Abstract
The objective of the present experiment was to evaluate the effect of bioactive peptides derived from cottonseed (BPC) on chicken performance, immunity, the total antioxidant activity of serum and intestinal morphology. A total of 280 one-day-old male broiler chicks (Ross 308) were randomly allocated into 1 of the following 7 experimental treatments (5 replicates per treatment with 8 broilers per pen). Five diets were formulated to contain 0 (control), 3, 4, 5 and 6 g BPC/kg of diet in comparison with control + 50 U excessive dietary vitamin E and control + 2 mg lincomycin. At 40 d the BW tended to improve in broilers supplemented with an antibiotic, 3, 5 and 6 g BPC/kg groups (p > .05). In the whole trial, supplementing 5 g BPC/kg increased feed intake of broilers in comparison to other groups (p < .05). In the whole trial broilers fed diets supplemented with 6 g BPC/kg had a significantly better FCR value (p < .001). Supplementation of 3 g BPC/kg increased antibody titres against Newcastle disease virus and sheep red blood cell (p < .01). Dietary supplementation of vitamin E, antibiotic, 3, 4 and 5 g BPC/kg significantly (p < .001) increased total antioxidant activity of serum compared with those fed the basal diet. In conclusion, the results indicated that supplementation of 6 g BPC/kg in broiler diets could induce favourable influences on growth performance, immune responses and total antioxidant activity of serum and it could be used in broiler diets as an alternative to antibiotics.
Broilers fed diets supplemented with 6 g bioactive peptides (BPC)/kg had better FCR.
Supplementation of BPC increased humoral immune responses.
HIGHLIGHTS
Introduction
In-feed antibiotics (IFA) have been used in poultry feeds for many years widespread at sub-therapeutic doses for their affirmative influences on growth performance, and health status of the birds (Franti et al. Citation1971; Ghalamkari et al. Citation2012; Goodarzi et al. Citation2014; Yazdi et al. Citation2014a, Citation2014b; Foroutankhah et al. Citation2019). The IFA are purported to enhance the performance of the birds by decreasing the proliferation of pathogenic bacteria in the gut, resulting in greater digestion, absorption and metabolism of nutrients (Gheisari et al. Citation2017; Kheiri et al. Citation2018). Although including IFA in poultry diets resulted in some beneficial influences on poultry performance, the continuous utilisation resulted in increment of cross-resistance and multifold antibiotic resistance in pathogenic bacteria (Sørum and Sunde Citation2001), deposition of drug in poultry meat, and dysbacteriosis (Andremont Citation2000). Because of tremendous demand for appropriate IFA substitutions, probiotics (Landy and Kavyani Citation2014; Toghyani et al. Citation2015), prebiotics (Ceylan and Çiftçl Citation2003), essential oils and natural products (Landy et al. Citation2012) and bioactive peptides (Dhama et al. Citation2014) have received considerable attention.
Bioactive peptides are peptides that have biological functions apart from their nutritional value (Hou et al. Citation2017). Numerous studies indicated that bioactive peptides have therapeutic benefits, such as antimicrobial (Osman et al. Citation2016; Wald et al. Citation2016), antioxidant (Power et al. Citation2013; Hisham et al. Citation2018), antihypertensive (Zambrowicz et al. Citation2015; Ryder et al. Citation2016), and immunomodulatory (Kotzamanis et al. Citation2007) activities based on the amino acid profile and molecular weight (Hou et al. Citation2017). Abdollahi et al. (Citation2017) reported that the addition of soybean bioactive peptide (SBP) in broiler diets significantly enhanced production performance through improving feed conversion ratio (FCR) although they did not compare the results with a positive control group. The betterment in feed efficiency of broilers fed bioactive peptides has been ascribed to the improvement of small intestinal morphology, improvement of intestinal microflora, and enhanced digestive enzymes activity (Jin et al. Citation2008; Tang et al. Citation2008). The affirmative efficacy of bioactive peptides on the intestinal histology of broilers has been reported in several research trials (Liu et al. Citation2008; Bao et al. Citation2009; Wen and He Citation2012). Similarly, Abdollahi et al. (Citation2018) reported that dietary supplementation of SBP had the potential to improve FCR in broiler chickens as a result of improvement in small intestinal morphology. Osho et al. (Citation2019) reported that supplementation of SBP to broiler diet enhanced the relative weight of spleen as an indicator of immune organ development. Hisham et al. (Citation2018) observed the ability of bioactive peptides of casein and whey proteins of camel milk to significantly increase the tolerance of yeast cells against peroxide-induced oxidative stress, but they did not compare the potential of bioactive peptides with a positive control such as vitamin E. Despite the mentioned pharmaceutical benefits, there has been a dearth of information on the effect of bioactive peptides derived from cottonseed (BPC) on broiler growth performance, carcase characteristics, gut development, total antioxidant activity of serum and immune responses in comparison with an IFA and excessive dietary vitamin E.
Materials and methods
Ethics approval
This experiment was performed in Pishgam Damparvar Sepahan company farm which is located around the Isfahan city with an altitude of 1,680 m. All procedures including blood sampling and slaughtering the birds were performed in accordance of the ethical guidelines of the Animal Care and Welfare Committee of the Islamic Azad University, Shahrekord Branch, Iran (approval ref no. 2019-064).
Animals and dietary treatments
The aim of the current trial was to examine the effect of bioactive peptides derived from cottonseed (Fortide, Chengdu Mytech Biotech Co. Ltd., Chengdu, Sichuan, China) in comparison with an in-feed antibiotic and excessive dietary vitamin E on chicken growth performance, carcase traits, immunity, antioxidant capacity and intestinal morphology.
A total of 280 days-old male broiler chickens (Ross 308) were obtained from a commercial hatchery, individually weighed and divided into 35 Pens (120 × 120 × 80 cm3) of the 7 groups, each with 5 replicates pens (8 birds/replicate pen). Five dietary treatments were formulated to contain 0 (control), 3, 4, 5, and 6 g bioactive peptides derived from cottonseed (BPC)/kg of diet in comparison with control + 50 U excessive dietary vitamin E and control + 2 mg lincomycin. The BPC is a functional protein source made from cottonseed protein with an enzymatic hydrolysis process. The dietary treatments were formulated to meet the nutrient requirements of Ross 308 strain (Aviagen Citation2019) and were fed in mash form during the trial in 3 phases, 0 to 10 d (Table ), 11 to 24 d (Table ), and 25 to 40 d (Table ). The broilers were raised in an environmentally controlled room and feed and water were offered ad libitum throughout the whole trial. The room was enclosed and continuous lighting was provided by incandescent bulbs. The initial temperature of the broiler house was set at 33 °C and was reduced by 3 °C each week during the first, second, third, fourth and fifth weeks to finally be fixed at 21 °C.
Table 1. Ingredients and calculated content of dietary treatments in starter period.
Table 2. Ingredients and calculated content of dietary treatments in grower period.
Table 3. Ingredients and calculated content of dietary treatments in finisher period.
Analysis of bioactive peptides derived from cottonseed
Foregoing to preparing the formula, corn, soybean meal, and BPC were evaluated for the level of crude protein (Method 990.03; AOAC Citation2006), and the number of total amino acids (Methods 982.30E a, b, and c; AOAC Citation2006). Calcium and total P of BPC were measured by Inductively coupled plasma – optical emission spectrometry (Method 2011.14; AOAC Citation1965) at the Shahrekord university Laboratories (Table ). The molecular weight distribution of the BPC was measured by a Superdex peptide HR 10/30 column as described by Jung et al. (Citation2006).
Table 4. Analysis of the protein hydrolysates of cottonseed.
Performance and carcase components
The body weight (BW) was determined at days 1, 10, 24 and termination of the experiment, average daily weight gain (DWG) was calculated for starter, grower, finisher and the entire experimental period thereafter. Daily feed intake (DFI) was measured in different growth periods and corrected for dead birds. FCR was calculated as the DFI to DWG ratio.
At the termination of the experiment, 2 broilers were chosen based on the average BW of the pen. The broilers were individually weighed and slaughtered by cutting the jugular vein. Carcase yield was calculated using the eviscerated weight over live weight. Empty proventriculus, empty gizzard, empty small intestine, liver, heart, pancreas, spleen and bursa of Fabricius were removed from carcases, weighed and computed as a percentage of live weight.
Jejunal histology
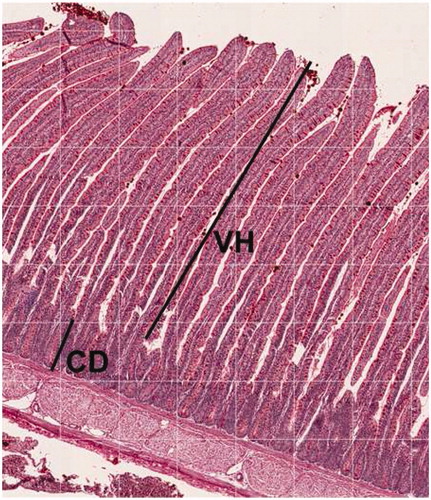
At the termination of the experiment, two birds per replicate, based on the average BW of the group, were chosen and killed, and their gastrointestinal tracts were removed from carcases thereafter. As described by Iji et al. (Citation2001) around 2 cm of the proximal part of jejunum were fixed in 10% neutral formalin, and dehydrated in a graded ethanol series, and before embedding in paraffin. Paraffin segment at 6 m width was tinged with haematoxylin and eosin, and examined by light microscopy (Olympus Co. Ltd., BX 50, F-3, Tokyo, Japan) to evaluate each preparation. The villus height (VH) was measured from the root to the upside of the villi, villi width (VW) was measured as the distance betwixt hands of the villi at the middle section of the villi and crypt depth (CD) was measured from the root of the villi to root of the crypt (Figure ). VH to CD ratio was calculated by dividing VH to CD.
FCR was calculated as the DFI to DWG ratio.
Immunity
Chicks were orally vaccinated with attenuated Newcastle disease virus (NDV) vaccine at 7 (B 1), 14 (B 1) and 21 (LaSota) d of age. At 25 d of age, 2 broilers per cage were inoculated by intravenous injection with 1 mL of 1% sheep red blood cells (SRBC) suspension. At day 6 post-SRBC, blood samples were collected to examine the ability of broilers to produce antibody against SRBC. Plasma SRBC antibody titres were measured by the microtiter method as described by Landy et al. (Citation2011). Antibody titres to SRBC antigen were expressed as the log2 values of the reciprocal of the highest dilution in which there was hemagglutination (Wegmann and Smithies Citation1966). Blood samples were drawn at day 7 post-vaccination (28 d) by venipuncture to measure antibody titres against NDV. Antibodies were detected using the hemagglutination inhibition test (HI), and HI antibody titres to NDV were then converted to log2 (Landy et al. Citation2011).
At the termination of the experiment, two birds per cage were chosen and blood samples were drawn from the brachial vein into heparinised syringes to avoid blood clot formation. Blood smears were stained by May–Greenwald–Giemsa stain (Lucas and Jamroz Citation1961). One hundred leukocytes, per sample including granular and nongranular cells, were computed under an optical microscope (Nikon, Tokyo, Japan), and the heterophil-to-lymphocyte ratio (H/L) also was computed (Gross and Siegel Citation1983). The percentage of haematocrit or packed cell volume (PCV) was measured by microhematocrit method (Keçeci et al. Citation1998). Also total white blood cell (WBC) counts were determined by brilliant cresyl blue dye (Haddad and Mashaly Citation1990).
At 40 d of age, two blood samples per pen were collected by puncture of wing vein and total protein concentration in serum was determined as described by Cannon et al. (Citation1974). The serum albumin concentration was determined as described by Doumas et al. (Citation1971). Globulin concentration in serum was estimated as the difference between albumin and total protein. Albumin to globulin ratios were also determined.
Total antioxidant activity of serum
At 40 d of age, two blood samples per pen were collected by puncture of wing vein and serum samples were separated. Total antioxidant capacity (T-AOC), were assayed in the serum samples by using BioAssay Systems Commercial kit (Re et al. Citation1999).
Statistical analysis
This study was conducted as a completely randomised design, and all collected data were analysed using the General Linear Model procedures of SAS (Citation2012). Means were compared using a post-hoc Tukey test at 5% significance.
Results
Performance and carcase traits
No mortalities occurred during the experiment. The effects of different inclusion rates of BPC in comparison with an in-feed antibiotic and excessive dietary vitamin E on chicken growth performance are summarised in Table . During the starter phase (1–10 d), broilers fed diets supplemented with the antibiotic had the highest BW compared with other groups (p < .01). During the starter phase supplementation of different levels of BPC in broiler’s diet could not significantly increase BW, though it tended to improve in broilers fed diets supplemented with 3, 5 and 6 g BPC/kg. In the grower phase (11–24 d), supplementation of antibiotics and 5 g BPC/kg significantly increased BW compared with other groups (p < .001). During the finisher phase (25–40 d), the BW tended to improve in broilers supplemented with an antibiotic, 3, 5 and 6 g BPC/kg, but the differences compared with control were not statistically significant. During the starter period, DFI of broilers did not differ between the treatment groups (p > .05). During the grower period the highest DFI obtained in the groups fed diets containing antibiotics or 5 g BPC/kg in comparison with those fed the basal diet, the basal diet supplemented with an excessive level of vitamin E, and those fed 4 and 6 g BPC/kg, but did not differ from those fed diets containing 3 g BPC/kg (p < .001). In the finisher period, the highest DFI obtained in the groups fed diets containing the excessive level of vitamin E and 5 g BPC/kg compared with those fed 4 g BPC/kg, but did not significantly differ from other groups (p < .01). In the whole experiment, the highest DFI obtained in the groups fed diets containing 5 g BPC/kg compared with those fed 4 g BPC/kg, but did not significantly differ from other groups (p < .05). During the starter period, FCR of broiler did not significantly (p > .05) differ between the treatment groups, though it tended to improve in broilers fed antibiotic or 3 g BPC/kg. In the grower phase broilers fed diets containing 5 g BPC/kg had significantly (p < .01) better FCR in comparison with those fed diets containing an excessive level of vitamin E, but did not significantly differ from other groups. In the finisher phase, the best FCR obtained in broilers fed diets containing 6 g BPC/kg (p < .01). In the whole trial broilers fed diets supplemented with 6 g BPC/kg had significantly (p < .001) better FCR value in comparison with those fed dietary excessive level of vitamin E or 4 g BPC/kg, but did not differ from other groups.
Table 5. Influence of dietary treatments on performance indices of broiler chickens at different ages.
Table illustrated carcase yield and relative weights of organs as a percentage of live BW at slaughter. Carcase yield and relative weight of proventriculus, liver, pancreas, small intestinal, heart, and spleen were not significantly affected by the dietary treatments. The highest percentage of gizzard obtained in the group supplemented with excessive level of vitamin E in comparison with those fed 5 g BPC/kg, but did not differ from other groups (p < .05). The highest percentage of the bursa of fabricius obtained in the group supplemented with 4 g BPC/kg (p < .05).
Table 6. Influence of dietary treatments on carcase yield and internal relative organ weight of broilers at 40 d of age.
Morphometric analysis of the jejunum
The effects of dietary treatments on VH, CD, VW, VH to CD ratio and epithelial thickness of jejunum are summarised in Table . Supplementation of an antibiotic to the basal diet significantly (p < .01) increased VH compared with those fed diets containing 4 or 6 g BPC/kg but did not significantly differ from other groups. Dietary treatments failed to induce any marked effects on CD, although it tended to enhance in broilers fed 4 g BPC/kg (p > .05). Treatments failed to induce any significant (p > .05) effects on VH to CD ratio, though it tended to improve in broilers fed diets containing antibiotics and 5 g BPC/kg. Supplementation of 6 g BPC/kg significantly (p < .001) increase epithelial cell thickness compared with those fed the basal diet, the basal diet supplemented with 4 and 5 g BPC/kg.
Table 7. Influence of dietary treatments on villus height (VH), villus width, crypt depth (CD), VH/CD ratio and epithelial thickness in jejunum of broiler chickens at 40 d of age.
Immune responses and haematology
The effect of experimental treatments on antibody titres against NDV, and SRBC and H/L and albumin to globulin ratios are presented in Table . Supplementation of vitamin E, antibiotic and 3 g BPC/kg significantly (p < .001) increased albumin to globulin ratios in comparison with those fed the basal diet, but did not significantly differ from other groups. Treatments failed to induce any effect on H/L ratio (p > .05). Supplementation of 3 and 6 g BPC/kg significantly increased antibody titres against NDV (p < .01). The addition of 3 and 4 g BPC/kg significantly (p < .05) increased antibody titres against SRBC compared with those fed basal diets but did not differ from other groups.
Table 8. Influence of dietary treatments on humoral immune responses and serum antioxidant status of broiler chickens.
The hematological data are shown in Table . Treatments failed to have any significant effect on hematological parameters of broiler chickens. The means of monocytes and eosinophils values tended to increase in broilers fed diets containing 3 and 4 g BPC/kg (p > .05).
Table 9. Influence of dietary treatments on hematological responses of broiler chickens.
Total antioxidant capacity of serum
As shown in Table , the serum antioxidant status of broilers was affected by the dietary treatments. Dietary supplementation of vitamin E, antibiotic and 3, 4, and 5 g BPC/kg significantly (p < .001) increased T-AOC of serum compared with the control group but did not differ from those fed 6 g BPC/kg.
Discussion
Performance and carcase characteristics
In the current experiment, the BW obtained in different growth periods was lower than the breed standard. This experiment was performed in Pishgam Damparvar Sepahan company research farm with an altitude of 1,590 m. Julian (Citation2007) mentioned that the pressure of oxygen drops nearly 2.5% per 1,000 m increase in altitude. Beker et al. (Citation2003), reported that broiler chickens raised under low pressure of oxygen had low final BW as a result of low DFI. Besides rearing in high altitude, feeding diet in mash form was another reason for the results. In the current trial, supplementing 5 g BPC/kg increased the daily feed intake of broilers in comparison with other groups during the entire experimental period. Abdollahi et al. (Citation2017) investigated the effects of 1.0, 2.0, 3.0, 4.0, 5.0 and 6.0 g of a commercial soybean bioactive peptide (SBP) product/kg of feed (Fortide, Chengdu Mytech Biotech Co. Ltd., Chengdu, Sichuan, China) on the performance of broiler chickens. The results indicated that the inclusion of different levels of SBP had not any significant effects on DFI of broilers. Similarly, Abdollahi et al. (Citation2018) reported that the addition of different levels of SBP in broiler diets had no significant effects on DFI. The differences between our findings in DFI and obtained results by Abdollahi et al. (Citation2017, Citation2018) may be due to the form of feed. As previously mentioned we provided the feed in mash form whereas Abdollahi et al. (Citation2018) provided it in pelleted form. As reported by Nagodawithana et al. (Citation2010) the use of enzymes to hydrolysis vegetable proteins is a new technology to promote the sensory characteristics of vegetable proteins. So enhanced DFI obtained in the current trial may be resulted from sensory flavours provided by supplementation of BPC in the feed. In the present study, final BW of broilers tended to improve in broilers supplemented with antibiotics, 3, 5 and 6 g BPC/kg. In contrast with our results, Abdollahi et al. (Citation2017, Citation2018) reported that supplementation of different levels of SBP in broiler diet had not any significant effects on the final BW of broilers. In agreement with our results, Osho et al. (Citation2019) reported that by supplementing SBP in broiler diets final BW and BW gain of broilers was enhanced at 15 and 22 d post-hatching. Similarly, Wang (Citation2005) reported an improvement in final BW, and BW gain of broilers fed diets containing SBP at day 21 post hatch. In the current trial, the enhanced final BW of broilers could be explained by increased DFI in broilers fed diets containing 5 g BPC/kg and an improvement in VH to CD ratio as indicated in Table , though differences between the control group and broilers fed diets containing 5 g BPC/kg were not statistically significant. Similarly, Osho et al. (Citation2019) reported that VH to CD ratios of broilers in the jejunum and ileum were enhanced by increasing the inclusion rate of SBP in the diet. Abdollahi et al. (Citation2017) also reported an improvement in VH and CD of the duodenum by supplementing SBP in broilers’ diet. As reported by Caspary (Citation1992) an enhancement in VH occurs in parallel with an enhancement in the digestive, absorptive functions, and expression of brush border enzymes. So observed improvement in FCR of broilers fed diets containing 6 g BPC/kg (during finisher phase) may be due to heightened intestinal enzyme activities due to bioactive peptide supplementation because none of the morphological related parameters were not improved in the treatment. As reported by Feng et al. (Citation2007), supplementation of fermented soybean meal in broiler diets could increase activation of intestinal trypsin, lipase and protease enzymes. Additional investigations are needed to identify the effects of BPC on intestinal enzyme activities which can influence digestion and absorption of nutrients.
In the current study, the addition of lincomycine in broilers diet tended to improve the final BW and VH to CD ratio, though the results were not statistically significant. Similarly, Kavyani et al. (Citation2012) reported that supplementation of 4.5 mg flavophospholipol/kg of diet could improve the FCR of broilers. As Bedford (Citation2000) observed, antibiotics could control and restrict the formation of Bacterial colonies in the chicks’ gut. This may lead to better efficiency in the usage of feed, resulting in greater growth and feed yield.
In the present study, there was no significant (p > .05) effect of BPC on the carcase yield, the relative weight of the heart and digestive organs. Similarly, Abdollahi et al. (Citation2017) reported that the addition of different levels of SBP in broiler diets had not any marked effects on carcase traits. In the current trial addition of an excessive level of vitamin E in broiler diets increased the relative weight. Similar to our results, Mazur-Kuśnirek et al. (Citation2019) reported higher weight of gizzard and pH of gizzard digesta in broilers fed diets supplemented with vitamin E.
Morphometric analysis of the jejunum
In the present study, VH and VH to CD ratio tended to increase in comparison to the control group. Similarly, Abdollahi et al. (Citation2017) reported that VH in broilers fed diets containing 3 or 6 g SBP/kg tended to increase, though treatments failed to induce any marked effects on CD, epithelial thickness, and goblet cell number in the duodenum. Osho et al. (Citation2019) reported that VH to CD ratio in the jejunum and VH in the ileum were enhanced by the addition of SBP in broilers’ diet. In several trials, affirmative effects of bioactive peptides on small intestinal morphology of broiler has been documented (Liu et al. Citation2008; Bao et al. Citation2009; Wen and He Citation2012). As VH to CD ratio is a useful index for estimating the digestive capacity of the small intestine, thus this may be an explanation for higher final BW obtained in the groups supplemented with antibiotics and BPC in the present trial.
Immune responses and haematology
In the present study addition of 4 g BPC/kg significantly enhanced the relative weight of bursa of Fabricius. Abdollahi et al. (Citation2017) reported that the relative weight of lymphoid organs including the spleen and bursa of Fabricius tended to increase in broilers fed diets containing SBP. In another trial relative spleen weight was enhanced with the addition of SBP in broiler diets (Osho et al. Citation2019). The bursa of Fabricius is a primal lymphoid organ in broilers which is responsible for the augmentation and dissociation of B lymphoid; thus its development directly impacts the immune function. It can be concluded that in the present trial higher antibody titres against SRBC and NDV are related to more development of bursa of Fabricius as a lymphoid organ. Cheng et al. (Citation2017) investigated the effects of vitamin E supplementation on T-AOC of serum in cyclophosphamide (CY) immunosuppressed broilers; the results indicated that vitamin E increased T-AOC of serum, and alleviated the immune damage of the bursa of Fabricius. So in the current trial may immune responses were increased by an increment in T-AOC of serum. Osho et al. (Citation2019) investigated the effects of using SBP in poultry nutrition on immune-related gene expression during coccidia challenge; the results indicated that SBP has the potential for the amelioration of coccidia infection. The basic mechanism of immunomodulatory effects of bioactive peptides can be explained by increasing the production of TNF-α, IL-8, IL-10, and IL-6 as a result of stimulating Toll-like receptors (Osho et al. Citation2019).
In the current study, treatment failed to induce any effects on hematological parameters. According to Whitehair and Thompson (Citation1956) the attendance of a serious health challenge is necessary to disclose the efficiency of a feed additive on blood haematology, while the present trial was carried out in optimised status.
Total antioxidant capacity of serum
In the present study, the highest T-AOC of serum obtained in the group which received an excessive level of vitamin E. Similarly, Cheng et al. (Citation2017) reported that inclusion of excessive level of vitamin E enhanced T-AOC level, enzymatic and non-enzymatic antioxidants compared with the CY immunosuppressed broilers. T-AOC consists of a numeral of antioxidant enzymes and the associated biomolecules intricately in scavenging free radicals (Ren et al. Citation2012). In the current trial supplementation of 3, 4, and 5 g BPC/kg significantly increased T-AOC of serum. According to Jang et al. (Citation2008) report, bioactive peptides have the potential to donate hydrogen from amino acids to break the oxidation chain reaction. Similarly, Girgih et al. (Citation2015) reported that antioxidant peptides with a high content of aromatic amino acids such as Tyr, Trp, and Phe have the potential to donate electrons. Hisham et al. (Citation2018) investigated caseins and whey protein of camel milk for their potential as nutraceuticals or therapeutic peptides for the prevention and treatment of oxidative stress. The results indicated that both protein sources containing bioactive peptides with radical-scavenging activities. Antioxidant peptides which have hydroxyl radical scavenging capacity are known with high contents of the hydrophobic amino acid residues (AA) such as His, Cys and Met (Davalos et al. Citation2004). As indicated in Table protein hydrolysate derived from cottonseed contain a considerable amount of hydrophobic AA. Besides as reported by Ruiz-Ruiz et al. (Citation2013) and Wattanasiritham et al. (Citation2016) low molecular weight peptides (<10 kDa) can be more efficient antioxidant peptides in comparison to high molecular weight peptides, as indicated in Table the bioactive peptides which we supplemented to the basal diet contain at least 18 Peptides with molecular weight <1,000 Da.
Conclusions
In conclusion, the results indicated that supplementation of 6 g BPC/kg in broiler diets could induce favourable influences on growth performance, immune responses and total antioxidant activity of serum and it can be used in broiler diets as a replacement for IFA.
Ethical approval
The birds were raised in accordance with the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals. Besides, the sampling procedures complied with the ethical guidelines of the Shahrekord University’s Ethical Committee, Islamic Azad University, Shahrekord branch, Iran (approval ref no. 2018-005).
Acknowledgements
The authors thank Pishgam Damparvar Sepahan Co. Ltd. for supplying broiler house, feed and skilled workers.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abdollahi MR, Zaefarian F, Gu Y, Xiao W, Jia J, Ravindran V. 2017. Influence of soybean bioactive peptides on growth performance, nutrient utilisation, digestive tract development and intestinal histology in broilers. J Appl Anim Nutr. 5:1–7.
- Abdollahi MR, Zaefarian F, Gu Y, Xiao W, Jia J, Ravindran V. 2018. Influence of soybean bioactive peptides on performance, foot pad lesions and carcass characteristics in broilers. J Appl Anim Nutr. 0:1–7.
- Andremont A. 2000. Consequences of antibiotic therapy to the intestinal ecosystem. Ann Fr Anesth Reanim. 19(5):395–402.
- AOAC. 1965. Official methods of analysis. 10th ed. Washington (DC): AOAC.
- AOAC. 2006. Official methods of analysis. 18th ed. Washington (DC): AOAC.
- Aviagen. 2019. Ross Broiler Management Manual. Midlothian (UK): Aviagen Ltd.
- Bao H, She R, Liu T, Zhang Y, Peng KS, Luo D, Yue Z, Ding Y, Hu Y, Liu W, et al. 2009. Effects of pig antibacterial peptides on growth performance and intestine mucosal immune of broiler chickens. Poult Sci. 88(2):291–297.
- Bedford M. 2000. Removal of antibiotic growth promoters from poultry diets: implications and strategies to minimize subsequent problems. Worlds Poult Sci J. 56(4):347–365.
- Beker A, Vanhooser SL, Swartzlander JH, Teeter RG. 2003. Graded atmospheric oxygen level effects on performance and ascites incidence in broilers. Poult Sci. 82(10):1550–1553.
- Cannon DC, Olitzky I, Inkpen JA, Henry RJ, Winkelman JE. 1974. Protein Cli Chem. In: Cannon DC, Winkelman JE, editors. Clinical chemistry principles and techniques. 2th ed. Harper and Row, Hagerstown, Md; p. 407–421.
- Caspary WF. 1992. Physiology and pathophysiology of intestinal absorption. Am J Clin Nutr. 55(1):299S–308S.
- Ceylan N, Çiftçl I. 2003. The Effects of some alternative feed additives for antibiotic growth promoters on the performance and gut microflora of broiler chicks. Turk J Vet Anim Sci. 27:727–733.
- Cheng K, Song ZH, Zheng XC, Zhang H, Zhang JF, Zhang LL, Zhou YM, Wang T. 2017. Effects of dietary vitamin E type on the growth performance and antioxidant capacity in cyclophosphamide immunosuppressed broilers. Poult Sci. 96(5):1159–1166.
- Davalos A, Miguel M, Bartolome B, Lopez-Fandino R. 2004. Antioxidant activity of peptides derived from egg white proteins by enzymatic hydrolysis. J Food Prot. 67(9):1939–1944.
- Dhama K, Tiwari R, Khan RU, Chakrabort S, Gopi M, Karthik K, Saminathan M, Desingu PA, Sunkara LT. 2014. Growth promoters and novel feed additives improving poultry production and health, bioactive principles and beneficial applications. Int J Pharmacol. 10(3):129–159.
- Doumas BT, Watson WA, Bigg HG. 1971. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta. 31(1):87–96.
- Franti CE, Adler HE, Julian LM. 1971. Antibiotic growth promotion: effects of bacitracin and oxytetracycline on intestines and selected lymphoid tissues of New Hampshire cockerels. Poult Sci. 50(1):94–99.
- Feng J, Liu X, Xu ZR, Wang YZ, Liu JX. 2007. Effects of fermented soybean meal on digestive enzyme activities and intestinal morphology in broilers. Poult Sci. 86(6):1149–1154.
- Foroutankhah M, Toghyani M, Landy N. 2019. Evaluation of Calendula officinalis L. (marigold) flower as a natural growth promoter in comparison with an antibiotic growth promoter on growth performance, carcass traits and humoral immune responses of broilers. Anim Nutr. 5(3):314–318.
- Ghalamkari GH, Toghyani M, Landy N, Tavalaeian E. 2012. Investigation the effects using different levels of Mentha pulegium L. (pennyroyal) in comparison with an antibiotic growth promoter on performance, carcass traits and immune responses in broiler chickens. Asian Pac J Trop Biomed. 2(3):S1396–S1399.
- Gheisari A, Shahrvand S, Landy N. 2017. Effect of ethanolic extract of propolis as an alternative to antibiotics as a growth promoter on broiler performance, serum biochemistry, and immune responses. Vet World. 10(2):249–254.
- Girgih AT, He R, Hasan FM, Udenigwe CC, Gill TA, Aluko RE. 2015. Evaluation of the in vitro antioxidant properties of a cod (Gadus morhua) protein hydrolysate and peptide fractions. Food Chem. 173:652–659.
- Goodarzi M, Nanekarani SH, Landy N. 2014. Effect of dietary supplementation with onion (Allium cepa L.) on performance, carcass traits and intestinal microflora composition in broiler chickens. Asian Pac J Trop Dis. 4:S297–S301.
- Gross WB, Siegel PS. 1983. Evaluation of heterophil to lymphocyte ratio as a measure of stress in chickens. Avian Dis. 27(4):972–979.
- Haddad EE, Mashaly MM. 1990. Effect of thyrotropin-releasing hormone, triiodothyronine, and chicken growth hormone on plasma concentrations of thyroxine, triiodothyronine, growth hormone, and growth of lymphoid organs and leukocyte populations in immature male chickens. Poult Sci. 69(7):1094–1102.
- Hisham RI, Isono H, Miyata T. 2018. Potential antioxidant bioactive peptides from camel milk proteins. Anim Nutr. 4(3):273–280.
- Hou Y, Wu ZH, Dai ZH, Wang G, Wu G. 2017. Protein hydrolysates in animal nutrition: industrial production, bioactive peptides, and functional significance. J Anim Sci Biotech. 8:24.
- Iji PA, Hughes RJ, Choct M, Tivey DR. 2001. Intestinal structure and function of broiler chickens on wheat-based diets supplemented with a microbial enzyme. Asian Australas J Anim Sci. 14(1):54–60.
- Jang A, Liu XD, Shin MH, Lee BD, Lee SK, Lee JH, Jo C. 2008. Antioxidative potential of raw breast meat from broiler chicks fed a dietary medicinal herb extract mix. Poult Sci. 87(11):2382–2389.
- Jin Z, Yang YX, Choi JY, Shinde PL, Yoon SY, Hahn TW, Lim HT, Park Y, Hahm KS, Joo JW, et al. 2008. Potato (Solanum tuberosum L. cv. Golden valley) protein as a novel antimicrobial agent in weanling pigs. J Anim Sci. 86(7):1562–1572.
- Julian RJ. 2007. The response of heart and pulmonary arteries to hypoxia, pressure and volume: a short review. Poult Sci. 86(5):1006–1011.
- Jung WK, Mendis E, Je JY, Park PJ, Son BW, Kim HC, Choi YK, Kim SK. 2006. Angiotensin I-converting enzyme inhibitory peptide from yellowfin sole (Limanda aspera) frame protein and its antihypertensive effect in spontaneously hypertensive rats. Food Chem. 94(1):26–32.
- Kavyani AZ, Shahne A, PorReza J, Jalali Haji-Abadi SMA, Landy N. 2012. Evaluation of dried powder of mushroom (Agaricus bisporus) as an antibiotic growth promoter substitution on performance, carcass traits and humoral immune responses in broiler chickens. J Med Plants Res. 6:94–100.
- Keçeci T, Oğuz H, Kurtoğlu V, Demet O. 1998. Effects of polyvinylpolypyrrolidone, synthetic zeolite and bentonite on serum biochemical and haematological characters of broiler chickens during aflatoxicosis. Br Poult Sci. 39(3):452–458.
- Kheiri F, Faghani M, Landy N. 2018. Evaluation of thyme and ajwain as antibiotic growth promoter substitutions on growth performance, carcass characteristics and serum biochemistry in Japanese quails (Coturnix japonica). Anim Nutr. 4(1):79–83.
- Kotzamanis YP, Gisbert E, Gatesoupe FJ, Zambonino Infante J, Cahu C. 2007. Effects of different dietary levels of fish protein hydrolysates on growth, digestive enzymes, gut microbiota, and resistance to Vibrio anguillarum in European sea bass (Dicentrarchus labrax) larvae. Comp Biochem Physiol A Mol Integr Physiol. 147(1):205–214.
- Landy N, Ghalamkari GH, Toghyani M. 2011. Performance, carcass characteristics, and immunity in broiler chickens fed dietary neem (Azadirachta indica) as alternative for an antibiotic growth promoter. Livest Sci. 142(1–3):305–309.
- Landy N, Ghalamkari GH, Toghyani M. 2012. Evaluation of St John’s Wort (Hypericum perforatum L.) as an antibiotic growth promoter substitution on performance, carcass characteristics, some of the immune responses, and serum biochemical parameters of broiler chicks. J Med Plants Res. 6:510–515.
- Landy N, Ghalamkari GH, Toghyani M, Moattar F. 2011. The effects of Echinacea purpurea L. (purple coneflower) as an antibiotic growth promoter substitution on performance, carcass characteristics and humoral immune response in broiler chickens. J Med Plants Res. 5:2332–2338.
- Landy N, Kavyani A. 2014. Effect of using multi-strain probiotic on performance, immune responses, and cecal microflora composition in broiler chickens reared under heat stress condition. Iran J Appl Anim Sci. 3:703–708.
- Liu T, She R, Wang K, Bao H, Zhang Y, Luo D, Hu Y, Ding Y, Wang D, Peng K. 2008. Effect of rabbit sacculus rotundus antimicrobial peptides on the intestinal mucosal immunity in chicken. Poult Sci. 87(2):250–254.
- Lucas AM, Jamroz C. 1961. Atlas of avian hematology. Washington (DC): USDA.
- Mazur-Kuśnirek M, Antoszkiewicz Z, Lipiński K, Kaliniewicz J, Kotlarczyk S. 2019. The effect of polyphenols and vitamin E on the antioxidant status and meat quality of broiler chickens fed low-quality oil. Arch Anim Breed. 62(1):287–296.
- Nagodawithana TW, Nelles L, Trivedi NB. 2010. Protein hydrolysates as hypoallergenic, flavors and palatants for companion animals. In: Pasupuleki VK, Demain AL, editors. Protein hydrolysates in biotechnology. New York (NY): Springer Science; p. 191–207.
- Osho SO, Xiao WW, Adeola O. 2019. Response of broiler chickens to dietary soybean bioactive peptide and coccidia challenge. Poult Sci. 0:1–10.
- Osman A, Goda HA, Abdel-Hamid M, Badran SM, Otte J. 2016. Antibacterial peptides generated by Alcalse hydrolysis of goat whey. LWT-Food Sci Technol. 65:480–486.
- Power O, Jakeman P, FitzGerald RJ. 2013. Antioxidative peptides: enzymatic production, in vitro and in vivo antioxidant activity and potential applications of milk-derived antioxidative peptides. Amino Acids. 44(3):797–820.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 26(9–10):1231–1237.
- Ren WK, Yin YL, Liu G, Yu XL, Li YH, Yang G, Li TJ, Wu GY. 2012. Effect of dietary arginine supplementation on reproductive performance of mice with porcine circovirus type 2 infection. Amino Acids. 42(6):2089–2094.
- Ruiz-Ruiz J, Dávila-Ortíz G, Chel-Guerrero L, Betancur-Ancona D. 2013. Angiotensin I-converting enzyme inhibitory and antioxidant peptide fractions from hard-to-cook bean enzymatic hydrolysates. J Food Biochem. 37(1):26–35.
- Ryder K, Bekhit AE-D, McConnell M, Carne A. 2016. Towards generation of bioactive peptides from meat industry waste proteins: generation of peptides using commercial microbial proteases. Food Chem. 208:42–50.
- SAS. 2012. SAS for Windows. Cary (NC): SAS Institute Inc.
- Sørum H, Sunde M. 2001. Resistence to antibiotics in the normal flora of animals. Vet Res. 32(3–4):227–241.
- Tang Z, Yin Y, Zhang Y, Huang R, Sun Z, Li T, Chu W, Kong X, Li L, Geng M, et al. 2008. Effects of dietary supplementation with an expressed fusion peptide bovine lactoferrampin on performance, immune function and intestinal mucosal morphology in piglets weaned at age 21 d. Br J Nutr. 101(7):998–1005.
- Toghyani M, Mosavi SK, Modaresi M, Landy N. 2015. Evaluation of kefir as a potential probiotic on growth performance, serum biochemistry and immune responses in broiler chicks. Anim Nutr. 1(4):305–309.
- Wald M, Schwarz K, Rehbein H, Bußmann B, Beermann C. 2016. Detection of antibacterial activity of an enzymatic hydrolysate generated by processing rainbow trout by-products with trout pepsin. Food Chem. 205:221–228.
- Wang FQ. 2005. Effects of bioactive peptide as feed additive on the performance, immune function and protein metabolism rate in broiler chicken [Dissertation]. Beijing (China): China Agricultural University.
- Wattanasiritham L, Theerakulkait C, Wickramasekara S, Maier CS, Stevens JF. 2016. Isolation and identification of antioxidant peptides from enzymatically hydrolyzed rice bran protein. Food Chem. 192:156–162.
- Wegmann TG, Smithies O. 1966. A simple hemagglutination system requiring small amounts of red blood cells and antibodies. Transfusion. 6(1):67–73.
- Wen LF, He JG. 2012. Dose–response effects of an antimicrobial peptide, a cecropin hybrid, on growth performance, nutrient utilisation, bacterial counts in the digesta and intestinal morphology in broilers. Br Poult Sci. 108:1756–1763.
- Whitehair CK, Thompson CM. 1956. Observations in raising “disease-free swine”. J Am Vet Med Assoc. 128(2):94–98.
- Yazdi FF, Ghalamkari GH, Toghiani M, Modaresi M, Landy N. 2014a. Anise seed (Pimpinella anisum L.) as an alternative to antibiotic growth promoters on performance, carcass traits and immune responses in broiler chicks. Asian Pac J Trop Dis. 4(6):447–451.
- Yazdi FF, Ghalamkari GH, Toghiani M, Modaresi M, Landy N. 2014b. Efficiency of Tribulus terrestris L. as an antibiotic growth promoter substitute on performance and immuneresponses in broiler chicks. Asian Pac J Trop Dis. 4(2):S1014–S1018.
- Zambrowicz A, Pokora M, Setner B, Dąbrowska A, Szołtysik M, Babij K, Szewczuk Z, Trziszka T, Lubec G, Chrzanowska J. 2015. Multifunctional peptides derived from an egg yolk protein hydrolysate: isolation and characterization. Amino Acids. 47(2):369–380.