Abstract
Although selenium (Se) supplementation is a common practice in poultry, the best source and level has not been established yet. Thus, a 42-day experiment involving diets with three levels (0.1, 0.2 and 0.5 mg/kg) of supplemental Se from sodium selenite (SS), selenised yeast (SY) and nanoelemental Se (SN) was conducted to evaluate the possible differential responses of broiler chickens to inorganic, organic and nano Se sources relative to a control diet. Throughout the experiment, broilers receiving Se supplements had higher feed intake, body weight gain (BWG) and survival rate than the control. Broilers fed dietary SY or SN had improved BWG compared with those fed the SS-supplemented diets. Broilers treated with Se supplementation had increased serum glutathione peroxidase (GPx) and thioredoxin reductase (TrxR) activities and produced higher antibody responses to avian influenza virus (AIV) and sheep red blood cell (SRBC) versus the control. These effects were enhanced with increasing Se addition, except for GPx that responded equally to all supplemental Se levels. Also, broilers receiving supplementary SY or SN exhibited higher anti-AIV and anti-SRBC titres along with more elevated serum Se, TrxR activity and total antioxidant capacity compared with those receiving SS. At the same time, SN had the most increasing effect on anti-SRBC titre. To conclude, diet supplementation with 0.5 mg/kg of Se in the form of SY or SN was capable of meeting the Se demands of broiler chickens for optimum growth and antioxidant capability, while SN seemed to be the most effective Se source in enhancing immunity.
Diet supplementation with SN and SY, especially at 0.5 mg/kg diet, improved broiler performance and carcase quality of chickens compared with SS.
SY and SN showed more favourable effects on oxidative status and immunocompetence.
The best anti-SRBC titre was observed with SN.
Highlights
Introduction
Selenium (Se) is a nutritionally indispensable trace mineral that requires a regular supply in animal and is known to be essential to support normal growth, fertility and health in poultry (Canoğullari et al. Citation2010; Baylan et al. Citation2011; Habibian et al. Citation2014). It is an integral part of more than 25 selenoproteins, of which glutathione peroxidases (GPx) and thioredoxin reductases (TrxR) have roles in detoxification and maintenance of the redox potential (Yuan et al. Citation2012). Furthermore, Se involves in virtually all aspects of immune function (reviewed by Dalgaard et al. (Citation2018) and Nabi et al. (Citation2020)). Se deficiency influences unfavourably immune cell development and differentiation in both innate and acquired immune defences, as manifested with atrophy of lymphoid tissues, decreased number of T and B lymphocytes, and impaired immune functions including lymphocyte proliferation, pro-inflammatory cytokine secretions, antibody responses, macrophage phagocytic activity and specific functions of heterophils (Liu et al. Citation2016; Yang et al. Citation2016; Pan et al. Citation2018; Yiming et al. Citation2020). However, the impact of Se supplementation on the immune system varies under different environments (Habibian et al. Citation2014; Rama Rao et al. Citation2016) and is highly influenced by the level and source of dietary Se (Boostani et al. Citation2015; Wang et al. Citation2016; Shabani et al. Citation2019; Shojadoost et al. Citation2020).
The National Research Council (NRC Citation1994) estimated the minimum requirement of Se for growing chickens to be 0.1 mg/kg of diet. Practically, however, feed manufacturers supply higher levels of Se than that specified by the NRC (Citation1994), and there is ongoing research into alternative Se sources and alternative Se supplementation levels. Currently, Se is fed to poultry as inorganic salts, such as sodium selenite (SS) and selenate, or organ compounds like selenised yeast (SY), in which selenomethionine (SM) is the predominant form of Se (Xiao et al. Citation2016). The highest authorised Se inclusion level from these sources is 0.2 mg/kg of diet in the United States (AAFCO Citation2011), and the present total maximum European Union permissible level of Se in a feed is 0.5 mg/kg (European Union Citation2004). Usually, SM shows a higher bioavailability and antioxidant capacity than SS and a higher threshold for toxicity (Zia et al. Citation2018). The significant benefits of these effects in broiler diet include increased Se transfer to the muscle tissue and accumulation of Se stores in the body, which is expected to lead to higher resistance to stressors and to have a positive impact on immune function and carcase quality (Heindl et al. Citation2010). However, one disadvantage of SY is that its SM level is very variable (60–84%: Rayman Citation2004). There is no evidence that the remaining part of Se has any advantage over SS for chickens. The main disadvantage of pure SM is its instability when included in diets and premixes (Rayman Citation2004; Surai et al. Citation2018).
Nanoelemental Se (SN) is a new form of mineral that has been produced and marketed with the recent development of nanotechnology. The transition of metal from microparticles to nanoparticles leads to increases in surface area, surface functionality, catalytic property and adsorbing capacity as well as lower poisoning rate (Zhang et al. Citation2008; Nabi et al. Citation2020). An earlier study with broilers detected that the range of optimal and toxic doses of SN was wider than that of SS, and SN retained more effectively in the body than SS (Wang Citation2009). This result has been confirmed in recent experiments (Selim et al. Citation2015; Bakhshalinejad et al. Citation2018, Li et al. Citation2018). Also, Zhang et al. (Citation2008) showed that SN has comparable efficiency to SS in upregulating selenoenzymes while exhibiting lower toxicity. However, whether SN shows different effects versus organic Se sources has not been well explored in broiler chickens. According to Selim et al. (Citation2015) and Bakhshalinejad et al. (Citation2018), organic forms of Se are superior to nanoforms of Se in terms of utility. In contrast, there is no difference between the forms of Se according to Gangadoo et al. (Citation2020).
Based on these findings, this study was performed to compare the effects of SS, SY and SN at currently advocated and maximum allowable levels on growth efficiency, carcase traits, antibody responses, serum Se and antioxidant status of broiler chickens.
Materials and methods
Selenium sources
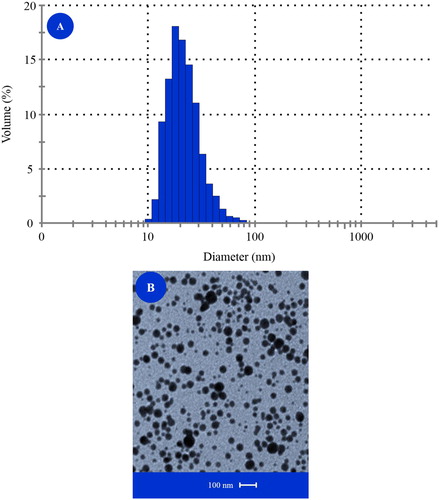
Anhydrous SS (99% purity) was purchased from Sigma (Sigma-Aldrich, St. Louis, MO) and used as the source of inorganic Se, whereas SY was the source of organic Se and obtained from Alltech (Sel-Plex®, Alltech Co. Ltd., Chicago, IL). The SN product was synthesised following the chemical method of Zhang et al. (Citation2001) through the addition of bovine serum albumin to the redox system of selenite and glutathione (GSH), as described elsewhere in details (Hu et al. Citation2012). The mean size of the SN particles was determined by dynamic light scattering using a zeta potential analyser (Malvern Instruments, Malvern, UK) and found to be ranged from 10 to 80 nm (Figure ). Transmission electron microscopy (Philips FEI, Oregon, OR) verified the determined diameter and revealed that the particles had a spherical shape (Figure ). Analysed total selenium contents were 452 mg/kg in SS, 231 mg/kg in SY and 345 mg/kg in SN, respectively, as obtained by a graphite furnace atomic absorption spectrometer (AAnalyst200, Waltham, MA). The method has been detailed previously by Amoako et al. (Citation2007) and Wang (Citation2009).
Treatments and broiler husbandry
All experimental protocols were approved and conducted in accordance with the guidelines for the care and use of experimental animals endorsed by our institutional animal care committee (approval NO. 23850103972005). In total, 900 one-day-old male broiler chicks (Ross 308) were purchased from a local supplier and housed in floor pens of 1.4 m2. Each pen was covered with a 5 cm layer of sawdust and fitted with one bell drinker and one hanging tube feeder. The temperature was held at 34 °C on Day 1 and gradually reduced by 1 °C every 2 days until 21 °C was reached. The lighting regimen was implemented based on the Ross 308 management handbook (Aviagen Citation2014b).
Birds were allotted to 10 treatment groups with six replicate pens of 15 birds each. All groups were approximately equal in body weight (BW) at the beginning of the experiment (42 ± 1 g). The first group served as the control and was given corn–soya bean basal diets, while the other treatment groups fed control diets supplemented with 0.1, 0.2 or 0.5 mg/kg of Se (either as SS, SY or SN). The feeding programme was portioned into a starter, a grower and a finisher phase, which lasted from 0–10, 11–24 and 25–42 days of the experiment, respectively. The starter diet was in crumble form, and the grower and finisher diets were in pellet form. Feed and water were provided for ad libitum access. Basal diets (Table ) were prepared to be adequate in all nutrients for Ross 308 broilers (Aviagen Citation2014a), except Se. The actual Se content of diets (Table ) was analysed using the same atomic absorption instrument described for the Se supplements.
Table 1. Ingredients and calculated composition of the basal diets.
Table 2. Analysed Se contents in starter, grower and finisher diets.
Sampling and measurements
Birds and feeds were weighed at 0, 10, 24 and 42 days of the experiment on a pen basis. Feed intake (FI), body weight gain (BWG) and feed conversion ratio (FCR) were determined for each rearing phase. The survival rate was determined by counting the living birds in each pen.
At 42 days of age, 12 birds per treatment (two broilers per replicate) were chosen, based on their BW, and slaughtered by exsanguination after electrical stunning to determine the yields (% of live BW) of the carcase, breast, thigh, abdominal fat pad, liver, heart, bursa of Fabricius, spleen and thymus.
Broilers were immunised using intramuscular injection with an emulsified mixture of killed vaccine of Newcastle disease and avian influenza viruses (NDV and AIV) at 9 days of the experiment, and orally against NDV at 21 and 30 days. Also, at 25 and 30 days of the experiment, two broilers per replicate were injected (through the brachial vein) with 1 mL of 0.5% sheep red blood cell (SRBC) solution in normal saline. At 32 and 42 days of the experiment, blood specimens were obtained and centrifuged (1500 ×g for 10 min) to separate the serum. Antibody titres against NDV, AIV and SRBC were quantified by microtitration methods as used earlier (Wegmann and Smithies Citation1966; King and Hopkins Citation1983). The titres were represented in log2 of the reciprocal of the highest dilution giving positive agglutination. Prechallenge antibody titres to NDV were typically <2 log2, while anti-SRBC and anti-AIV titres were zero before the challenge.
Serum GPx and TrxR activities and total antioxidant capacity (TAC) were measured according to previously published methods (Luthman and Holmgren Citation1982; Rice-Evans and Miller Citation1994). Serum Se concentrations were also analysed in the same manner as described above.
Statistical analysis
Statistical analysis of data was carried out using the GLM procedure of SAS Institute (Citation2003). Firstly, Dunnett's tests were used to check for significant differences among the means of the control and each supplemental Se treatment. Then the outcome variables excluding the control values were assessed using a 3 × 3 arrangement of treatment design, and the main effects and two-way interactions of Se source and added Se level were estimated. Mean comparisons were made using Tukey's studentised range test. Responses to dietary Se source were also investigated using linear and quadratic polynomial contrasts based on Se level supplemented to the diets. The statistical significance level was claimed at p < .05. Day-32 and day-42 NDV titres were adjusted using prechallenge titres as the covariate.
Results
Performance characteristics
The effects of dietary treatments on FI and BWG of broilers are depicted in Tables and , respectively. During the starter period (0–10 days), FI responded linearly to increases in dietary Se from either source (p < .05). Similarly, a linear response in FI was evident to the rise of dietary Se dose in the form of SS during the grower (11–24 days), finisher (25–42 days) and overall (0–42 days) periods (p < .05). In contrast, responses to SY and SN were both linear and quadratic (p < .05). All broilers fed diets containing supplemental Se consumed significantly higher feed than the control (p < .05), except those receiving 0.1 mg/kg of Se as SS. Furthermore, throughout the experiment, a linear and quadratic relationship between the level of dietary Se and broiler BWG was observed (p < .05). Relative to the control, BWG of broilers was markedly improved by all supplemental Se sources and levels (p < .05). FI and BWG were also different between Se sources and Se levels (p < .05). In addition to having higher BWG in the total period (p < .05), broilers fed diets supplemented with SY or SN had a higher FI compared with those receiving diets supplemented with SS during the grower period (p < .05). Moreover, broilers fed diets supplemented with 0.2 or 0.5 mg/kg of Se had higher FI than those offered with the lowest level of added Se (0.1 mg/kg) during the starter, finisher and overall periods (p < .05), but this effect was not observed during the grower period. The benefits of higher levels of Se (0.2 or 0.5 mg/kg) on BWG were, however, durable over the experimental period (p < .05). Furthermore, a 0.5 mg/kg level of Se was more effective on BWG than 0.2 mg/kg during the entire 42 days of the experiment (p < .05). There was no apparent observable interaction between Se source and Se level on FI and BWG.
Table 3. Effects of dietary treatments on feed intake (FI) in broilers at different periods of experiment.
Table 4. Effects of dietary treatments on body weight gain (BWG) in broilers at different periods of experiment.
Table lists the effects of dietary treatments on FCR and the survival rate of broilers. There was both a linear and a quadratic response in FCR during the starter period (p < .05), and broilers in Se treatment groups showed lower FCR compared with the control (p < .05). However, this effect was not sustained through the rest of the experimental period. Dietary supplementation with Se also improved survival rate throughout the experimental period (p < .05). There were no significant main effects or interactions between Se source and Se level on FCR and survival rate.
Table 5. Effects of dietary treatments on feed conversion ratio (FCR) and survival rate in broilers at different periods of experiment.
Carcase traits
As shown in Table , the carcase, thigh and heart yields were not influenced by dietary treatments. However, the breast yield was substantially increased compared with the control when 0.2 or 0.5 mg/kg of Se was provided from either SY or SN (p < .05). This observation was also confirmed by significant linear contrasts (p < .05). Conversely, the liver yield was reduced linearly and abdominal fat yield was decreased linearly and quadratically under the control levels when birds reared on Se-supplemented diets (p < .05). Also, the breast and abdominal fat yields responded to Se levels and sources. Broilers given diets supplemented with 0.2 or 0.5 mg/kg of Se from SY or SN exhibited higher breast yields than those consuming the other Se-supplemented diets (p < .05). Additionally, a much lower abdominal fat yield was found in broilers given diets with 0.2 or 0.5 mg/kg of Se when compared with those fed diets added with only 0.1 mg/kg of Se (p < .05), and the effects of SY and SN were superior to SS (p < .05). The decreasing effect of additional increments of dietary Se on liver yield was, however, independent of dietary Se source (p < .05).
Table 6. Effects of dietary treatments on relative carcase part weights in broiler chickens at 42 days of age.
The effects of dietary treatments on lymphoid organ yields are summarised in Table . Dietary treatments did not affect the bursa of Fabricius yield, while the spleen and thymus yields were substantially increased by all supplemental Se treatments compared with the control (p < .05). The same results were supported by contrast analysis. Furthermore, while the increased spleen yield did not differ between Se levels and sources, the increased thymus yield was affected by both (p < .05). The thymus yield increased as the level of Se from either source increased (p < .05), but the increase was more significant for broilers fed the diets supplemented with SY or SN (p < .05). No interaction between Se source and Se level was detected on lymphoid organ yields.
Table 7. Effects of dietary treatments on relative immune organ weights in broilers at 42 days of age.
Antibody responses
Table illustrates the effects of dietary treatments on antibody responses against AIV, NDV and SRBC. Compared with the control, higher antibody titres against AIV and SRBC were noticed in Se-treated birds at both 32 and 42 days of the experiment (p < .05). This was also confirmed by significant linear and quadratic trends (p < .05, only linear effect of SN was significant on anti-AIV titre at day 42). Furthermore, antibody titres against AIV and SRBC showed an association with dietary Se source and level (p < .05). With both antigens, antibody titres were increased steadfastly as Se risen from 0.1 to 0.5 mg/kg in the diet (p < .05). Moreover, broilers receiving diets supplemented with SY or SN had significantly higher antibody titres against AIV and SRBC compared with those fed SS-supplemented diets (p < .05), and SN was the most effective source of Se in increasing antibody production against SRBC (p < .05). No difference in antibody titre against NDV could be detected among the treatment groups.
Table 8. Effects of dietary treatments on antibody titres against avian influenza virus (AIV), Newcastle disease virus (NDV) and sheep red blood cell (SRBC) in broilers at 32 and 42 days of age.
Serum Se content and antioxidant activity
The data on serum Se content and oxidative status of broilers are listed in Table . Increasing dietary Se level from either source exerted a linear and quadratic impact on serum Se level, GPx activity and TAC, and a linear effect on serum TrxR activity (p < .05). Broilers fed with added Se represented significantly higher serum Se levels, improved GPx and TrxR activities and greater TAC values than the control at both 32 and 42 days of the experiment (p < .05). The Se, TAC and TrxR values were also increased with increasing of Se level (p < .05), and by passing from SS to SY and SN (p < .05). However, the increased GPx activity was not influenced by either Se source or level. The interaction of Se source and level was not significant on serum Se or antioxidant indices.
Table 9. Effects of dietary treatments on serum Se concentration and antioxidative status in broiler chickens at 32 and 42 days of age.
Discussion
In this study, the growth response of broiler chickens was improved by dietary Se supplementation. Such results are similar to those of Selim et al. (Citation2015), Rama Rao et al. (Citation2016) and Bakhshalinejad et al. (Citation2018), who found that adding supplemental Se improved FI and BWG in different broiler strains. In contrast, other researchers found that the addition of Se treatment from various sources had no significant effects on BWG, FI and FCR of broilers (Cai et al. Citation2012; Briens et al. Citation2013; Habibian et al. Citation2014; Chadio et al. Citation2015). These different results may likely be a function of differences in Se content of the feeding stuffs. The basal diets used in this study contained about 0.06 mg/kg of Se, which is 40% below the minimum recommended level of 0.1 mg/kg (NRC Citation1994). In growing chicks, dietary Se deficiency is known to stimulate various stress responses, leading to anorexia, retarded growth rate, pancreatic fibrosis, muscle myopathies, humoral and cell-mediated immune dysfunction and, ultimately, increased morbidity and mortality (Yang et al. Citation2016; Cao et al. Citation2017; Xu et al. Citation2017; Pan et al. Citation2018). These findings were confirmed in this study, as the birds receiving the basal diet (without any additional Se source) showed the Se deficiency symptoms such as lower FI, BWG and survival rate. At the same time, 0.1 mg/kg of Se supplementation was sufficient to maintain the survival of chickens.
However, the improvements of FI and BWG also varied according to Se source and level. During the entire 42-day period, broilers given diets with 0.2 or 0.5 mg/kg of Se consumed higher feed and exhibited higher BWG than those fed diets added with 0.1 mg/kg of Se. Furthermore, a 0.5 mg/kg level of Se was more effective in increasing BWG than 0.2 mg/kg level. Also, broilers fed diets supplemented with SY or SN gained more BW than those receiving dietary Se in the form of SS. These results support previous findings that SY and SN are more useful than SS as sources of Se to achieve maximum, genetically determined, growth rate in broiler chickens (Bakhshalinejad et al. Citation2018; Mohammadi et al. Citation2019). Our BWG results are largely consistent with the observations in serum Se content, indicating that only part of the enhancing effects of SN and SY on broiler growth is due to increased FI and the rest is due to improved Se status per se. Ha et al. (Citation2019) suggested that SM, the main Se compound of SY, might have a superior nutritional value as it can be taken up across the epithelial barrier of the small intestine by the same active sodium-dependent process as methionine, compared with SS that is absorbed via passive diffusion into epithelial cells (Heindl et al. Citation2010). Moreover, in addition to providing Se for the synthesis of cellular selenoproteins, SM can be incorporated directly into body proteins and form a Se pool (Behne et al. Citation2009). In comparison, inorganic Se is either utilised for the synthesis of selenoproteins or excreted in urine (Kobayashi et al. Citation2001). The higher absorption rate of nanosized Se in comparison with inorganic Se salts has also been established (Surai et al. Citation2018), but the exact underlying mechanism has yet to be understood. Recent research has proposed that the higher absorption efficiency of nanoparticles may be attributed to their tiny particle size and wider surface space per unit mass, which maximises their contact with mucosal surfaces and favours the formation of nanoemulsion droplets (Nabi et al. Citation2020). Menon et al. (Citation2018) reported that the Se nanoparticles could cross the intestinal epithelium by paracellular and endocytosis pathways. The superiority of SY and SN in increasing BWG and serum Se content might therefore be relied on higher absorption rates and/or different metabolic pathways. Supplementing a broiler diet with 0.5 mg/kg of Se as either SY or SN could be suggested by the current results.
Furthermore, in this study, it was found that upon dietary Se supplementation, serum TAC was significantly increased, in parallel with increases in enzymatic activities of GPx and TrxR. These results were not unexpected as altered Se status has long been known to be associated with impairment of antioxidant enzyme activities and augmentation of oxidants (Hu et al. Citation2012; Yang et al. Citation2016; Woods et al. Citation2020). Se acts throughout the cytoplasm as a part of selenoenzyme GPx to neutralise reactive radicals (Yuan et al. Citation2012), though all the observed effects cannot be explained by this function alone. Hu et al. (Citation2012) showed that the serum GPx activity increased upon an increase in the level of dietary Se for both SS and SN and reached a plateau at dietary Se content of 0.15 to 1.2 mg/kg. These results indicate that the serum GPx activity reflects dietary Se level, but the most remarkable response to dietary Se occurs with supplementation of 0.15 mg/kg. Additional dietary Se does not stimulate the further activity of the enzyme. In this study, the source or level of Se did not affect the serum GPx activity, because the level of Se in our diets was always higher than 0.15 mg/kg. Regarding the serum TrxR; however, it showed more heightened activity with the increase of dietary Se level, and the enzyme activity was also affected by the source of Se: there was an increasing effect by passing from SS to SY or SN. These effects were associated with increasing TAC values. Our findings are in line with those of another report (Karunasinghe et al. Citation2006), which showed that the serum Se levels of a group of men were correlated with serum TrxR activity but not with GPx activity. These results also find support from the work of Yuan et al. (Citation2012), where broiler breeder hens that given with 0.15 mg/kg of Se from SY or SM showed higher gene expression of TrxR1 in the liver and kidney than those given Se in the form of SS, whereas no differences were found in gene expression of GPx1. TrxR is a Se-dependent enzyme, which in addition to its regulatory actions in thiol redox homeostasis and intracellular signalling, also has a central role in Se metabolism, reducing Se compounds and thereby providing selenide to the synthesis of all selenoproteins (Madeja et al. Citation2005). Research has provided evidence that TrxR is also responsible for the generation of selenide required for its own synthesis (Hashemy et al. Citation2006). For these reasons, the TrxR activity is very sensitive not only to the level of Se intake, but also to the metabolism of ingested Se form (Čobanová et al. Citation2017). Together, these findings indicate that the activity of TrxR in combination with the concentration of Se in serum may be a more accurate indicator of Se adequacy and antioxidant status than GPx activity in serum of broiler chickens.
The results of this study demonstrated no effect of source or level of Se on carcase and thigh yields, which is corroborated by the reports of Mohammadi et al. (Citation2019). Meanwhile, broilers receiving 0.2 or 0.5 mg/kg of Se from SY or SN had higher breast yields than those fed the other diets, a finding which has been noted previously (Marković et al. Citation2018). This effect of dietary Se might result from an enhanced protein deposition in the breast tissue, as reported by Baltić et al. (Citation2015). However, other authors have found no supporting evidence for this relationship (Hada et al. Citation2013). A similar inconsistency is seen in the other carcase traits. Boostani et al. (Citation2015) reported that supplementing a broiler diet with 0.3 mg/kg of Se (as SS) increased liver yield, whereas Woods et al. (Citation2020) observed no change in liver yield by diet supplementation with the same level of Se as SS or SY. Otherwise, in this study, the liver weight declined by all the investigated Se sources, and the lowest liver weight was found in broiler groups that were given with 0.5 mg/kg of Se. The abdominal fat yield also showed a decreasing trend as the level of dietary Se increased. However, both SY and SN were more effective than SS in the reduction of abdominal fat. Similarly, Khajali et al. (Citation2010) found a decreased abdominal fat yield in broilers that treated with a dietary addition of 0.3 mg/kg of Se from an organic origin. Safdari-Rostamabad et al. (Citation2017) also reported lower liver and abdominal fat yields in broilers receiving 0.6 or 1.2 mg/kg of Se in the form of SN in comparison with those fed a control diet. The liver is the main site of fatty acid biosynthesis in avian systems (Wang et al. Citation2017). Accordingly, the reduction of liver yield in broilers receiving Se supplementation could also be due to lower lipogenesis in this organ. Thus, a positive change in body composition from fat to lean mass could be achieved by the higher intake of Se, mostly, in the form of SY or SN.
Dietary treatments exerted distinct effects on the yields of different lymphoid organs. The bursa of Fabricius yield was not affected by Se supplementation, which is supported by the previous studies (Singh et al. Citation2006; Niu et al. Citation2009). However, the yields of spleen and thymus were increased by all supplemental Se treatments than the control. The raise of thymus yield was more pronounced in broilers fed the higher levels of dietary Se and those receiving Se supplementation in the form of SY or SN: suggesting that the latter may improve the immune response in broilers. This is synonymous with evidence from Hussain et al. (Citation2004), who reported that Se deficiency had adverse effects on the development of spleen and thymus in broiler chickens. Our results are also compatible with the report of Boostani et al. (Citation2015), in which an elevation in spleen and thymus weights occurred by diet supplementation with different levels (0.25 to 1.0 mg/kg) of Se from SS, SY or SN. Also, Wang et al. (Citation2016) observed higher spleen and thymus yields in broilers receiving dietary Se supplementation in the form of SM compared with those receiving SS. In birds, thymus is a primary lymphoid organ that plays a pivotal role in the development and differentiation of T lymphocytes, whereas spleen is a secondary lymphoid organ that functions in maturation and activation of both B and T lymphocytes (Yiming et al. Citation2020). An explanation for the considerable increase of spleen and thymus yields with dietary Se treatment could be that Se helps protect the proliferating immature bursal B lymphocytes and splenic T lymphocytes against oxidative damage (Biswas et al. Citation2011). As a result, there would be a larger army of adult immune cells available to send out to the rest of the body. However, why only the thymus yield responded to increasing dietary Se levels and more bioavailable Se sources (SY and SN) might be explained based on the findings of Chang et al. (Citation1994). They indicated that the impaired immune capability of vitamin E- and Se-deficient chickens is associated primarily with problems in the maturation of T lymphocytes within the thymus.
Increased numbers of B and T lymphocytes are expected to have an influence on the immune system. In this way, it may be explained why broilers receiving Se supplementation gave higher antibody titres to AIV and SRBC than the control. Additionally, the anti-AIV and anti-SRBC titres were augmented steadfastly as Se supplementation increased from 0.1 to 0.5 mg/kg in the diet. Furthermore, broilers fed Se in the form of SY or SN revealed higher antibody titres against AIV and SRBC than those fed Se as SS. Also, SN produced a higher anti-SRBC titre compared with SY. Our results are in good comparison with the findings of other researchers (Niu et al. Citation2009; Habibian et al. Citation2014; Boostani et al. Citation2015; Safdari-Rostamabad et al. Citation2017; Bakhshalinejad et al. Citation2018), that showed higher antibody responses to SRBC following dietary intake of SY, SM or SN. Also confirming our findings, Shabani et al. (Citation2019) found that SN was more effective in enhancing the antibody response against SRBC than SY, and Shojadoost et al. (Citation2020) showed that SY had a higher ability to improve titres against low pathogenicity AIV compared with SS. In contrast, Bakhshalinejad et al. (Citation2018) reported higher antibody titre increases to SRBC with supplementary SY versus the same level (0.1 or 0.4 mg/kg diet) of nanosized Se, which might originate from their larger size (50 to 70 nm) of nanoparticles. On the other hand, and perhaps surprisingly, in this study, the anti-NDV titres were not affected by dietary Se, although similar findings have been reported (Hussain et al. Citation2004; Singh et al. Citation2006; Torki et al. Citation2015). Singh et al. (Citation2006) and Torki et al. (Citation2015) found no difference in total and NDV specific antibody titres when broilers fed various concentrations (0 to 1.0 mg/kg) of Se from SS, and in the same way, Hussain et al. (Citation2004) reported no differences in anti-NDV titres when broilers fed diets containing 0.2 or 0.4 mg/kg of Se from SS or SY. These results are in sharp contrast to those of Swain et al. (Citation2000), who found that supplemental Se (0.25 to 1.0 mg/kg in the form SS) improved immune function of broiler chickens by enhancing serum immunoglobulin levels and antibody titres to NDV. Also, an increasing trend in anti-NDV titres was found by supplementing the broiler diets with SN in the range of 0.15 to 1.2 mg/kg (Wang et al. Citation2008). We do not have the exact explanation for this discrepancy, and its elucidation will require future investigations. Based on our results, it may be suggested that the effect of Se on the immune response depends on the type of antigen with which the bird is challenged. Still, it should be noted that the antibody response, even to a particular antigen, can be influenced by the dose, route and schedule of immunisation, as well as the age, sex and immune status of the flock (Khorajiya et al. Citation2015).
Conclusions
In this study, the growth rate, survivability and carcase traits of broiler chickens could be enhanced by dietary supplementation with both SN and SY, especially at 0.5 mg/kg diet. This level of supplementation also had the most favourable effects on body Se and antioxidant status, and increased antibody titres against AIV and SRBC. Furthermore, SN was the most effective Se source in enhancing anti-SRBC titre. A recommendation for supplementing the broiler diets with SN to supply 0.5 mg/kg of Se is asserted by our results.
Ethical approval
All experimental procedures were approved by the Animal Care Committee at the Islamic Azad University, Isfahan (Khorasgan) Branch.
Acknowledgements
The authors thank the Islamic Azad University (Department of Animal Science) for providing research facilities and infrastructure.
Disclosure statement
The authors do not have any conflict of interest.
References
- AAFCO. 2011. AAFCO model guidance document: official guidelines suggested for contaminants in individual mineral feed ingredients. Olympia (WA): Official publication, Association of American Feed Control Officials.
- Amoako PO, Kahakachchi CL, Dodova EN, Uden PC, Tyson JF. 2007. Speciation, quantification and stability of selenomethionine, S-(methylseleno) cysteine and selenomethionine Se-oxide in yeast-based nutritional supplements. J Anal At Spectrom. 22:938–946.
- Aviagen. 2014a. Ross 308: broiler nutrition specification. Scotland (UK): Aviagen.
- Aviagen. 2014b. Ross broiler management handbook. Scotland (UK): Aviagen.
- Bakhshalinejad R, Akbari Moghaddam Kakhki R, Zoidis E. 2018. Effects of different dietary sources and levels of selenium supplements on growth performance, antioxidant status and immune parameters in Ross 308 broiler chickens. Br Poult Sci. 59:81–91.
- Baltić MŽ, Starčević MD, Bašić M, Zenunović A, Ivanović J, Marković R, Janjić J, Mahmutović H. 2015. Effects of selenium yeast level in diet on carcass and meat quality, tissue selenium distribution and glutathione peroxidase activity in ducks. Anim Feed Sci Technol. 210:225–233.
- Baylan M, Canogullari S, Ayasan T, Copur G. 2011. Effects of dietary selenium source, storage time, and temperature on the quality of quail eggs. Biol Trace Elem Res. 143:957–964.
- Behne D, Alber D, Kyriakopoulos A. 2009. Effects of long-term selenium yeast supplementation on selenium status studied in the rat. J Trace Elem Med Biol. 23:258–264.
- Biswas A, Ahmed M, Bharti VK, Singh SB. 2011. Effect of antioxidants on physio- biochemical and hematological parameters in broiler chicken at high altitude. Asian Australas J Anim Sci. 24:246–249.
- Boostani A, Sadeghi AA, Mousavi SN, Chamani M, Kashan N. 2015. Effects of organic, inorganic, and nano-Se on growth performance, antioxidant capacity, cellular and humoral immune responses in broiler chickens exposed to oxidative stress. Livest Sci. 178:330–336.
- Briens M, Mercier Y, Rouffineau F, Vacchina V, Geraert PA. 2013. Comparative study of a new organic selenium source V. seleno-yeast and mineral selenium sources on muscle selenium enrichment and selenium digestibility in broiler chickens. Br J Nutr. 110:617–624.
- Cai SJ, Wu CX, Gong LM, Song T, Wu H, Zhang LY. 2012. Effects of nano-selenium on performance, meat quality, immune function, oxidation resistance, and tissue selenium content in broilers. Poult Sci. 91:2532–2539.
- Canoğullari S, Ayaşan T, Baylan M, Çopur G. 2010. The effect of organic selenium on performance characteristics, egg production parameters and egg selenium content of laying Japanese quail. Kafkas Univ Vet Fak Derg. 16:743–749.
- Cao C, Fan R, Zhao J, Zhao X, Yang J, Zhang Z, Xu S. 2017. Impact of exudative diathesis induced by selenium deficiency on LncRNAs and their roles in the oxidative reduction process in broiler chick veins. Oncotarget. 8:20695–20705.
- Chadio SE, Pappas AC, Papanastasatos A, Pantelia D, Dardamani A, Fegeros K, Zervas G. 2015. Effects of high selenium and fat supplementation on growth performance and thyroid hormones concentration of broilers. J Trace Elem Med Biol. 29:202–207.
- Chang WP, Hom JS, Dietert RR, Combs GF, Marsh JA. 1994. Effect of dietary vitamin E and selenium deficiency on chicken splenocyte proliferation and cell surface marker expression. Immunopharmacol Immunotoxicol. 16:203–223.
- Čobanová K, Faix Š, Plachá I, Mihaliková K, Váradyová Z, Kišidayová S, Grešáková Ľ. 2017. Effects of different dietary selenium sources on antioxidant status and blood phagocytic activity in sheep. Biol Trace Elem Res. 175:339–346.
- Dalgaard TS, Briens M, Engberg RM, Lauridsen C. 2018. The influence of selenium and selenoproteins on immune responses of poultry and pigs. Anim Feed Sci Technol. 238:73–83.
- European Union. 2004. List of the authorized additives in feeding stuffs, Article 9t (b) of Council Directive 70/524/EEC concerning additives in feeding stuffs. Off J Eur Union. 50:1–144.
- Gangadoo S, Dinev I, Willson NL, Moore RJ, Chapman J, Stanley D. 2020. Nanoparticles of selenium as high bioavailable and non-toxic supplement alternatives for broiler chickens. Environ Sci Pollut Res Int. 27:16158–16159.
- Ha HY, Alfulaij N, Berry MJ, Seale LA. 2019. From selenium absorption to selenoprotein degradation. Biol Trace Elem Res. 192:26–37.
- Habibian M, Ghazi S, Moeini MM, Abdolmohammadi A. 2014. Effects of dietary selenium and vitamin E on immune response and biological blood parameters of broilers reared under thermoneutral or heat stress conditions. Int J Biometeorol. 58:741–752.
- Hada FH, Malheiros RD, Silva JDT, Marques RH, Gravena RA, Silva VK, Moraes VMB. 2013. Effect of protein, carbohydrate, lipid, and selenium levels on the performance, carcass yield, and blood changes in broilers. Braz J Poult Sci. 15:385–394.
- Hashemy SI, Ungerstedt JS, Avval FZ, Holmgren A. 2006. Motexafin gadolinium, a tumor-selective drug targeting thioredoxin reductase and ribonucleotide reductase. J Biol Chem. 281:10691–10697.
- Heindl J, Ledvinka Z, Tumova E, Zita L. 2010. The importance, utilization and sources of selenium for poultry: a review. Sci Agric Bohemi. 41:55–64.
- Hu CH, Li YL, Xiong L, Zhang HM, Song J, Xia MS. 2012. Comparative effects of nano elemental selenium and sodium selenite on selenium retention in broiler chickens. Anim Feed Sci Technol. 177:204–210.
- Hussain MI, Khan SA, Chaudhary ZI, Aslam A, Ashraf K, Rai MF. 2004. Effect of organic and inorganic selenium with and without vitamin E on immune system of broilers. Pak Vet J. 24:1–4.
- Karunasinghe N, Ferguson LR, Tuckey J, Masters J. 2006. Hemolysate thioredoxin reductase and glutathione peroxidase activities correlate with serum selenium in a group of New Zealand men at high prostate cancer risk. J Nutr. 136:2232–2235.
- Khajali F, Raei A, Aghaei A, Qujeq D. 2010. Evaluation of a dietary organic selenium supplement at different dietary protein concentrations on growth performance, body composition and antioxidative status of broilers reared under heat stress. Asian Australas J Anim Sci. 23:501–507.
- Khorajiya JH, Pandey S, Ghodasara PD, Joshi BP, Prajapati KS, Ghodasara DJ, Mathakiya RA. 2015. Patho-epidemiological study on genotype-XIII Newcastle disease virus infection in commercial vaccinated layer farms. Vet World. 8:372–381.
- King DJ, Hopkins SR. 1983. Evaluation of the hemagglutination-inhibition test for measuring the response of chickens to avian infectious bronchitis virus vaccination. Avian Dis. 27:100–112.
- Kobayashi Y, Ogra Y, Suzuki KT. 2001. Speciation and metabolism of selenium injected with 82Se-enriched selenite and selenate in rats. J Chromatogr B Biomed Sci Appl. 760:73–81.
- Li JL, Zhang L, Yang ZY, Zhang ZY, Jiang Y, Gao F, Zhou GH. 2018. Effects of different selenium sources on growth performance, antioxidant capacity and meat quality of local Chinese Subei chickens. Biol Trace Elem Res. 181:340–346.
- Liu Z, Qu Y, Wang J, Wu R. 2016. Selenium deficiency attenuates chicken duodenal mucosal immunity via activation of the NF-κB signaling pathway. Biol Trace Elem Res. 172:465–473.
- Luthman M, Holmgren A. 1982. Rat liver thioredoxin and thioredoxin reductase: purification and characterization. Biochemistry. 21:6628–6633.
- Madeja Z, Sroka J, Nyström C, Björkhem-Bergman L, Nordman T, Damdimopoulos A, Nalvarte I, Eriksson LC, Spyrou G, Olsson JM, et al. 2005. The role of thioredoxin reductase activity in selenium-induced cytotoxicity. Biochem Pharmacol. 69:1765–1772.
- Marković R, Ćirić J, Drljačić A, Šefer D, Jovanović I, Jovanović D, Milanović S, Trbović D, Radulović S, Baltić MŽ, et al. 2018. The effects of dietary selenium-yeast level on glutathione peroxidase activity, tissue selenium content, growth performance, and carcass and meat quality of broilers. Poult Sci. 97:2861–2870.
- Menon S, Ks SD, Santhiya R, Rajeshkumar S, Kumar V. 2018. Selenium nanoparticles: a potent chemotherapeutic agent and an elucidation of its mechanism. Colloids Surf B. 170:280–292.
- Mohammadi A, Ghazanfari S, Sharifi SD. 2019. Comparative effects of dietary organic, inorganic, and Nano-selenium complexes and rosemary essential oil on performance, meat quality and selenium deposition in muscles of broiler chickens. Livest Sci. 226:21–30.
- Nabi F, Arain MA, Hassan F, Umar M, Rajput N, Alagawany M, Syed SF, Soomro J, Somroo F, Liu J. 2020. Nutraceutical role of selenium nanoparticles in poultry nutrition: a review. World's Poult Sci J. 17:1–3.
- National Research Council (NRC). 1994. Nutrient requirements of poultry. 9th Rev ed. Washington (DC): National Academy Press, National Research Council.
- Niu ZY, Liu FZ, Yan QL, Li L. 2009. Effects of different levels of selenium on growth performance and immunocompetence of broilers under heat stress. Arch Anim Nutr. 63:56–65.
- Pan T, Liu T, Tan S, Wan N, Zhang Y, Li S. 2018. Lower selenoprotein T expression and immune response in the immune organs of broilers with exudative diathesis due to selenium deficiency. Biol Trace Elem Res. 182:364–372.
- Rama Rao SV, Prakash B, Raju MVLN, Panda AK, Kumari RK, Reddy EP. 2016. Effect of supplementing organic forms of zinc, selenium and chromium on performance, anti-oxidant and immune responses in broiler chicken reared in tropical summer. Biol Trace Elem Res. 172:511–520.
- Rayman MP. 2004. The use of high-selenium yeast to raise selenium status: how does it measure up? Br J Nutr. 92:557–573.
- Rice-Evans C, Miller NJ. 1994. Total antioxidant status in plasma and body fluids. Meth Enzymol. 234:279–293.
- Safdari-Rostamabad M, Hosseini-Vashan SJ, Perai AH, Sarir H. 2017. Nanoselenium supplementation of heat-stressed broilers: effects on performance, carcass characteristics, blood metabolites, immune response, antioxidant status, and jejunal morphology. Biol Trace Elem Res. 178:105–116.
- SAS Institute. 2003. SAS user’s guide: statistics. Cary (NC): SAS Institute.
- Selim NA, Radwan NL, Youssef SF, Eldin TS, Elwafa SA. 2015. Effect of inclusion inorganic, organic or nano selenium forms in broiler diets on: 2-physiological, immunological and toxicity statuses of broiler chicks. Int J of Poultry Science. 14:144–155.
- Shabani R, Fakhraei J, Yarahmadi HM, Seidavi A. 2019. Effect of different sources of selenium on performance and characteristics of immune system of broiler chickens. Braz J Anim Sci. 48:e20180256.
- Shojadoost B, Taha-Abdelaziz K, Alkie TN, Bekele-Yitbarek A, Barjesteh N, Laursen A, Smith TK, Shojadoost J, Sharif S. 2020. Supplemental dietary selenium enhances immune responses conferred by a vaccine against low pathogenicity avian influenza virus. Vet Immunol Immunopathol. 227:110089.
- Singh H, Sodhi S, Kaur R. 2006. Effects of dietary supplements of selenium, vitamin E or combinations of the two on antibody responses of broilers. Br Poult Sci. 47:714–719.
- Surai PF, Kochish II, Fisinin VI, Velichko OA. 2018. Selenium in poultry nutrition: from sodium selenite to organic selenium sources. J Poult Sci. 55:79–93.
- Swain BK, Johri TS, Majumdar S. 2000. Effect of supplementation of vitamin E, selenium and their different combinations on the performance and immune response of broilers. Br Poult Sci. 41:287–292.
- Torki M, Habibian M, Rostami T, Moradi A. 2015. Effects of high dietary levels of selenium and copper on growth performance, selected blood biochemical parameters and antibody production against Newcastle disease vaccine virus in broiler chickens. Iran J Appl Anim Sci. 5:707–713.
- Wang Y. 2009. Differential effects of sodium selenite and nano-Se on growth performance, tissue Se distribution, and glutathione peroxidase activity of avian broiler. Biol Trace Elem Res. 128:184–190.
- Wang G, Kim WK, Cline MA, Gilbert ER. 2017. Factors affecting adipose tissue development in chickens: a review. Poult Sci. 96:3687–3699.
- Wang F, Ren H, Zhu F, Sun J, Jiang J, Li W. 2008. Effects of nano-selenium on the immune functions and antioxidant abilities of broiler chickens. Chin Agric Sci Bull. 2:831–835.
- Wang Y, Wang H, Zhan X. 2016. Effects of different DL‐selenomethionine and sodium selenite levels on growth performance, immune functions and serum thyroid hormones concentrations in broilers. J Anim Physiol Anim Nutr. 100:431–439.
- Wegmann TG, Smithies O. 1966. A simple hemagglutination system requiring small amounts of red cells and antibodies. Transfusion. 6:67–73.
- Woods SL, Sobolewska S, Rose SP, Whiting IM, Blanchard A, Ionescu C, Bravo D, Pirgozliev V. 2020. Effect of feeding different sources of selenium on growth performance and antioxidant status of broilers. Br Poult Sci. 61:274–280.
- Xiao X, Yuan D, Wang YX, Zhan XA. 2016. The protective effects of different sources of maternal selenium on oxidative stressed chick embryo liver. Biol Trace Elem Res. 172:201–208.
- Xu J, Wang L, Tang J, Jia G, Liu G, Chen X, Cai J, Shang H, Zhao H. 2017. Pancreatic atrophy caused by dietary selenium deficiency induces hypoinsulinemic hyperglycemia via global down-regulation of selenoprotein encoding genes in broilers. PLoS One. 12:e0182079.
- Yang Z, Liu C, Zheng W, Teng X, Li S. 2016. The functions of antioxidants and heat shock proteins are altered in the immune organs of selenium-deficient broiler chickens. Biol Trace Elem Res. 169:341–351.
- Yiming Z, Qingqing L, Hang Y, Yahong M, Shu L. 2020. Selenium deficiency causes immune damage by activating the DUSP1/NF-κB pathway and endoplasmic reticulum stress in chicken spleen. Food Funct. 11:6467–6475.
- Yuan D, Zhan XA, Wang YX. 2012. Effect of selenium sources on the expression of cellular glutathione peroxidase and cytoplasmic thioredoxin reductase in the liver and kidney of broiler breeders and their offspring. Poult Sci. 91:936–942.
- Zhang JS, Gao XY, Zhang LD, Bao YP. 2001. Biological effects of a nano red elemental selenium. Biofactors. 15:27–38.
- Zhang J, Wang X, Xu T. 2008. Elemental selenium at nano size (nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with Se-methylselenocysteine in mice. Toxicol Sci. 101:22–31.
- Zia WM, Khalique A, Naveed S, Hussain J. 2018. Organic and inorganic selenium in poultry: a review. Ind J Anim Res. 52:483–489.