Abstract
This study was aimed to evaluate the impact of different monochromatic LED light colour (white ‘WL’, green ‘GL’, blue ‘BL’ and mixed green and blue ‘GL × BL’) and two levels of light intensity (5 lx and 20 lx) on growth performance, physiological responses (haematological and biochemical), behaviour and fear reactions in broiler. A 4 × 2 factorial treatment structure was used to evaluate the impact of 4 LED light colours (WL, GL, BL and GL × BL) and 2 levels of light intensity (5 lx and 20 lx) in triplicates. One-day-old Cobb Avian-48 chicks (N = 384, 36.29 ± 0.092 g) were used. Results revealed a significant effect of light colour on performance (final weight, weight gain and feed conversion ratio [FCR]), haematological parameters (haemoglobin, RBCs, PCV, TLC and H/L ratio), biochemical parameters (TP, albumin (AL), and AL/globulin (GL) ratio), tonic immobility (TI) duration and open field (OF) test (latency to first step, mobility duration, immobility duration, immobility sitting, immobility standing, pecking and dropping) with the best results observed in GL × BL. Light intensity had no effects on studied parameters except for H/L ratio, TI duration, latency to first step, immobility sitting, immobility standing and pecking with better findings detected in 5 lx. There was a significant light colour × light intensity interaction on final weight, weight gain, AL, TI duration, mobility duration, immobility duration, immobility sitting, immobility standing and pecking. Conclusively, monochromatic LED light (GL × BL, BL and GL) particularly of low intensity could be a source of light in broiler houses that might have the potential to improve broiler welfare.
LED light is an important factor in the broiler production.
Mixed blue and green, blue or green light of 5 lx improves broiler performance and health status.
Mixed blue and green, blue or green light of low intensity reduced fear reaction in broilers.
Highlights
Introduction
Light quality, duration, intensity and wavelength have been known as major environmental stimuli that affects physiology, behaviour, immunity and growth performance of birds (Olanrewaju, Miller, et al. Citation2016; Olanrewaju, Purswell, et al. Citation2016). In the last few decades, several types of lighting systems have been used in the poultry production houses including fluorescent, compact fluorescent and incandescent lighting systems.
Similar to most governments around the world, the Egyptian government encouraged the use of light-emitting diode (LED) bulbs in human and industrial purposes, especially because LED bulbs are more sustainable than current electricity sources, which include fuels or power from the high dam station (Waide Citation2010). LED bulbs are small in size, have a long operating life, are resistance to moisture, and are available in different wavelengths. Monochromatic LEDs as well have adjustable light intensity, and a low thermal output with high photoelectric conversion efficiency (Craford Citation1985; Olanrewaju, Miller, et al. Citation2016). These advantages make LED desirable for usage in the poultry industry, particularly for broiler chickens.
However, it is become difficult to choose the best method of lighting to maximise broiler production. Intensity and colour of light affect production differently throughout the stages of production (Olanrewaju, Miller, et al. Citation2016). Some reports from studies of modern broiler production suggest that light colour, as determined by wavelength, can affect broiler behaviour, with blue light having a calming effect, red light reducing feather pecking and cannibalism, and blue-green single or mixed LED light stimulating growth performance (Rozenboim et al. Citation2004; Kim et al. Citation2013; Mohamed, El-Kholya, et al. Citation2017). However, Sadrzadeh et al. (Citation2011) found that green LED light reduced body weight and red LED light has no effect on final body weight (FBW) of broilers. Yang et al. (Citation2018) showed that monochromatic LED light was induced a significant increase in broiler body weight irrespective to strain and sex, light sources, light intensity and LED light system. Conversely, food conversion ratio responses to monochromatic LED light interacted with the strain and sex of broilers, light sources, light intensity and LED light system (Parvin et al. Citation2014). In brief, there are still several gaps between published results regarding monochromatic LED light colour, light intensity and their effects on broiler chicken growth and welfare. These gaps resulted in failure in quantifying the relationships between monochromatic LED light colour, light intensity and broilers response.
Yet, further investigations are needed to understand how light intensity in combination with different colours affects broiler production and welfare. Therefore, the present experiment was designed to determine the impact of different monochromatic LED light colour (white ‘WL’, green ‘GL’, blue ‘BL’ and mixed green and blue ‘GLxBL’) and light intensity (5 lx and 20 lx) on growth performance, physiological responses, behaviour and fear reactions of broiler chickens during their growth period extending from day 1 to day 36.
Materials and methods
Experimental design
A triplicate 4 × 2 factorial treatment structure in a randomised complete block design was used to evaluate the impact of four LED light colours (WL, GL, BL and GL × BL) and two levels of light intensity (5 lx and 20 lx) on broiler chicks from day 1 to day 36.
Birds and husbandry system
One-day-old Cobb Avian-48 mixed-sex chicks (N ex 84) were used in this experiment. Directly after arrival, all chicks were inspected for any deformities or abnormalities and weighed to determine the initial body weight (36.29 ± 0.092 g, BW ± SEM). Chicks were randomly distributed into eight environmentally controlled and light-proof rooms (four rooms for four light colours at low light intensity and the other four rooms for four light colours at high light intensity), with three pens in each room (1.5 × 1.5 × 2.8 m) and each pen was stocked with 16 chicks. Each room was cleaned daily and had deep litter floor containing a layer of 10–15 cm wood shavings with ad-libitum access to a standard diet (NRC Citation1994) and fresh water. Ambient temperature and humidity were measured daily at bird height (25 cm) inside each room. Chicks were brooded in a temperature of 33 ± 1.06 °C and a relative humidity of 55 ± 3.8% during the first three days of age. The temperature was gradually reduced to the room temperature (23 °C) at 21 d of age while the relative humidity remained constant throughout the experimental period. Chicks remained in this housing from day 1 to day 36.
Light treatments and management
Chicks were randomly allocated to one of four broiler specific monochromatic LED light colour treatments as measured by light wavelength. Treatments included white light (WL, ∼400:770 nm), green light (GL, ∼560 nm) (BL, ∼460 nm), and mixed monochromatic blue and green light (GL × BL, ∼460 −L, ∼46). Each one of the LED colour treatments operated under one of two different light intensity (5 and 20 lx) treatments. The different light intensities were selected from the American poultry industry’s usage of 5 lx versus the European poultry industry’s usage of 20 lx. To maintain a uniform and fixed intensity, the light strips were cleaned weekly and light intensity was measured at the midpoint and the four corners of each room at bird level using a portable digital lux metre (HI97500, HANNA instrument, Woonsocket, RI). The light strips were purchased from Venus electric instruments, Cairo, Egypt (http://www.venus-electric.net) and were adjusted to equal intensity according to the spectral sensitivity of broilers (Prescott and Wathes Citation1999). The light/dark cycle was adjusted to 23 h L: 1 h D. From day 1 to 3, all chicks were reared under 40 lx. From day 4 to 8, all chicks were reared under a gradually reduced-intensity till reaching 20 or 5 lx at day 8 and were kept fixed till the end of experiment. This gradual reduction in the light intensity was to achieve successful adaptation of the birds to the new light system and to avoid any leg problems. To ensure the negative effect of reduced light intensity in birds, all birds were weighed at the beginning of day 9 and the average weight in birds reared under 20 and 5 lx were 184.3 ± 1.724g and 182.6 ± 1.495 g, respectively, without any significant differences between the groups using student T-test (p = .163).
Growth performance
At the end of the experiment, birds were individually weighed using a digital balance (PW Balance, Adam Equipment Co., Oxford, CT) to obtain FBW. Feed intake (FI) was calculated daily for each treatment. The other growth performance parameters were calculated according to the standard method: Body weight gain (BWG) = FBW − IBW; Feed conversion ratio (FCR) = FI/BWG.
Physiological response
Blood sampling
Blood was aspirated between 09:00 and 11:00 a.m. for the assessment of haematological and biochemical parameters at day 35 of the experiment. For blood sampling, a 4 mL of blood was collected from the wing vein of four randomly selected birds per replicate. The process of blood aspiration was finished within 45 s for each bird and the bleeding was arrested by gentle pressure on the site of vein puncture using a piece of cotton soaked in alcohol 70%. The birds were then returned to the appropriate rooms without unnecessary discomfort using proper upright handling techniques.
Out of 4 mL blood, 2 mL was injected into graduated tubes containing EDETA as anticoagulant and the other 2 mL were stored in empty tubes without anticoagulant.
Haematological parameters
Blood samples with EDETA anticoagulant were analysed within 2 h of collection for packed cell volume (PCV), haemoglobin, total erythrocyte count, total leukocyte count and differential leukocyte count (as described by Dein (Citation1984)) . Two thin smears were prepared from each blood sample on clean microscope slides and were allowed to air-dry. Smears were stained with a modified Wright’s stain, and were then cover slipped. A total of 100 cells were counted under ×100 oil immersion lens and the number of heterophils and lymphocytes was determined. The H: L ratios were calculated by dividing the number of heterophils by the number of lymphocytes (Gross and Siegel Citation1983).
Biochemical parameters
Blood samples without anticoagulant were stored in 4 °C in ice tank and immediately transported to laboratory. The blood was left to clot at 4 °C for 2 h. The tubes were centrifuged at 3000 rpm for 10 min for serum separation. The serum was separated in Eppendorf tubes, frozen and stored at − tu °C for further analyses of glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT) enzymes (Reitman and Frankel Citation1957), serum total proteins (TP), albumin (AL), globulin (GL), AL: GL ratio and serum total blood glucose (Armstrong and Carr Citation1964).
Behaviour and fear reactions
To avoid any possible effect of blood sampling on behaviour, the scheduled time of behavioural observations were 2 d after the end of blood sampling. All behavioural observation tests were conducted under the same light colour and intensity as in the experimental design. Behavioural observations were conducted on10 randomly selected birds/replicate. Unnecessary noise or movement by the operator was avoided to avoid attraction of the bird attention during testing.
Tonic immobility (TI) test
The birds were transported to a separate room and were subjected to TI measurements. The TI was induced by inverting the bird on its back on a clean table with its neck rested on u-shape soft clothes and applying a manual restraint for 10 s until the bird stopped struggling. The TI duration was defined as the length of time from the moment the bird became immobile until the bird righted itself. Direct eye contact between the observer and the bird should be avoided as it has been shown to prolong TI duration. The measure of TI was terminated if the bird had remained immobile for 600 s (TI = 600) or if it failed to remain at all immobile after five attempts of inducing TI (TI = 0). The number of attempts required to perform TI was recorded for each individual bird (Jones Citation1986).
Open field (OF) test
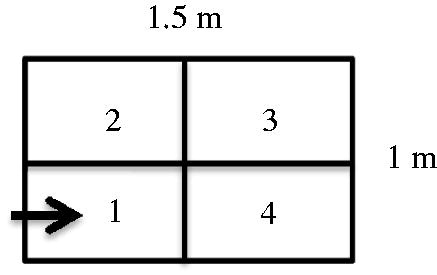
An OF arena with dimensions of 1 × 1.5 m was used for testing of each individual bird, and behavioural observations were recorded over 3 min testing time. The arena was subdivided into four equal zones by a perpendicular and horizontal line (Figure ). The individual bird was always placed in zone 1 of the arena. Number of visited zones, latency to first step, immobility duration (sitting and standing duration), walking duration, pecks directed to the floor or the walls of the arena and frequency of elimination (droppings) were recorded using a video camera (Mohamed, Abou-Ismail, et al. Citation2017).
Statistical analysis
Data were tested for normality, linearity and homogeneity of variance. Log-transformation of the raw data was used for some parameters because of the large range of the data. Data were analysed using Graph Pad Prism version 6 (San Diego, CA) and all results were reported as means and SEM. Data was compared by two-way ANOVA and the main effects of LED light colour and light intensity. The interactions of the two factors were tested by the Tukey’s multiple comparison post-hoc test when appropriate. The level of significance was set at p ≤ .05.
Results
Growth performance
The effect of LED light colour and intensity on growth performance of broilers from day 1 to day 36 is presented in Table . There was a significant effect of light colour on FBW (p = .001) and BWG (p = .001) of broiler. The GL × BL, BL and GL birds showed a significant higher FBW and BWG in respect to WL. Moreover, there was a significant light colour × light intensity effect on FBW (p = .018) and BWG (p = .018). There was a significant effect of light colour on FI (p = .022) and FCR (p = .047) with the birds reared under the GL × BL that recording the lowest FI and FCR followed by BL, GL and WL. There was no effect to the light colour × light intensity on FI and FCR. Similarly, there was no effect of light intensity on broiler growth performance. However, birds experiencing the low light intensity (5 lx) level showed higher FBW and BWG and lower FI and FCR compared to those experiencing high light intensity (20 lx).
Table 1. Effects of monochromatic LED light colour and intensity on growth performance of broilers from day 1 to day 36.
Physiological response
Haematological parameters
The influence of monochromatic LED light colour and intensity on haematological parameters of broilers is presented in Table . There was a significant effect of light colour on haemoglobin (p = .001), RBCs (p = .001), PCV (p = .001), TLC (p = .001) and H/L ratio (p = .001). The birds of the BL treatment showed the highest significant haemoglobin content when compared to WL and GL. In addition, the birds reared under BL showed the highest significant RBCs count in respect to GL × BL, GL and WL. The highest significant PCV was reported in GL × BL compared to the other groups. The WL treatment recorded the highest significant values of TLC and H/L ratio in respect to the other experimental groups. For light intensity, there was no effect on haemoglobin, RBCs, PCV and TLC while there was a significant effect of light intensity on H/L ratio (p = .021) with birds in the low light intensity (5 lx) showing lower H/L ratio compared to those in the high light intensity (20 lx). In addition, there was no light colour × light intensity effect on haematological parameters of broilers.
Table 2. Effects of monochromatic LED light colour and intensity on haematological parameters of broilers from day 1 to day 36.
Biochemical parameters
The effect of monochromatic LED light colour and intensity on biochemical parameters of broilers is summarised in Table . There was a significant effect of light colour on TP (p = .010), AL (p = .001) and A/G ratio (p = .042). The birds in the BL treatment showed the highest values of TP, and GLO and the lowest values of GPT while those of the GL × BL treatment showed the lowest GOT and glucose concentration and the highest A/G ratio. There was no effect of light intensity on broiler biochemical parameters where birds in the low light intensity (5 lx) showed lower serum glucose compared to their conspecifics in the high light intensity (20 lx). Likewise, there was a no significant light colour × light intensity effect.
Table 3. Effects of monochromatic LED light colour and intensity on biochemical parameters of broilers from day 1 to day 36.
Behaviour and fear reactions
Tonic immobility (TI) test
As shown in Table , there was a significant effect of light colour on TI duration (p = .001). The birds in the BL treatment showed the significant lowest TI duration followed by those in GL and GL × BL in respect to WL. There was a significant effect of light intensity on broiler TI duration (p = .011) with the birds in the low light intensity (5 lx) showing lower TI duration compared to those in the high light intensity (20 lx). In addition, there was a significant light colour × light intensity effect on TI duration (p = .005) of broilers. In contrast, there was no effect of light colour, light intensity and their interaction on the number of TI induction.
Table 4. Effects of LED light colour and intensity on tonic immobility test of broilers from day 1 to day 36.
Open field (OF) test
Rearing broilers under different light colours significantly affected their behaviour in OF test as latency to first step (p = .001), mobility duration (p = .001), immobility duration (p = .015), immobility sitting (p = .001), immobility standing (p = .001), pecking (p = .001) and droppings (p = 0.001). The birds in the WL treatment recorded the highest values in latency to first step, immobility duration, immobility sitting and number of dropping, but recorded the lowest values in number of visited zones, mobility duration, immobility standing and pecking compared to those in the GL, BL and GL × BL treatments. Furthermore, there was a significant effect of light intensity on broiler latency to first step (p = .021), immobility sitting (p = .001), immobility standing (p = .001) and number of pecking (p = .002). The birds in the low light intensity (5 lx) group showed lower latency to first step, immobility sitting and higher immobility standing and pecking compared to those in the high light intensity (20 lx) group. Similarly, there was a significant light colour × light intensity effect on mobility duration (p = .002), immobility duration (p = .021), immobility sitting (p = .002), immobility standing (p = .001), and number of pecking (p = .001) as presented in Table . The results in Figures and revealed that broilers reared under low light intensity (5 lx) showed higher pecking percentage and lower dropping percentage compared to those in the high light intensity (20 lx). In addition, the WL birds showed the lowest pecking percentage and the highest dropping percentage compared to those in the GL, BL and GL × BL.
Figure 2. Percentages of broiler chicks pecking (black = yes, white = no) in the open field test. WL: white light; GL: green light; BL: blue light; GL × BL: mixed green and blue light.
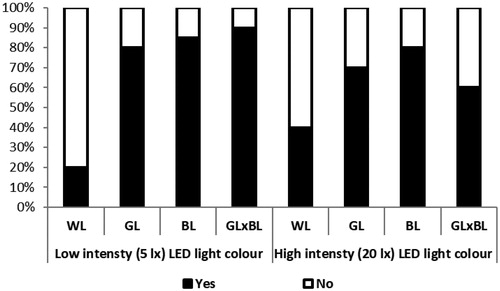
Figure 3. Percentages of broiler chicks dropping (black = yes, white = no) in the open field test. WL: white light; GL: green light; BL: blue light; GL × BL: mixed green and blue light.
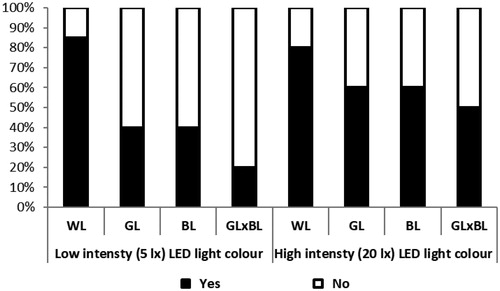
Table 5. Effects of LED light colour and intensity on open field test of broilers from day 1 to day 36.
Discussion
Recently, it has become unacceptable to use only one measurable indicator to assess the welfare state of an animal. Therefore, to achieve an accurate and scientific evaluation of the welfare of an animal, a multidisciplinary approach that involves production, physiological and behavioural indicators should be used. To achieve this multidisciplinary approach, a 4 × 2 factorial treatment structure was used to evaluate the impact of four LED light colours and two levels of light intensity on broiler welfare from day 1 to day 36. In addition, production, physiological and behavioural indicators were collected and evaluated. To the best of our knowledge, this is the first trial to evaluate the effect of LED light colours × light intensity on broiler welfare.
Growth performance and production parameters (FBW and BWG), feed utilisation and feed efficiency (FI and FCR) were significantly improved in broilers reared under mixed GL × BL, BL and GL compared to those reared under WL with the best performance achieved in mixed GL × BL. These results are in agreement with previous studies that recommended the use of LED light sources in poultry houses without adverse effects on growth performance and production parameters (Riber Citation2015; Mohamed, El-Kholya, et al. Citation2017). These findings may be attributed to higher proliferation of skeletal muscle satellite cells (Halevy et al. Citation2006) in broiler reared under GL × BL, BL and GL that resulted in heavier body weights (Rozenboim et al. Citation2004). While, higher FI and lower FCR in broiler reared under GL × BL, BL and GL compared to those in WL may be due to the calming effect of BL and GL that makes, birds less active and less fearful (Mohamed et al. Citation2014; Mohamed, Abou-Ismail, et al. Citation2017).
Dim lights are used in modern commercial broiler houses to adjust FCR to the lowest value and to improve energy utilisation. In this study, there was no significant effect of light intensity on growth performance between 5 and 20 lx while, the broilers reared under the low light intensity (5 lx) showed better growth performance compared to those reared under the high light intensity (20 lx). These results are in agreement with those of Deep et al. (Citation2010) and Olanrewaju, Miller, et al. (Citation2016) who found no significant effects of different light intensities on growth performances and welfare of broiler chickens. Similarly, Blatchford et al. (Citation2009) found that broilers reared in 5, 50 and 200 lx showed no significant differences in growth performance and immune response.
Lighting programmes (colour, intensity and duration) can affect many aspects of broiler physiology including blood haematology and biochemistry. Haematological parameters (haemoglobin, RBCs, PCV, TLC and H/L ratio) of broilers were significantly influenced by light colour. The higher RBC counts and PCV recorded in broilers reared under mixed GL × BL, BL and GL are obvious as these blood parameters are closely associated with each other. An increase in the number of erythrocytes of the same mean corpuscular volume always leads to an increase in PVC value. However, chickens that were characterised by a greater number of RBCs and higher PCV showed higher haemoglobin amount in blood. Consequently, mixed GL × BL, BL and GL influenced the haematological measures that were ultimately reflected in better health condition and growth response. Similarly, the lower TLC and H/L ratio in broilers reared under mixed GL × BL, BL and GL indicated that these light colours provide a suitable environment resulting in healthy and less stressful birds. These findings are in consistent with those reported by Mohamed, El-Kholya, et al. (2017); Mohamed, Abou-Ismail, et al. (Citation2017). This may be due to the calming effect of BL and GL (Prayitno et al. Citation1997). Mohamed et al. (Citation2014) documented that rearing broiler chickens under BL reduced H/L ratio, improved stress response and reduced bird physical activity. Therefore, rearing broilers under BL and GL influenced the haematological parameters that appeared in form of improvement in health condition and high growth performance. On the other hand, Kim et al. (Citation2013) found that monochromatic light might have a beneficial effect on growth performance but is inconclusive for haematological parameters of broilers. There was no significant effect of light intensity (5, 20 lx) on haematological parameters of broilers. These findings agree with those of Olanrewaju et al. (Citation2010) who reported no effects of light intensity (0.5, 3 and 20 lx) on most of the haematological parameters of broilers.
Biochemical parameters (TP, AL, GL, A/G ratio, GOT, GPT and glucose) were influenced by light colour with better findings in broilers reared under GL × BL, BL and GL compared to those experienced WL. These findings may be attributed to the improvements in growth performance and health condition (Kim et al. Citation2013; Yang et al. Citation2016) and immune response (Xie et al. Citation2008) of broilers reared under mixed GL × BL, BL and GL. There was no significant effect of light intensity (5, 20 lx) on biochemical parameters of broilers. This finding may indicate that high light intensity (20 lx) constitutes a stressful environment to broiler chickens. These findings agree with those of Olanrewaju et al. (Citation2010) who found no significant effects of the light intensity (0.5, 3 and 20 lx) on serum glucose levels of broilers reared under different light sources.
Light is a powerful exogenous microclimate factor that affects bird activity, behaviour and wellbeing. Rearing broiler chickens under different monochromatic light colours influenced their TI reactions and responses in OF arena. Tonic immobility (TI) duration was significantly influenced by light colour with the shortest duration recorded in BL and longest one in WL. Similar results were demonstrated by Mohamed et al. (Citation2014) and Mohamed, El-Kholya, et al. (2017). This finding may be due to the calming effect of BL (Prayitno et al. Citation1997) which might play a role in modifying stress in broilers and could therefore reduce their fear responses (Mohamed Abou-Ismail et al. Citation2017). This study documented that broilers reared under low light intensity (5 lx) showed shorter TI duration and lower levels of fear and stress compared to those reared in high light intensity (20 lx). The birds spent shorter time at the feeders and visited the feeders more frequently under 5 lx LED light (Li et al. Citation2018), therefore, they ate more and recorded heavier body weight (Yang et al. Citation2016), and became less active, and more calm (Prayitno et al. Citation1997).
Light colour significantly influenced the behaviour of broiler in OF arena. The broiler reared under GL × BL, BL and GL showed shorter latency to first step, longer mobility duration and immobility standing, shorter immobility duration and immobility sitting and lower dropping rate, indicating that they were experiencing lower levels of fear compared to those reared in WL. The lower degree of fear-indicating behaviours in mixed GL × BL, BL and GL broiler might be due to high rates of pecking activity directed to the floor or the walls of the arenas and could reflect high levels of exploration in the novel environments (Mohamed, Abou-Ismail, et al. Citation2017). This high level of exploration is associated with low level of fear and stress (Faure et al. Citation1983). Further studies are needed to explain the effect of LED light colour and light intensity and their interaction especially on the level of transcriptomic response of the broilers.
Conclusions
It could, therefore, be concluded that monochromatic blue, green and mixed LED light (GL × BL) particularly those with low intensity (5 lx) could be used as a light source in broiler poultry houses that might have the potential not only to decrease broilers fear reactions and stress responses but also to improve their growth performance, health conditions and ultimately their welfare.
Ethical approval
The experimental protocol used for broiler in this study was conducted in accordance with Egyptian Legislation on Ethics in Broiler Use and Handling and the committee of animal care and use in research, Kafrelsheikh University.
Acknowledgements
The authors thank Usama Abou-Ismail and Reuben Don for their helpful comments and revising the language of the article.
Disclosure statement
The authors declare that there is no conflict of interest associated with the article. All authors contributed equally in this work (conception, acquisition, samples analysis, statistical analysis, data interpretation, manuscript drafting and manuscript revision).
Additional information
Funding
References
- Armstrong W, Carr C. 1964. Physiological chemistry laboratory directions. 3rd ed. Minneapolis (MN): Burges.
- Blatchford R, Klasing K, Shivaprasad H, Wakenell P, Archer G, Mench J. 2009. The effect of light intensity on the behaviour, eye and leg health, and immune function of broiler chickens. Poultr Sci. 88(1):20–28.
- Craford MG. 1985. Light-emitting diode displays. Flat-panel displays and CRTs. Berlin, Germany: Springer; p. 289–331.
- Deep A, Schwean-Lardner K, Crowe T, Fancher B, Classen H. 2010. Effect of light intensity on broiler production, processing characteristics, and welfare. Poult Sci. 89(11):2326–2333.
- Dein FJ. 1984. Laboratory manual of avian hematology. Association of avian hematology, association of avian veterinarians. East Northport, NY, USA.
- Faure J, Jones R, Bessei W. 1983. Fear and social motivation as factors in open-field behaviour of the domestic chick. A theoretical consideration [behaviour, open-field test]. Biol Behav. 8:103-116
- Gross W, Siegel H. 1983. Evaluation of the heterophil/lymphocyte ratio as a measure of stress in chickens. Avian Dis. 27(4):972–979.
- Halevy O, Piestun Y, Rozenboim I, Yablonka-Reuveni Z. 2006. In ovo exposure to monochromatic green light promotes skeletal muscle cell proliferation and affects myofiber growth in posthatch chicks. Am J Physiol Regul Integr Comp Physiol. 290(4):R1062–R1070.
- Jones RB. 1986. The tonic immobility reaction of the domestic fowl: a review. World’s Poult Sci J. 42(1):82–96.
- Kim MJ, Parvin R, Mushtaq MMH, Hwangbo J, Kim JH, Na JC, Kim DW, Kang HK, Kim CD, Cho KO, et al. 2013. Growth performance and hematological traits of broiler chickens reared under assorted monochromatic light sources. Poult Sci. 92(6):1461–1466.
- Li G, Zahao Y, Purswell JL, Liang Y, Lowe JW. 2018. feeding behaviors of broilers at chicken-perceived vs. human- perceived light intensities under two light spectrums. In2018 ASABE Anual International Meeting (p. 1). American Society of Agricultural and Biological Engineers.
- Mohamed RA, Abou-Ismail UA, Shukry M. 2017. Effects of different monochromatic LED light colours on fear reactions and physiological responses in Mulard ducks. Anim Prod Sci. 57(6):1128–1136.
- Mohamed RA, El-Kholya SZ, Shukry M, El-Kassas S, El Saidy NR. 2017. Manipulation of broiler growth performance, physiological and fear responses using three monochromatic LED lights. Alexandria J Vet Sci. 53(1):57–62.
- Mohamed R, Eltholth M, El-Saidy N. 2014. Rearing broiler chickens under monochromatic blue light improve performance and reduce fear and stress during pre-slaughter handling and transportation. Bio Anim Husb. 30(3):457–471.
- NRC. 1994. Nutrient requirements of poultry. 9th ed. Washington (DC): National Academy Press.
- Olanrewaju HA, Purswell J, Collier S, Branton SL. 2016. Effects of light sources and intensity on broilers grown to heavy weights: hematophysiological and biochemical assessment. Int J Poult Sci. 15:384–393.
- Olanrewaju H, Miller W, Maslin W, Collier S, Purswell J, Branton S. 2016. Effects of light sources and intensity on broilers grown to heavy weights. Part 1: growth performance, carcass characteristics, and welfare indices. Poult Sci. 95(4):727–735.
- Olanrewaju H, Purswell J, Collier S, Branton S. 2010. Effect of ambient temperature and light intensity on physiological reactions of heavy broiler chickens. Poult Sci. 89(12):2668–2677.
- Parvin R, Mushtaq M, Kim M, Choi H. 2014. Light emitting diode (LED) as a source of monochromatic light: a novel lighting approach for behaviour, physiology and welfare of poultry. World's Poult Sci J. 70(3):543–556.
- Prayitno D, Phillips C, Stokes D. 1997. The effects of color and intensity of light on behavior and leg disorders in broiler chickens. Poult Sci. 76(12):1674–1681.
- Prescott N, Wathes C. 1999. Spectral sensitivity of the domestic fowl (Gallus g. domesticus). Br Poult Sci. 40(3):332–339.
- Reitman S, Frankel S. 1957. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 28(1):56–63.
- Riber AB. 2015. Effects of colour of light on preferences, performance, and welfare in broilers. Poultr Sci. 94(8):1767–1775.
- Rozenboim I, Biran I, Chaiseha Y, Yahav S, Rosenstrauch A, Sklan D, Halevy O. 2004. The effect of a green and blue monochromatic light combination on broiler growth and development. Poult Sci. 83(5):842–845.
- Sadrzadeh A, Brujeni GN, Livi M, Nazari MJ, Sharif MT, Hassanpour H, Haghighi N. 2011. Cellular immune response of infectious bursal disease and Newcastle disease vaccinations in broilers exposed to monochromatic lights. Afr J Biotechnol. 10(46):9528–9532.
- Waide P. 2010. Phase out of incandescent lamps: implications for international supply and demand for regulatory compliant lamps. Paris, France: International Energy Agency.
- Xie D, Wang Z, Dong Y, Cao J, Wang J, Chen J, Chen Y. 2008. Effects of monochromatic light on immune response of broilers. Poultr Sci. 87(8):1535–1539.
- Yang Y, Pan C, Zhong R, Pan J. 2018. The quantitative models for broiler chicken response to monochromatic, combined, and mixed light-emitting diode light: a meta-analysis. Poultr Sci. 97(6):1980–1989.
- Yang Y, Yu Y, Pan J, Ying Y, Zhou H. 2016. A new method to manipulate broiler chicken growth and metabolism: response to mixed LED light system. Sci Rep. 6:25972.