos research farm and the laboratory staff of AGRIS
Disclosure statement
None of the authors has a financial or personal relationship with other people or organisations that could inappropriately influence this publication.
References
- Addis M, Fiori M, Manca C, Riu G, Scintu MF. 2013. Muscle colour and chemical and fatty acid composition of ‘Agnello di Sardegna’ PGI suckling lamb. Small Ruminant Res. 115(1-3):51–55.
- Alves SP, Marcelino C, Portugal PV, Bessa RJB. 2006. The nature of heptadecenoic acid in ruminant fats. J Dairy Sci. 89(1):170–173.
- Arsalane A, El Barbri N, Tabyaoui A, Klilou A, Rhofir K. 2019. The assessment of fresh and spoiled beef meat using a prototype device based on GigE Vision camera and DSP. Food Measure. 13(3):1730–1738.
- Buccioni A, Decandia M, Minieri S, Molle G, Cabiddu A. 2012. Lipid metabolism in the rumen: New insights on lipolysis and biohydrogenation with an emphasis on the role of endogenous plant factors. Anim Feed Sci Technol. 174(1-2):1–25.
- Cabiddu A, Lee MRF, Decandia M, Molle G, Salis L, Vargiu M, Winters AL. 2014. Characterization of polyphenol oxidase activity in a range of forage ecotypes with different phenol substrates. Grass Forage Sci. 69(4):678–692.
- Daley CA, Abbott A, Doyle PS, Nader GA, Larson S. 2010. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr J. 9:10–12.
- Danezis GP, Tsagkaris AS, Camin F, Brusic V, Georgiou CA. 2016. Food authentication: Techniques, trends & emerging approaches. Trends Anal Chem. 85:123–132.
- De Maesschalck R, Jouan-Rimbaud D, Massart DL. 2000. The Mahalanobis distance. Chemom. Intell. Lab. 50(1):1–18.
- Doreau M, Bauchart D, Chilliard Y. 2011. Enhancing fatty acid composition of milk and meat through animal feeding. Anim Prod Sci. 51(1):19–29.
- Duncan AJ, Poppi DP. 2008. Nutritional ecology of grazing and browsing ruminants. In: Gordon IJ, Prins HHT, editors. The ecology of browsing and grazing ecological studies. vol. 195. Berlin, Germany: Springer-Verlag; p. 89–116.
- Enjalbert F, Combes S, Zened A, Meynadier A. 2017. Rumen microbiota and dietary fat: a mutual shaping. J Appl Microbiol. 123(4):782–797.
- Esteves C, Livramento KG, Paiva LV, Peconick AP, Garcia IFF, Garbossa CAP, Faria PB. 2019. The polymorphisms of genes associated with the profile of fatty acids of sheep Arq. Arq Bras Med Vet Zootec. 71 (1):303–313.
- Fruet APB, Trombetta F, Stefanello FS, Speroni CS, Donadel JZ, De Souza ANM, Rosado Júnior A, Tonetto CJ, Wagner R, De Mello A, et al. 2018. Effects of feeding legume-grass pasture and different concentrate levels on fatty acid profile, volatile compounds, and off-flavor of the M. Longissimus Thoracis. Meat Sci. 140:112–118.
- Granato D, Putnik P, Bursać Kovačević D, Sousa Santos J, Calado V, Silva Rocha R, Gomes Da Cruz A, Jarvis B, Ye Rodionova O, Pomerantsev A. 2018. Trends in chemometrics: food authentication. Microbiol Effects Process Compreh Rev Food Sci Food Safety. 17(3):663–677.
- Griinari JM, Bauman DE. 1999. Biosynthesis of conjugated linoleic acid and its incorporation into meat and milk in ruminants. In: Advances in Conjugated Linoleic Acid Research Volume I Ed: Yurawecz MP, Mossoba MM, Kramer JKG, Pariza MW, Nelson GJ AOCS press, Illinois, Chapter 13, pp. 180–200.
- Huang Y, Andueza D, de Oliveira L, Zawadzki F, Prache S. 2015. Visible spectroscopy on carcass fat combined with chemometrics to distinguish pasture-fed, concentrate-fed and concentrate-finished pasture-fed lambs. Meat Sci. 101:5–12.
- Jami E, Mizrahi I. 2012. Similarity of the ruminal bacteria across individual lactating cows. Anaerobe. 18(3):338–343.
- Kong Y, Teather R, Forster R. 2010. Composition, spatial distribution, and diversity of the bacterial communities in the rumen of cows fed different forages. FEMS Microbiol Ecol. 74(3):612–622.
- Laverroux S, Glasser F, Gillet M, Joly C, Doreau M. 2011. Isomerization of vaccenic acid to cis and trans C18:1 isomers during biohydrogenation by rumen microbes. Lipids. 46(9):843–850.
- Lee HJ, Jung JY, Oh YK, Lee S-S, Madsen EL, Jeon CO. 2012. Comparative survey of rumen microbial communities and metabolites across one caprine and three bovine groups, using barcoded pyrosequencing and 1 H nuclear magnetic resonance spectroscopy. Appl Environ Microbiol. 78 (17):5983–5993.
- Lisitsyn AB, Chernukha IM, Ivankin AN. 2013. Comparative study of fatty acid composition of meat material from various animal species. Scientif J Anim Sci. 2(5):124–131.
- López-Pedrouso M,M, Rodríguez-Vázquez R, Purriños L, Oliván M, García-Torres S, Sentandreu MA, Manuel Lorenzo J, Zapata C, Franco D. 2020. Sensory and physicochemical analysis of meat from bovine breeds in different livestock production systems, pre-slaughter handling cond ageing time. Foods. 9(2):176.
- Lourenco M, Van Ranst G, Vlaeminck B, De Smet S, Fievez V. 2008. Influence of different dietary forages on the fatty acid composition of rumen digesta as well as ruminant meat and milk. Anim Feed Sci Technol . 145(1-4):418–437.
- Maia MRG, Chaudhary LC, Bestwick CS, Richardson AJ, McKain N, Larson TR, Graham IA, Wallace RJ. 2010. Toxicity of unsaturated fatty acids to the biohydrogenating ruminal bacterium, Butyrivibrio fibrisolvens. BMC Microbiol. 10:52.
- Mardia KV, Bookstein FL, Moreton IJ. 2000. Statistical assessment of bilateral symmetry of shapes. Biometrika. 87(2):285–300.
- Moloney AP, O’ Riordan EG, Schmidt O, Monahan FJ. 2018. The fatty acid profile and stable isotope ratios of C and N of muscle from cattle that grazed grass or grass/clover pastures before slaughter and their discriminatory potential. Irish J Agric Food Res. 57(1):84–94.
- Monahan FJ, Schmidt O, Moloney AP. 2018. Meat provenance: authentication of geographical origin and dietary background of meat. Meat Sci. 144:2–14.
- Moon YH, Ok JU, Lee SJ, Ha JK, Lee SS. 2010. A comparative study on the rumen microbial populations, hydrolytic enzyme activities and dry matter degradability between different species of ruminant. Anim Sci J. 81(6):642–647.
- Nudda A, Palmquist DL, Battacone G, Fancellu S, Rassu SPG, Pulina G. 2008. Relationships between the contents of vaccenic acid, CLA and n − 3 fatty acids of goat milk and the muscle of their suckling kids. Livestock Sci. 118(3):195–203.
- Osorio MT, Downey G, Moloney AP, Röhrle FT, Luciano G, Schmidt O, Monahan FJ. 2013. Beef authentication using dietary markers: Chemometric selection and modelling of significant beef biomarkers using concatenated data from multiple analytical methods. Food Chem. 141(3):2795–2801.
- Piasentier E, Valusso R, Camin F, Versini G. 2003. Stable isotope ratio analysis for authentication of lamb meat. Meat Sci. 64(3):239–247.
- Prache S, Martin B, Coppa M. 2020. Review: authentication of grass-fed meat and dairy products from cattle and sheep. Animal. 14 (4):854–863.
- Rohman A. 2019. The employment of Fourier transform infrared spectroscopy coupled with chemometrics techniques for traceability and authentication of meat and meat products. J Adv Vet Anim Res. 6 (1):9–17.
- Santos-Silva J, Francisco A, Alves SP, Portugal P, Dentinho T, Almeida J, Soldado D, Jerónimo E, Bessa RJB. 2019. Effect of dietary neutral detergent fibre source on lambs growth, meat quality and biohydrogenation intermediates. Meat Sci. 147:28–36.
- Scollan ND, Hocquette JF, Nuernberg K, Dannenberger D, Richardson I, Moloney A. 2006. Innovations in beef production systems that enhance the nutritional and health value of beef lipids and their relationship with meat quality. Meat Sci. 74(1):17–33.
- Sinanoglou VJ, Batrinou A, Mantis F, Bizelis I, Miniadis-Meimaroglou S. 2013. Lipid quality indices: differentiation of suckling lamb and kid breeds reared by traditional sheep farming. Small Ruminant Res. 113 (1):1–10.
- Smith SB, Prior RL. 1986. Comparisons of lipogenesis and glucose metabolism between ovine and bovine adipose tissues. J Nutr. 116(7):1279–1286.
- Toral PG, Bernard L, Belenguer A, Rouel J, Hervás G, Chilliard Y, Frutos P. 2016. Comparison of ruminal lipid metabolism in dairy cows and goats fed diets supplemented with starch, plant oil, or fish oil. J Dairy Sci. 99(1):301–316.
- Troegeler-Meynadier A, Nicot MC, Bayourthe C, Moncoulon R, Enjalbert F. 2003. Effects of pH and concentrations of linoleic and linolenic acids on extent and intermediates of ruminal biohydrogenation in vitro. J Dairy Sci. 86(12):4054–4063.
- Ulbricht TLV, Southgate DAT. 1991. Coronary heart disease: seven dietary factors. Lancet. 338(8773):985–992.
- Vasta V, Luciano G, Dimauro C, Röhrle F, Priolo A, Monahan FJ, Moloney AP. 2011. The volatile profile of longissimus dorsi muscle of heifers fed pasture, pasture silage or cereal concentrate: Implication for dietary discrimination. Meat Sci. 87(3):282–289.
- Vlaeminck B, Dufour C, Van Vuuren AM, Cabrita ARJ, Dewhurst RJ, Demeyer D, Fievez V. 2005. Use of odd and branched-chain fatty acids in rumen contents and milk as a potential microbial marker. J Dairy Sci. 88(3):1031–1042.
- Wu D, Xu L, Tang S, Guan L, He Z, Guan Y, Tan Z, Han X, Zhou C, Kang J, et al. 2016. Influence of oleic acid on rumen fermentation and fatty acid formation in vitro. PLoS One. 11(6):e0156835.
- Xu L, Deng DH, Cai CB, Yang HW. 2011. Automatic discrimination of the geographical origins of milks by excitation-emission fluorescence spectrometry and chemometrics. J Autom Methods Manag Chem. 2011:323196.
- Zhou M, Hernandez-Sanabria E, Guan LL. 2009. Assessment of the microbial ecology of ruminal methanogens in cattle with different feed efficiencies. AEM. 75(20):6524–6533.
