Abstract
The present experiment was conducted to evaluate the effect of enzymatically treated Artemisia annua L. (EA) on intestinal digestive function and immune capacity in weaned pigs. Firstly, a total of 300 21-day-old piglets were randomly allotted to five groups, including CON, EA1, EA2, EA3, and EA4 groups (basal diet supplemented with 0, 0.5, 1, 2, 4 g/kg EA, respectively). At 50 days of age, the results showed that pigs in the EA3 group presented significant improvements (p < .05) of growth performance and health status when compared with those of the CON group. According to the results above, we considered 2 g/kg EA as the optimal dose for pig diets. Therefore, we selected 12 pigs from the CON and EA3 groups (n = 6) at 51 days of age for further investigation in vivo. Compared with the CON group, EA3 group significantly increased (p < .05) small intestinal length of pigs, enhanced (p < .05) the activities of amylase, trypsin and Na+-K+-ATPase and the concentrations of glucose transporter in both jejunum and ileum. EA3 group had lower (p < .05) concentrations of interleukin 1β, interleukin 6 and tumour necrosis factor α in the jejunum and higher (p < .05) concentrations of interleukin 10, secretory immunoglobulin A and immunoglobulin G in the ileum of pigs than the CON group. These results demonstrated that EA provided a dietary nutritional means to improve intestinal function of weaned pigs and 2 g/kg was the optimal dose for weaned pigs in the present study.
Highlights
Dietary supplementation with 2 g/kg enzymatically treated Artemisia annua L. increased growth performance and nutrient digestibility, decreased the incidence of diarrhoea in weaned pigs.
The optimal dose of enzymatically treated Artemisia annua L. in weaned pigs in the current study was 2 g/kg.
Enzymatically treated Artemisia annua L. inclusion increased the digestive function of weaned pigs.
Diet supplemented with enzymatically treated Artemisia annua L. reduced the inflammation and enhanced the immunity of weaned pigs.
Introduction
With the rapid turnover of pig production, piglets are usually weaned at an early age (14–28 days after birth) in commercial production (Carroll et al. Citation1998; Tang et al. Citation2009). They are susceptible to numerous stresses, such as the changes in diet, separation from the sow, new environmental and social adaptation (Heim et al. Citation2014). The abrupt changes reduce the feed intake and break down the balance of the intestine. The intestinal imbalance causes the marked changes in gut homeostasis and function which include villous atrophy, crypt hyperplasia, intestinal inflammation and dysfunction (Pluske et al. Citation1997). The digestive and absorptive capacity of small intestine (SI) are inhibited after the weaning process, which finally affect the gut health and lead to a decrease of growth performance (Mcorist and Mellits Citation2010; Wijtten et al. Citation2012). Recent studies have shown that diet supplemented with phytogenic ingredient as an alternative of antibiotics can improve the growth, nutrient digestibility and intestinal structure and function in weaned pigs (Zhou et al. Citation2015; Mendoza et al. Citation2018; Wang et al. Citation2020). Therefore, it is important to apply a new nutritional strategy during the post-weaning period to ameliorate the intestinal development and health.
Artemisia annua L. (A. annua), which belongs to Artemisia species of Asteraceae family, is an annual herb native to Asia, most probably China, and now is distributed in many countries worldwide (Lee et al. Citation2002; Das Citation2012). There are numerous bioactive compounds isolated from A. annua, including polyphenols, terpenoids, flavonoids, coumarins, acetylenes, sterols, and sesquiterpene lactone (artemisinin), as well as some nutrients such as amino acids, vitamins and mineral elements (Brisibe et al. Citation2009; Brown Citation2010). Artemisinin, a main bioactive ingredient of A. annua, is known for antimalarial activity all over the world. Besides antimalarial effects, A. annua also has other biological activities such as antibacterial, anti-inflammatory, antitumor and antioxidant effects (Juteau et al. Citation2002; Wetzstein et al. Citation2014). However, because of the presence of cellulose and pectin, the plant cell wall of A. annua prevents the release of bioactive ingredients (Puri et al. Citation2012), which are the anti-nutritional factors in the cell wall of A. annua. Meanwhile, monogastric animals cannot synthesise enzymes that cleave such plant structural polysaccharides (Huang et al. Citation2013). Enzymatic treatment by cellulase and pectinase, either individually or in combination, degrades plant cell walls and facilitates the release of plant-based bioactive compounds, finally improving nutrients utilisation and growth performance (Omogbenigun et al. Citation2004; Puri et al. Citation2012).
In the present study, we used cellulase and pectinase to hydrolyse A. annua to produce enzymatically treated Artemisia annua L. (EA). Previous study demonstrated that broilers fed diet supplemented with 1 g/kg EA rather than 5 g/kg A. annua showed improved growth performance (Wan et al. Citation2016a). Studies also found that dietary inclusion of 1 g/kg EA exhibited beneficial effects on growth performance, intestinal morphology, digestive enzyme capacity, immune and oxidative status, and alleviated the intestinal inflammatory response of broilers challenged with heat stress (Song et al. Citation2017, Citation2018). However, the effect of EA on intestinal health of weaned pigs has not been studied. The aim of this study was to determine the optimal dose of EA and investigate the effect of dietary EA supplementation on intestinal digestive function and immunity of weaned pigs in vivo. The present study may be helpful in finding a new nutritional strategy for relieving the weaning stress and improving intestinal health of weaned pigs.
Materials and methods
All experimental design and procedures were approved by Institutional Animal Care and Use Committee of Nanjing Agricultural University following the requirements of the Regulations for the Administration of Affairs Concerning Experimental Animals of China (NJAU-CAST-2017-019).
Preparation of EA
In the present study, EA was produced and kindly provided by Kehu Biotechnology Research Centre (Guangzhou, China). Cellulase and pectinase were used for the enzymolysis of A. annua entire herb. The main components in EA were flavonoid, polyphenol, artemisin, artemisinic acid and deoxyartemisin. The detailed information of producing EA was outside the scope of this manuscript and cannot be described due to commercial sensitivity.
Experimental design and animal management
In this experiment, a completely randomised design was used for statistical analysis. Thirty healthy pregnant sows with similar expected dates of parity (second or third) were chosen during pregnancy. At 21 days of age, 300 piglets [Duroc × (Yorkshire × Landrace)] with similar initial body weight (5.62 ± 0.36 kg) from 30 litters (five barrows and five gilts per litter) were allotted into five groups with six replicates (pens) of 10 piglets each. The 10 piglets in each replicate consisted of five barrows and five gilts, which were randomly selected from five different litters to balance their litter of origin. At 50 days of age, no significant difference was observed in the body weight of each piglet per pen including barrows and gilts. Therefore, the factors of this experimental design, such as the litter of origin, body weight and sex, were tested and resulted in no significant. The dietary treatments included: control group (CON, basal diet), EA1 group (basal diet + 0.5 g/kg EA), EA2 group (basal diet + 1 g/kg EA), EA3 (basal diet + 2 g/kg EA) and EA4 (basal diet + 4 g/kg EA). The basal diet used in this experiment was formulated to meet or exceed the nutrient requirements of pigs according to the NRC (Citation2012). Every ten piglets were housed in an environmentally controlled and plastic floored pen (3.40 m × 3.60 m). House temperature was thermostatically maintained at 30 °C for the first seven days, and then gradually reduced by 2 °C per week until 50 days. All piglets were provided feed and water ad libitum through a self-feeder and nipple drinker throughout the whole experimental period. The composition and analysed nutrient contents of the experimental diets were shown in Table .
Table 1. Composition and nutrient content of basal diet (as-fed basis).
Growth performance and incidence of diarrhoea
Piglets were individually weighed at the beginning (21 days) and the end (50 days) of the experiment. Feed intake was recorded daily on a pen basis during the whole trial. Growth performance was evaluated in terms of feed intake (FI), body weight gain (BWG) and feed conversion ratio (FCR).
Pigs with diarrhoea were recorded during the experimental period (21–50 days of age). The incidence of diarrhoea was calculated according to the following formula: [total number of diarrhoea pigs on each day during the experiment/(total number of pigs × days of experiment)] × 100%. Pigs were not given veterinary treatments for diarrhoea during the experiment.
Digestibility trial
A digestibility trial was conducted using chromic oxide (0.2%) as an indicator for the determination of apparent total tract digestibility (ATTD) of dry matter (DM), organic matter (OM), nitrogen (N) and ether extract (EE) according to Fenton and Fenton (Citation1979). Pigs in all pens were fed diets mixed with chromic oxide on day 43, and fresh faecal samples were collected from each pen on day 47–50. All the faeces (50 g per pen) were pooled and mixed from each pen over a 3-day period. For chemical analysis, faecal samples were dried in a forced-air drying oven (FC610; Advantec, Toyo Seisakusho, Tokyo, Japan) at 60 °C for 72 h and ground to pass through a 1 mm screen. All feed and faecal samples were measured for DM, OM, N and EE in accordance with the AOAC (Citation2007) methods. Chromium was analysed using UV absorption spectrophotometry (UV-1201; Shimadzu, Kyoto, Japan) according to the procedure described by Fenton and Fenton (Citation1979). The ATTD of nutrients was calculated by the following formula: Digestibility (%) = [1−(Nf×Cd)/(Nd×Cf)] × 100, where Nf = Nutrient concentration in faeces (% DM), Nd = Nutrient concentration in diet (% DM), Cf = Chromium concentration in faeces (% DM), and Cd = Chromium concentration in diet (% DM).
Sample collection
According to the results on growth performance and incidence of diarrhoea in the feeding experiment, EA3 group was considered as the optimal dose group. Considering the animal ethics, pigs in the CON group and EA3 group were selected in order to limit the number of animals to sacrifice for further research in vivo. At 51 days, one pig within nearly average body weight of each pen from CON and EA3 groups (6 pigs per group) was weighed and then humanely slaughtered by injection of sodium pentobarbitone. The small intestine (SI) was taken out from abdominal cavity immediately and divided into duodenum (nearly first 10-cm segment of the SI), jejunum (40% of the SI below the duodenum), and ileum (60% of the SI below the jejunum) according to previous study (Wang et al. Citation2008). Then the lengths of every segment of SI were measured. After that, the sample of intestinal contents was collected by massaging the intestinal tract from both ends. Jejunal and ileal mucosa were scraped from the luminal surface using a glass microscope slide. The digesta and mucosa samples were immediately put into liquid nitrogen after collection. Finally, the samples of intestinal digesta and mucosa from liquid nitrogen were homogenised in an ice-cold phosphate-buffered saline-EDTA (2.0 M NaCl, 0.05 M Na3PO4, 2 × 10−3 M EDTA, pH7.4) with a ratio of 1:4 (wt/vol). Then the homogenate of each sample was centrifuged at 3000 × g at 4 °C for 15 min and the supernatant was stored at −20 °C until further analysis. The relative intestinal length was calculated according to the following formula: The relative intestinal length = the length of each segment intestine (cm)/the length of total small intestine (m).
Analysis of digestive enzyme activity
The activities of amylase (C016-1-1), trypsin (A080-2-2) and lipase (A054-1-1) of intestinal digesta (wt/vol = 1:4) were determined using corresponding diagnostic kits of Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China) according to the instructions of the manufacturer.
Analysis of disaccharidase, Na+-K+-ATPase and alkaline phosphatase activities
The activities of lactase (A082-1-1), sucrase (A082-2-1), maltase (A082-3-1), Na+-K+-ATPase (A016-2-1) and alkaline phosphatase (AKP, A059-2-2) of intestinal mucosa (wt/vol = 1:4) were determined using corresponding diagnostic kits of Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China) according to the instructions of the manufacturer.
Analysis of nutrient transporter function, intestinal cytokines and immunoglobulin concentrations
The concentrations of glucose transporter 2 (GLUT2), sodium-dependent glucose transporter 1 (SGLT1), interferon γ (IFN-γ), interleukin 1β (IL-1β), interleukin 6 (IL-6), tumour necrosis factor α (TNF-α), interleukin 4 (IL-4), interleukin 10 (IL-10) and immunoglobulin G (IgG) of intestinal mucosa were determined by ELISA methods using each antibody and biotinylated secondary antibody according to the instructions of the manufacturer (YILI Biological Technology Co., Ltd, Shanghai, China).
Gene expression analysis
Total RNA from the small intestinal mucosal samples was isolated using Trizol reagent (TaKaRa, Dalian, Liaoning, China). RNA integrity was checked on 1% agarose gel with ethidium bromide staining. The concentration of total RNA was quantified with a NanoDrop-ND 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Reverse transcription was performed immediately using the PrimeScript RT Master Mix (Perfect Real Time) kit (TaKaRa, Dalian, Liaoning, China) according to the manufacturer’s instructions. Quantitative real-time polymerase chain reaction (qRT-PCR) was carried out in duplicate on the StepOnePlus ABI Prism Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The complementary DNA (cDNA) samples were amplified with the TB Green Premix Ex Taq (Tli RNaseH Plus) kit (TaKaRa, Dalian, Liaoning, China) according to the manufacturer’s instructions. The primer sequences of Actb, Ifng, Il1b, Tnfa, Il6, Il4, Il10, Igg were presented in Table . The relative levels of mRNA expression were calculated using the 2−ΔΔCT method, in which the Actb gene was amplified as a housekeeping gene. The values of pigs in the CON group were used as a calibrator.
Table 2. Primer sequences of target genes.
Statistical analysis
A completely randomised design was used in this study. The pen was the experimental unit for all analyses. Growth performance, incidence of diarrhoea and nutrient digestibility were analysed by one-way analysis (ANOVA) using SPSS statistical software (Ver. 20.0 for windows, SPSS, Inc., Chicago, IL, USA). The statistical differences between treatments were determined by a Tukey test. The orthogonal polynomial contrast test was performed to determine linear and quadratic effects with increasing inclusion level of EA (0, 0.5, 1, 2, and 4 g/kg) in the diet. Differences between the CON and EA3 groups were analysed using independent t-test (Ver. 20.0 for windows, SPSS, Inc., Chicago, IL, USA). Data were presented as mean ± SEM. p values lower than .05 were considered statistically significant.
Results
Growth performance and incidence of diarrhoea
As shown in Table , diet supplemented with 2 g/kg EA significantly increased (p < .05) BWG compared with that of the CON group. Both FI and BWG in the EA4 group were lower (p < .05) than those of in the EA3 group. With regard to FCR, there was no significant difference (p > .05) among treatment groups. In addition, EA supplementation quadratically increased FI (p = .031) and BWG (p = .002). EA3 group showed the lowest (p < .05) incidence of diarrhoea among treatment groups. There were linear (p < .01) and quadratic (p = .06) responses on incidence of diarrhoea with increasing EA levels.
Table 3. Effect of diet supplemented with different doses of enzymatically treated Artemisia annua L. on growth performance in weaned pigs at 50 days of age.
Nutrient digestibility
As shown in Table , diet supplemented with different doses of EA significantly increased (p < .05) the ATTD of DM and OM compared to the CON. The ATTD of N and EE in the EA3 group were higher (p < .05) than those of in the CON group. There was a linear (p = .022 and p = .029) and quadratic (p = .003 and p = .006) influence on the ATTD of DM and OM of pigs with increasing levels of EA in the diet. The ATTD of N was shown a quadratic increase (p = .022) as supplemental EA level increased.
Table 4. Effect of diet supplemented with different doses of enzymatically treated Artemisia annua L. on nutrient digestibility in weaned pigs at 50 days of age.
The development of small intestine
The effect of diet supplemented with 2 g/kg EA on the development of small intestine in weaned pigs was presented in Table . Pigs fed the 2 g/kg EA increased (p < .05) the SI length compared with those fed the basal diet. The relative length of duodenum, jejunum and ileum showed no significant difference among treatments (p > .05).
Table 5. Effect of diet supplemented with 2 g/kg enzymatically treated Artemisia annua L. on the development of small intestine in weaned pigs at 51 days of age.
Digestive enzyme activity of intestinal digesta
The effect of diet supplemented with 2 g/kg EA on digestive enzyme activity of intestinal digesta was shown in Table . The activities of amylase and trypsin of EA3 group in both jejunum and ileum were higher (p < .05) than those of the CON group. There was no significant difference of lipase activities in jejunum and ileum of weaned pigs (p > .05).
Table 6. Effect of diet supplemented with 2 g/kg enzymatically treated Artemisia annua L. on digestive enzyme activity of intestinal digesta in weaned pigs at 51 days of age.
Disaccharidase, Na+-K+-ATPase and alkaline phosphatase activities of intestinal mucosa
The results of intestinal disaccharidase activity of intestinal mucosa were summarised in Table . Dietary 2 g/kg EA supplementation increased (p < .05) the activities of lactase and maltase in the ileum, and Na+-K+-ATPase activities in both jejunum and ileum of pigs. EA had no influence on the sucrase activity in the ileum of pigs (p > .05). No significant difference was observed for the disaccharidase activity in the jejunum and AKP activity in both jejunum and ileum of weaned pigs among the treatment groups (p > .05).
Table 7. Effect of diet supplemented with 2 g/kg enzymatically treated Artemisia annua L. on disaccharidase, Na+-K+-ATPase and AKP activities of intestinal mucosa in weaned pigs at 51 days of age.
Nutrient transporter function
The effect of diet supplemented with 2 g/kg EA on nutrient transporter function in weaned pigs was shown in Table . The concentrations of GLUT2 and SGLT1 in both jejunum and ileum of EA3 group were significantly increased (p < .05) compared with the CON group.
Table 8. Effect of diet supplemented with 2 g/kg enzymatically treated Artemisia annua L. on nutrient transporter function of intestinal mucosa in weaned pigs at 51 days of age.
Intestinal immune function
As presented in Table , pigs fed 2 g/kg EA diet significantly decreased (p < .05) the concentrations of IL-1β, IL-6 and TNF-α in the jejunum, and increased (p < .05) the concentrations of IL-10, sIgA and IgG in the ileum compared with those fed the basal diet. As shown in Figure g/kg EA supplementation down-regulated (p < .05) the mRNA expressions of Il1b, Il6 and Tnfa and up-regulated (p < .05) the mRNA expression of Igg in the jejunum compared to the CON group. As shown in Figure , the mRNA expression of Igg in the ileum of EA3 group was up-regulated (p < .05) compared to the CON group.
Figure 1. Effect of diet supplemented with 2 g/kg enzymatically treated Artemisia annua L. on the mRNA expressions of cytokines and immunoglobulin G in the jejunum of weaned pigs at 51 days of age. CON: control group, basal diet; EA3: basal diet + 2 g/kg enzymatically treated Artemisia annua L.; IFN-γ: interferon γ; IL-1β: interleukin 1β; IL-6: interleukin 6; TNF-α: tumour necrosis factor α; IL-4: interleukin 4; IL-10: interleukin 10; IgG: immunoglobulin G. All data were analysed using independent t-test and were presented as mean ± SEM (n = 6). A probability level of p < .05 was considered statistically significant.
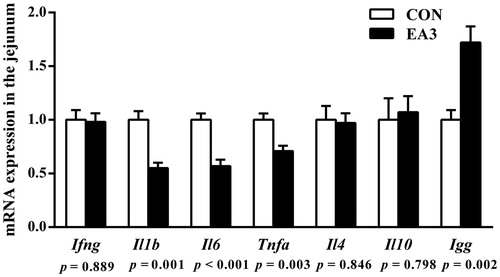
Figure 2. Effect of diet supplemented with 2 g/kg enzymatically treated Artemisia annua L. on the mRNA expressions of cytokines and immunoglobulin G in the ileum of weaned pigs at 51 days of age. CON: control group, basal diet; EA3: basal diet + 2 g/kg enzymatically treated Artemisia annua L; IFN-γ: interferon γ; IL-1β: interleukin 1β; IL-6: interleukin 6; TNF-α: tumour necrosis factor α; IL-4: interleukin 4; IL-10: interleukin 10; IgG: immunoglobulin G. All data were analysed using independent t-test and were presented as mean ± SEM (n = 6). A probability level of p < .05 was considered statistically significant.
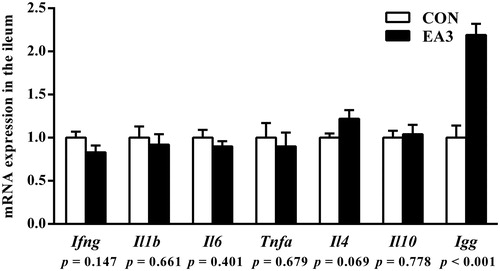
Table 9. Effect of diet supplemented with 2 g/kg enzymatically treated Artemisia annua L. on immune function of intestinal mucosa in weaned pigs at 51 days of age.
Discussion
A. annua is a traditional Chinese herb which processes anti-malaria, antibacterial, anti-inflammatory, anti-tumour and antioxidant capacities. Enzymatic hydrolysis technology can degrade plant cell wall, release active components and finally produce a marked effect on growth and metabolism. As far as we know, this is the first research to explore the effect of EA supplementation on growth performance, intestinal digestive function and immunity of weaned pigs.
In the present study, the results showed that diet supplemented with 2 g/kg EA significantly increased BWG compared with the CON group. BWG was quadratically improved by dietary EA supplementation. Wan et al. (Citation2016a) reported that broilers fed diet supplemented with 1 g/kg EA rather than 5 g/kg A. annua leaves showed increased average body weight gain in the starter phase and the whole trial. Wan et al. (Citation2017) also demonstrated that 0.75% EA diet increased BWG of broilers reared under heat stress. The crude protein, essential amino acids, minerals, vitamins, antioxidants and flavonoids in the A. annua leaves are important for growth and development of broilers, which is probably the reason for the enhancement of BWG (Brisibe et al. Citation2008). After treatment with enzymes, those active ingredients in A. annua were better released. In contrast, previous study showed that the growth performance of broilers were not affected by increasing of dietary EA (0.5%, 1%, 1.5%) during the 42 day experiment (Wan et al. Citation2016b). The reason for these differences could be attributed to the different experimental conditions, animal species and the diversity of origin and dosage of A. annua. In addition, pigs fed 4 g/kg EA showed lower FI and BWG compared with the pigs fed 2 g/kg EA. The results indicated that the high dose of EA may have an adverse effect on growth of pigs. Pig with diarrhoea often occurs in the early period which has become a serious problem in the pig industry. In this study, diet supplemented with 2 g/kg EA exhibited the lowest incidence of diarrhoea in weaned piglets. EA may reduce the incidence of diarrhoea by resisting intestinal pathogens and improving intestinal health (Zhao et al. Citation2015).
The current study showed that dietary supplementation with different doses of EA increased the ATTD of DM and OM in pigs. Pigs fed 2 g/kg EA diet improved the ATTD of N and EE compared with pigs fed the basal diet. The results indicated that EA supplementation had a positive effect on nutrient digestibility of weaned pigs. Artemisinin, isolated from Artemisia annua, was reported to inhibit inflammatory response via regulating NF-κB and MAPK signalling pathways (Wang et al. Citation2017). Therefore, according to previous studies, we speculated that EA could enhance the intestinal health by exerting anti-bacterial and anti-inflammatory activities. The improvement of nutrient digestibility in the EA group might be related to the improved body weight gains.
The intestine length represents the levels of intestinal development. The present study showed that pigs fed 2 g/kg EA diet increased body weight and the SI length compared with those fed basal diet. The results of the present study were in agreement with previous studies that the longer is gut length, the better is nutrient absorption which result in a heavier body weight (Yusrizal and Chen1 Citation2003). These results suggested that the development of small intestine was improved after EA treatment in the diet of pigs.
The small intestine is the major site of nutrient digestion and absorption. The digestive function and digestive enzyme secretions are altered after weaning (Pluske et al. Citation1997). The intestinal enzymes play important roles in facilitating gut digestive function, which hydrolyse macromolecular substances into small molecules for intestinal absorption. The literatures about the effect of EA on digestive enzyme activities are scarce. Similar results were observed by Song et al. (Citation2018) who found that jejunal lipase and trypsin activities of broilers under heat stress were enhanced by EA supplementation. Herbs, which are known as growth promoters, are gradually applied in the diet of animal production to balance the gut microbial ecosystem, stimulate the secretion of endogenous digestive enzyme, improve the nutrient digestibility and finally improve growth performance (Williams Citation2001). The improvement of intestinal digestive enzyme activities might be associated with intestinal morphology and integrity by dietary supplementation of EA (Song et al. Citation2018).
Carbohydrates are the main source of energy for pigs. The disaccharides (sucrase, maltose and lactose) are hydrolysed into monosaccharides (mainly glucose) at the brush border membrane of intestine by disaccharidases (Marion et al. Citation2005). The nutrient transporters, such as SGLT1 and GLUT2, are crucial to the absorption of glucose in the small intestine. They are responsible for transporting glucose from the intestinal lumen to the enterocyte and then to the blood stream (Sangild et al. Citation2006). Na+-K+-ATPase is an intestinal membrane-bound enzyme that actively transports Na+ out of and K+ into cells and provides energy for the transmembrane transport of SGLT1 (Gal-Garber et al. Citation2003). AKP is an enzyme capable of hydrolysing phosphate esters in an alkaline medium and its hydrolytic activity is important for intestinal absorption of glucose (Roubaty and Portmann Citation1988). In the present study, diet supplemented with 2 g/kg EA increased the activities of lactase and maltase in the ileum, improved the activities of Na+-K+-ATPase and the concentration of GLUT2 and SGLT1 in both jejunum and ileum of pigs, suggesting that EA treatment could improve the digestibility of carbohydrate. The results were consistent with the research which showed that essential oils from aromatic plants and their extracts could enhance the enzyme secretions related to feed digestion (Brenes and Roura Citation2010). Previous study has demonstrated that the maintenance of the villous structure was very important for the digestion and absorption of carbohydrate in the suckling, weaning and growth stage of pigs (Tsukahara et al. Citation2013). This improvement might be associated with the improvement of nutrient digestibility after dietary EA treatment.
In the current study, we hypothesised that EA exerted its protective effect on gut health by attenuating intestinal inflammatory responses and improving intestinal immunity. Previous research reported that overproduction of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) had a negative effect on gut integrity and epithelial function (McKay and Baird Citation1999). It has been indicated that the anti-inflammatory cytokine IL-10 could maintain epithelial barrier in the presence of interferon γ (Madsen et al. Citation1997). Another study suggested that IL-10 gene-deficient mice exerted an intestinal permeability defect at two weeks of age (Madsen et al. Citation1999). In the present study, the mRNA expressions and protein levels of IL-1β, TNF-α, IL-6 in the jejunum were decreased when pigs fed EA diet. However, the mRNA expression of IL-10 was increased while no significant IL-10 concentration was observed in the ileum of pigs in EA3 group when compared to CON group. sIgA, which is mainly involved in the mucosal immune response, plays an important role in the defense of the gastrointestinal tract. The level of IgG reflects the humoral immunity of the body. In the present study, EA addition in the diet increased the concentrations of sIgA and IgG, and the mRNA expression of Igg in the ileum, suggesting that EA supplementation could enhance the intestinal immunity. Evidence has been accumulated that artemisinin from A. annua exerted anti-inflammatory capacity by reducing the pro-inflammatory cytokines (Ryu et al. Citation2013). Artemisinin was also ascertained to possess the immunomodulatory function (Li et al. Citation2012). The reason for the improvement of intestinal immunity and reduction of inflammation in EA3 group may be attributed to the artemisinin in the EA. Further studies are needed to clarify the mechanism of EA for relieving intestinal inflammation and improving the immunity.
Conclusions
In conclusion, dietary supplementation with 2 g/kg EA increased growth performance and nutrient digestibility and decreased the incidence of diarrhoea in weaned pigs. It was identified 2 g/kg as the optimal dose to be incorporated in a weaner diet. In addition, such dietary level increased the digestive function, reduced the inflammation and enhanced the immunity in the intestine of pigs during the post-weaning period. These results provided a relevant insight into the supplementation of EA to enhance the growth and intestinal health in the weaned pigs.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- AOAC. 2007. Official methods of analysis. 18th ed. Rockville (MD): AOAC International.
- Brenes A, Roura E. 2010. Essential oils in poultry nutrition: Main effects and modes of action. Anim Feed Sci Technol. 158:1–14.
- Brisibe EA, Umoren UE, Brisibe F, Magalhäes PM, Ferreira JFS, Luthria D, Wu X, Prior RL. 2009. Nutritional characterisation and antioxidant capacity of different tissues of Artemisia annua L. Food Chem. 115:1240–1246.
- Brisibe EA, Umoren UE, Owai PU, Brisibe F. 2008. Dietary inclusion of dried Artemisia annua leaves for management of coccidiosis and growth enhancement in chickens. Afr J Biotechnol. 7:4083–4092.
- Brown GD. 2010. The biosynthesis of artemisinin (Qinghaosu) and the phytochemistry of Artemisia annua L. (Qinghao). Molecules. 15:7603–7698.
- Carroll JA, Veum TL, Matteri RL. 1998. Endocrine responses to weaning and changes in post-weaning diet in the young pig. Domest Anim Endocrinol. 15:183–194.
- Das S. 2012. Artemisia annua (Qinghao): a pharmacological review. Int J Pharm Sci Res. 3:4573–4577.
- Fenton TW, Fenton M. 1979. An improved procedure for the determination of chromic oxide in feed and feces. Can J Anim Sci. 59:631–634.
- Gal-Garber O, Mabjeesh S, Sklan D, Uni Z. 2003. Nutrient transport in the small intestine: Na+,K+-ATPase expression and activity in the small intestine of the chicken as influenced by dietary sodium . Poult Sci. 82:1127–1133.
- Heim G, Walsh AM, Sweeney T, Doyle DN, O'Shea CJ, Ryan MT, O'Doherty JV. 2014. Effect of seaweed-derived laminarin and fucoidan and zinc oxide on gut morphology, nutrient transporters, nutrient digestibility, growth performance and selected microbial populations in weaned pigs. Br J Nutr. 111:1577–1585.
- Huang M, Li Z, Huang X, Gao W, Zhu C, Xu H, Yuan Y, Shuai L, Chen R, Wu Z. 2013. Co-expression of two fibrolytic enzyme genes in CHO cells and transgenic mice. Transgenic Res. 22:779–790.
- Juteau F, Masotti V, Bessiere JM, Dherbomez M, Viano J. 2002. Antibacterial and antioxidant activities of Artemisia annua essential oil. Fitoterapia. 73:532–535.
- Lee S, Kim KS, Jang JM, Park Y, Kim YB, Kim BK. 2002. Phytochemical constituents from the herba of Artemisia apiacea. Arch Pharm Res. 25:285–288.
- Li T, Chen H, Wei N, Mei X, Zhang S, Liu DL, Gao Y, Bai SF, Liu XG, Zhou YX. 2012. Anti-inflammatory and immunomodulatory mechanisms of artemisinin on contact hypersensitivity. Int Immunopharmacol. 12:144–150.
- Madsen KL, Lewis SA, Tavernini MM, Hibbard J, Fedorak RN. 1997. Interleukin 10 prevents cytokine-induced disruption of T84 monolayer barrier integrity and limits chloride secretion. Gastroenterology. 113:151–159.
- Madsen KL, Malfair D, Gray D, Doyle JS, Jewell LD, Fedorak RN. 1999. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis. 5:262–270.
- Marion J, Petersen YM, Rome V, Thomas F, Sangild PT, Dividich JL, Huerou-Luron IL. 2005. Early weaning stimulates intestinal brush border enzyme activities in piglets, mainly at the posttranscriptional level. J Pediatr Gastroenterol Nutr. 41:401–410.
- McKay D, Baird A. 1999. Cytokine regulation of epithelial permeability and ion transport. Gut. 44:283–289.
- Mcorist S, Mellits KH. 2010. The important lifetime effects of intestinal gut health of pigs at weaning. Vet J. 184:253–254.
- Mendoza SM, Murugesan G, Hendel EG, Stelzhammer S, Helm ET, Gabler NB. 2018. Evaluation of a phytogenic blend on nursery pig growth and nutrient digestibility. J Anim Sci. 96:155–156.
- NRC. 2012. Nutrient requirements of swine. 11th rev. ed. Washington (DC): National Academic Press.
- Omogbenigun FO, Nyachoti CM, Slominski BA. 2004. Dietary supplementation with multienzyme preparations improves nutrient utilization and growth performance in weaned pigs. J Anim Sci. 82:1053–1061.
- Pluske JR, Hampson DJ, Williams IH. 1997. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest Prod Sci. 51:215–236.
- Puri M, Sharma D, Barrow CJ. 2012. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 30:37–44.
- Roubaty C, Portmann P. 1988. Relation between intestinal alkaline phosphatase activity and brush border membrane transport of inorganic phosphate, D-glucose, and D-glucose-6-phosphate. Pflugers Arch. 412:482–490.
- Ryu JC, Park SM, Hwangbo M, Byun SH, Ku SK, Kim YW, Kim SH, Jee SY, Cho ILJ. 2013. Methanol extract of Artemisia apiacea hance attenuates the expression of inflammatory mediators via NF-κ B Inactivation. Evid Based Complement Alternat Med. 2013:494681.
- Sangild PT, Tappenden KA, Malo C, Petersen YM, Elnif J, Bartholome AL, Buddington RK. 2006. Glucagon-like peptide 2 stimulates intestinal nutrient absorption in parenterally fed newborn pigs. J Pediatr Gastroenterol Nutr. 43:160–167.
- Song ZH, Cheng K, Zhang LL, Wang T. 2017. Dietary supplementation of enzymatically treated Artemisia annua could alleviate the intestinal inflammatory response in heat-stressed broilers. J Therm Biol. 69:184–190.
- Song ZH, Cheng K, Zheng XC, Ahmad H, Zhang LL, Wang T. 2018. Effects of dietary supplementation with enzymatically treated Artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult Sci. 97:430–437.
- Tang ZR, Yin YL, Zhang YM, Huang RL, Sun ZH, Li TJ, Chu WY, Kong XF, Li LL, Geng MM, et al. 2009. Effects of dietary supplementation with an expressed fusion peptide bovine lactoferricin-lactoferrampin on performance, immune function and intestinal mucosal morphology in piglets weaned at age 21 d. Br J Nutr. 101:998–1005.
- Tsukahara T, Kishino E, Inoue R, Nakanishi N, Nakayama K, Ito T, Ushida K. 2013. Correlation between villous height and the disaccharidase activity in the small intestine of piglets from nursing to growing. Anim Sci J. 84:54–59.
- Wan X, Jiang L, Zhong H, Lu Y, Zhang L, Wang T. 2017. Effects of enzymatically treated Artemisia annua L. on growth performance and some blood parameters of broilers exposed to heat stress. Anim Sci J. 88:1239–1246.
- Wan XL, Niu Y, Zheng XC, Huang Q, Su WP, Zhang JF, Zhang LL, Wang T. 2016a. Antioxidant capacities of Artemisia annua L. leaves and enzymatically treated Artemisia annua L. in vitro and in broilers. Anim Feed Sci Technol. 221:27–34.
- Wan XL, Song ZH, Niu Y, Cheng K, Zhang JF, Ahmad H, Zhang LL, Wang T. 2016b. Evaluation of enzymatically treated Artemisia annua L. on growth performance, meat quality, and oxidative stability of breast and thigh muscles in broilers. Poult Sci. 96:844–850.
- Wang JJ, Chen LX, Li DF, Yin YL, Wang XQ, Li P, Dangott LG, Hu WX, Wu GY. 2008. Intrauterine growth restriction affects the proteomes of the small intestine, liver, and skeletal muscle in newborn pigs. J Nutr. 138:60–66.
- Wang KS, Li J, Wang Z, Mi C, Ma J, Piao LX, Xu GH, Li X, Jin X. 2017. Artemisinin inhibits inflammatory response via regulating NF-κB and MAPK signaling pathways. Immunopharmacol Immunotoxicol. 39:28–36.
- Wang HC, Li SS, Xu SY, Feng J. 2020. Betaine improves growth performance by increasing digestive enzymes activities, and enhancing intestinal structure of weaned piglets. Anim Feed Sci Technol. 267:114545.
- Wetzstein HY, Porter JA, Janick J, Ferreira JFS. 2014. Flower morphology and floral sequence in Artemisia annua (Asteraceae)1. Am J Bot. 101:875–885.
- Wijtten PJA, Langhout DJ, Verstegen MWA. 2012. Small intestine development in chicks after hatch and in pigs around the time of weaning and its relation with nutrition: a review. Acta Agric Scand A AnimSci. 62:1–12.
- Williams P. 2001. The use of essential oils and their compounds in poultry nutrition. World Poultry. 17:14–15.
- Yusrizal , Chen TC. 2003. Effect of adding chicory fructans in feed on broiler growth performance, serum cholesterol and intestinal length. Int J Poult Sci. 2:214–219.
- Zhao XJ, Li L, Luo QL, Ye MQ, Luo GQ, Kuang ZS. 2015. Effects of mulberry (Morus alba L.) leaf polysaccharides on growth performance, diarrhea, blood parameters, and gut microbiota of early-weanling pigs. Livest Sci. 177:88–94.
- Zhou H, Wang CZ, Ye JZ, Chen HX, Tao R. 2015. Effects of dietary supplementation of fermented Ginkgo biloba L. residues on growth performance, nutrient digestibility, serum biochemical parameters and immune function in weaned piglets. Anim Sci J. 86:790–799.
