Abstract
The aim of this trial was to investigate the effects of a blend of monoglycerides of short- and medium-chain fatty acids (SMCFA) on health status and growth of preweaned male calves from 16 to 72 days of age. The in vivo study was conducted as a complete randomised block design. Twenty Italian Holstein calves received starter administered ad libitum and 6 L of milk replacer/day (12% powder concentration) and were divided into treated (T) and control (C) groups. A preliminary in vitro antibiotic activity test of SMCFA was performed on several bacterial strains showing a minimum inhibiting concentration ranging from 0.8 to 12.5%. The group T received SMCFA (11 g/day) within the milk replacer while, to obtain isoenergetic liquid diets, the group C received additional 18 g/day of milk replacer powder. Body weight and morphological measurements of the calves were performed weekly. Health scores were checked daily. Faecal and blood samples were collected every two weeks for routine clinical investigations including serum concentration of non-esterified fatty acids and β-Hydroxybutyric acid. At the end of the trial calves were slaughtered and gastrointestinal tract (GIT) organs weighed. The group T reported a better health status and did not require Ab therapy. No differences in biometrical measurements, feed efficiency, blood parameters, and GIT measures were found between the two groups. At 44 days of age, group T showed higher β-Hydroxybutyric acid concentration compared to group C (p < .05). The SMCFA improved the calf health status avoiding the use of antimicrobics.
The monoglycerides of short and medium chain fatty acids improve calf health status.
The monoglycerides of short and medium chain fatty acids reduce the need for antibiotic treatment.
The monoglycerides of short and medium chain fatty acids reduce the negative impact of enteropathogens.
HIGHLIGHTS
Keywords:
Introduction
Neonatal calf diarrhoea is a worldwide disease, representing the first cause of death and losses of herd productivity and gain (NAHMS, Citation2014). Along with respiratory diseases, scours affects future performance, mainly due to heifers continuing to perform poorly as adults (Cho and Yoon Citation2014). The cause of neonatal diarrhoea is related to the pathogenic action of viruses, bacteria (Salmonella enterica, Escherichia coli, Clostridium perfringens), and protozoa; increasing the morbidity by the failure of immune passive transfer (FPT) as a non-infective factor (Cho and Yoon Citation2014; Marseglia et al. Citation2020).
Due to the high costs and animal health impacts of diarrhoea, many efforts have been made to investigate different managerial and therapeutical strategies to improve pre-weaned calf health status. In particular, many approaches based on the prevention or treatment of the interaction between enteropathogens and the gastro-intestinal tract (GIT) have been studied. Among these, the use of prebiotics (e.g., fructooligosaccharides, mannanoologosaccharides, lactulose, and inulin), probiotics (e.g., Lactobacillus spp, bifidobacteria, Enterococcus faecium, and Bacillus spp.), multifunctional proteins (e.g., lactoferrin), protein from hyper-immunized egg or spry-dried plasma as well as the transfusion of bovine plasma have been employed with variable results (Agazzi et al. Citation2014; Bresciani et al. Citation2016; Simoni et al. Citation2020). Moreover, the essential oils can be used as an antibiotic (Ab) alternative without reducing performance or increasing mortality of dairy calves (Soltan Citation2009). Grandi et al. (Citation2016) demonstrated that certain essential oils have the potential activity against unicellular parasites such as coccidia. The fatty acids (e.g., sodium butyrate) supplementation in milk replacer and starter diet is consider an Ab alternative since the positive effects on calves scouring days were reported (Gorka et al. Citation2009). Short and medium chain fatty acids (SMCFA) express antimicrobial activity in humans (Batovska et al. Citation2009) and other preliminary studies indicated beneficial effects on health and performance in broilers – improved average daily gain (Gutierrez Del Alamo et al. Citation2006) and pigs –reduced the occurrence of Brachyspira hyodysenteriae infection (Foresti et al. Citation2014). In the author opinion, these studies suggested the need to investigate the potential effects of SMCFA on calves.
Monoglycerides of SMCFA are the main components of numerous lipids, composed of fatty acid monoesters of glycerol (C6–C18) usually found in very low concentration in cell extracts as intermediate products in the degradation of triacylglycerides, or in industrial products obtained from plant oils. The SMCFA and their derivatives are part of a wide array of biomolecules such as plant storage lipids, but also of Ab and insect pheromones (van der Hoeven and Steffens Citation2000). Garret and Grisham (Citation1998) summarised that SMCFA can easily form micelles in water solutions; moreover they are rapidly absorbed by the intestinal epithelium, possess detergent properties and express antimicrobial activity (Batovska et al. Citation2009). The fatty acids and monoglycerides, have been thoroughly studied as active molecules against bacteria (Kabara and Marshall Citation2010).
The authors hypothesised that the addition of SMCFA to preweaned calf diets may reduce the occurrence of diarrhoea and subsequent respiratory diseases, thus improving GIT development and functions leading to increased growth. The study aimed to investigate the effects of the SMCFA supplementation in preweaned calf diets on health status and growth.
Materials and methods
This study was carried out in accordance with the Italian Legislation on animal care (DL 26 04/03/2014).
Evaluation of SMCFA antimicrobial properties
A liquid blend of SMCFA commercially available containing monopropionin, monobutyrin, monocaprylin, monocaprin and monolaurin (Patent FI A000257 2013, SILO S.p.a., Firenze, Italy) was evaluated.
A preliminary in vitro evaluation of the SMCFA antimicrobial activity was performed using the Minimum Inhibiting Concentration (MIC). This procedure followed the guidelines of the Clinical Laboratory Standard Institute (Clinical and Laboratory Standards Institute Citation2008), using a broth microdilution method against a panel of reference strains of pathogenic bacteria (Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Staphylococcus pseudintermedius ATCC 49444, MRSA ATCC 43300, Streptococcus agalactiae ATCC 55194) and field strains of Acinetobacter baumannii, Pasteurella multocida, Klebsiella pneumoniae, and Enterococcus faecium.
Animals and experimental design
A completely randomised block design was conducted using twenty Italian Holstein male calves that were divided into two groups (control – C- and treated – T-). Two experimental replicates were performed for each treatment, using five calves per sub-group. The experimental period consisted of 56 days and calves were evaluated from 16 to 72 days of age.
The calves enrolled in the study belonged to two dairy farms located in Parma province (Italy). Calves were similar for body weight and genetics merit. Moreover, a complete clinical examination was performed according to McGuirk (Citation2009). Briefly, the calves were evaluated for: attitude (alert and bright) hair coat appearance, mucous membranes (pink, moist and refill time less than 3 s), head, limbs and tongue (no swelling, edoema, or discoloration), respiratory rate (50–75 breath per minutes), primarily thoracic effort, hearth rate and pulse (100–150 beats/min, regular and pulse strong) and rectal temperature (38.3–38.8 °C). All the selected calves were considered healthy. Blood samples were collected for haematological evaluation and to determine the possible FPT through a sodium sulphite turbidity test (Tyler et al., Citation1996). To avoid an initial infective cross contamination, all the calves were subjected to intramuscular Ab treatment for three days prior to the experiment, using Ab combination of clavulanic acid and amoxicillin (Synulox® RTU, Pfizer, Italy; 1 mL/20 Kg of BW).
Each calf was housed individually (box; 1.20 × 1.40 m), then grouped by treatment and experimental replicate in four separate pens with natural ventilation and lighting. Bedding was replenished daily with wood shavings. A milk replacer (crude protein –CP, 25%; crude fat, 22%) was administered using a calf feeding bucket with a nipple at the dose of 3 L (12% powder concentration) at 40 °C, twice a day at 7:00 a.m. and 19:00 p.m. A pelleted starter (18% CP, 3.4% fat and 9.8% of crude fibre) was offered ad libitum from the beginning of the trial. Both the milk replacer and starter composition are reported in Table ; the chemical composition was verified through the NIRs technology (NIRs™ DS2500 F, Foss Italia S.r.l., Padova, Italy). Fresh water was available ad libitum throughout the trial. Group T received SMCFA (11 g/day) emulsified in the milk replacer once a day during morning feeding, while group C received 18 g/day of milk replacer powder to create an isoenergetic liquid diet between the groups. At the end of the trial the calves were sent to a commercial slaughterhouse and the GIT organs (rumen, reticulum, omasum, abomasum, small and large intestine) were collected and evaluated post-mortem.
Table 1. Ingredients and chemical composition of milk replacer and feedstuff fed in the experiment.
Health evaluation
The calf health status was monitored twice a day, before liquid feed administration, by the veterinary staff of the University Teaching Hospital (Department of Veterinary Science, University of Parma), based on the calf health scoring chart of the school of the veterinary medicine of the University of Wisconsin–Madison (https://www.vetmed.wisc.edu/dms/fapm/apps/chs.htm). This evaluation chart includes rectal temperature (Score 0: 37.8–38.3 °C; Score 3: ≥39.5), nasal discharge presence and characteristics (0: normal, 3: serous discharge), eye scores (0: Normal; 3: Heavy ocular discharge), ear scores (0: Normal; 3: head tilt or bilateral droop), cough scoring (0: none; 3: repeated, spontaneous cough), total respiratory scores (0: normal; 2: more than 2 clinical parameters that exceed score 2 McGuirk and Peek Citation2014), faecal scores (0: Normal; 3: Watery, sifts through bedding) and modified hide cleanliness scores (0: clean tail head region, 2: back end of calf, tail head region and legs manure littered).
In case of scours, with faecal score= 3 in two subsequent cheques, without fever (rectal temperature <39.5 °C) calves were subjected to restricted milk feeding or oral rehydrating therapy; milk was partially (50%) or completely replaced for a minimum of two days with a rehydrating product (Enerlyte plus®, Virbac Italia, Milano) at a concentration of 50 g/L. When calves were affected by scours with fever, Ab therapy was undertaken. Amoxicillin clavulanate (1 mL/20 Kg, Synulox®; Pfizer, Italy) was administered for almost three days in the presence of scours or respiratory symptoms with the addition of enrofloxacin (Baytril® 5% injectable solution, Bayer, Italy; - dose 2.5 mg/Kg BW).
Average score values, number of observations per score, scores frequency of daily clinical signs, and therapy administration was determined per treatment as a representation for the entire period.
Sampling and analysis
Blood
Blood samples were collected prior to morning feeding on 16, 30, 44, 58 and 72 days of age during the trial from the jugular vein into 10 mL lithium heparin vacuum tubes (Terumo Venosafe 10 mL VF-109SHL, Terumo Europe L.V., Leuven, Belgium).
White blood cell count (WBC), neutrophils (NEU), lymphocyte (LYM), monocyte (MONO), eosinophils (EOS), basophils (BASO), red blood cell count (RBC), haemoglobin (HGB), haematocrit (HCT), mean corpuscular volume (MCV), mean cell haemoglobin (MCH), mean cell haemoglobin concentration (MCHC), platelets, platelet distribution width, mean platelet volume, percentage of large platelets, plateletcrit, neutrophils, and red cell distribution width (RDW) were determined using the automated Cell Dyn 3500 plus haematology analyser (Abbott Diagnostics, Lake Forest, IL, USA).
Nonesterified fatty acids (NEFA) and β-Hydroxybutyric acid (BHBA) were analysed at 37 °C using a clinical auto-analyser (ILAB 600, Instrumentation Laboratory, Lexington, MA, USA) with kits purchased from Wako Chemicals GmbH Neuss, Germany (NEFA) and Randox Laboratories Ltd., Crumlin, Co. Antrim, United Kingdom (BHBA).
Faeces
Faecal samples (20 g) were collected from the rectum every two weeks and subsampled (0.1 g) for bacteriological investigations.
The subsample was suspended in a 50 mL tube containing saline solution (NaCl 0.85%) and vortexed. The initial faecal suspension was further diluted 50 times in the same saline buffer producing a concentration of 0.04 mg/mL. Twenty microliters of the final suspension for each faecal sample were plated onto MacConkey agar, MRS agar plates (Merck, KGaA, Darmstadt, Germany) and Columbia blood agar (Thermo Fisher Scientific, Waltham, Massachusetts, USA) to test Gram negative bacteria, Lactobacillus spp. and the haemolytic activity of E. coli. Each plate was incubated for 24 h at 37 °C in an aerobic environment (except for MRS agar, incubated under microaerophilic conditions). An additional blood agar plate was incubated for each sample for 48 h at 37 °C under anaerobic conditions to evaluate the growth of Clostridium spp. To evaluate the presence of Salmonella spp., 50 µl of the initial suspension were inoculated in 5 mL of peptone water and incubated aerobically (24 h; 37 °C), then 1 mL was transferred into Rappaport-Vassiliadis broth (Fisher Scientific Italia, Milano, Italy) and incubated aerobically (48 h; 42 °C). Lactose non-fermenting colonies were identified using API 20E biochemical test systems (bioMérieux, Marcy l’Etoile, France) and conventional biochemical tests (Quinn et al. Citation1994).
E. coli and Lactobacillus spp. were identified on the basis of bacterial colonies morphology. Colony forming unit (CFU) quantifications were performed directly on MacConkey agar and MRS agar plates, respectively. The presence of E. coli haemolytic activity – considered per se as indicative of the presence of pathogenic strains – was tested using a Columbia blood agar plate (Blanco et al., Citation1992). The presence of Clostridium spp. was evaluated through microscopic examination of the suspected colonies grown in an anaerobic environment and stained with Gram’s method. The sample was considered positive for Clostridium spp. when oval bulging spores located in terminal or sub-terminal position were detected (Quinn et al. Citation1994).
Intake, growth performances and morphological evaluation
Starter dry matter and liquid intake were measured daily. Shrunk body weight (SBW; weight before morning feeding) was measured weekly, starting from 16 until 72 days of age using a calibrated electronic scale to calculate growth rate, feed conversion rate (FCR) and feed efficiency (FE). In addition, height at withers, hearth girth circumference, hip width and body condition score (BCS) were measured throughout the trial.
At 72 days of age, the calves were sent to a slaughterhouse prior to morning feeding and killed through captive bolt stunning and exsanguinated. The rumen, reticulum, omasum, abomasum, small intestine and large intestine were separated, emptied, rinsed repeatedly with water, drained, and weighed to perform a morphological evaluation.
Statistical analysis
Two outliers per treatment were identified and discarded from the statistical analysis of clinical, morphological, haematogical and feed efficiency parameters. The normality of data distribution was determined by the Shapiro–Wilk test. Average results for clinical score values, therapy administration, intake, BW, carcase and GIT segments weight and the relationships between GIT segments were subjected to ANOVA using the General Linear Model approach of SPSS (Windows software package; version 21.0; SPSS Inc., Chicago, IL). Clinical data on score frequency was compared between treatments using the Chi Squared Test. Clinical data on the number of calves medically treated, number and the average days of therapy were analysed using the Fisher exact test, Chi squared test and t-test, respectively. Weekly measurements (growth, intake, FCR, FE and blood parameters) were analysed as repeated measures using the mixed model procedure of the SPSS; treatment, interval and their interaction were fixed factors while subjects and groups were random effects. Regression analysis was used to determine relationships between the average count of haemolytic E. coli in faeces during the whole trial and the relative final BW (% of initial BW) as well as the average prevalence of Clostridium spp. measured on each calf to its relative final BW. Linear models were fitted and the parallel lines test was conducted to compare the slope coefficients between the two treatments. The average prevalence of Clostridium spp. in faeces was compared between treatments using the one-tail z-test while the t-test was applied in regards to the average haemolytic E. Coli and Lactobacillus spp. CFU. Statistical significance was set at p ≤ .05.
Results
The preliminary in vitro evaluation of the SMCFA to measure antimicrobial activity showed MIC values of 0.8% against S. pseudintermedius, 3.2% against A. baumanii, S. agalactiae and MRSA, 6.3% against E. coli, P. multocida, K. pneumoniae and E. faecium and 12,5% against P. aeruginosa.
All the animals were negative to the FPT test, indicating a proper immune status at the beginning of the trial. Clinical observations are summarised in Table . The T group calves showed a higher average score for nasal discharge (p = .009), ear bearing (p = .007) and faecal consistency (p ≤ .001), while a lower score for eye appearance (p ≤ .001), cough (p ≤ .001) and total respiratory score (p ≤ .001).
Table 2. Average score values, number of observations per score (n-Sc) and scores frequency (Sc%) of daily clinical sign evaluation of male calves (from 16 to 72 days of age) affected by short and medium chain fatty acids (SMCFA) added to milk.
Calves in group T did not required Ab therapy, while it was necessary for three calves in group C. As a consequence, the total days of Ab therapy administration was 19 days higher in group C (p ≤ .01) resulting in an average of 2.37 days of Ab therapy (p = .033). No differences were observed between groups regarding treatment through rehydrating products and restricted feeding (Table ).
Table 3. Therapy used in case of calf illness (from 16 to 72 days of age) affected by short and medium chain fatty acids (SMCFA) added to milk.
No differences were observed between groups for blood parameters that fell within normal physiological ranges (Table ). No significant interactions were found. The interval was significant only for EOS, BASO and HCT.
Table 4. Blood parameters of male calves (from 16 to 72 days of age) affected by short and medium chain fatty acids (SMCFA) added to milk.
Biochemical evaluation of blood samples showed a gradual increase in BHBA content and an irregular variation of NEFA throughout the trial. The overall BHBA values were 0.164 and 0.192 mmol/L, while the overall NEFA values were 0.186 and 0.195 mmol/L for group C and T respectively, with no difference between groups for both parameters (Table ). However, at 44 days of age the BHBA values were significantly higher for group T (p < .05).
Table 5. β-hydroxybutyrate (BHBA) and nonesterified fatty acids (NEFA) fluctuations in blood of male calves (from 16 to 72 days of age) affected by short and medium chain fatty acids (SMCFA) added to milk.
Biometrical and efficiency parameters are reported in Table . No differences were found for body measurements, increasing linearly from 16 to 72 days of age. However, it should be noted that a significant interaction was found between the group and interval for SBW. Both height at withers and hip height showed a similar bi-phasic pattern of growth between groups, with lower rates from 16 to 44 days of age, followed by an increase in rates from 44 to 72 days of age. No differences were observed between groups for solid and liquid FE parameters. Solid FE decreased and FCR increased showing irregular variations, while liquid FE and liquid FCR rate showed more regular trends.
Table 6. Body development measurements: growth, feed efficiency (FE) and feed conversion rate (FCR) of male calves (from 16 to 72 days of age) affected by short and medium chain fatty acids (SMCFA) added to milk.
No differences were found for measurements of total experimental intake, body, carcase and GIT organs weight at 72 days (Table ).
Table 7. Individual intake in the 56 days of experimental period, final body weight (BW) and gut macroscopic measurements (at 72 days of age) of male calves as affected by milk added with short and medium chain fatty acids (SMCFA).
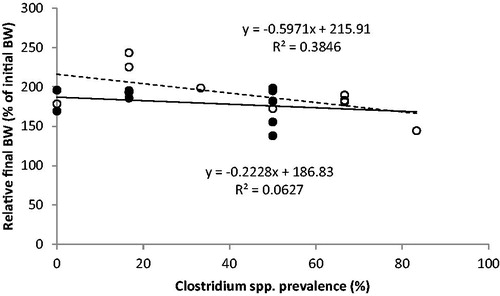
The impact of the investigated enteropathogens bacterial strains on calf growth are represented in Figures and . Figure shows that there was no significant difference in the regression slope (p = .853) of haemolytic E. coli count to relative final BW; however, there was a numerically lower slope of the regression fitting the group T data points. Figure suggests no significant difference between the slope of the two regressions (Clostridium spp. prevalence to relative final BW) even if the slope of the regression relative to group T was numerically lower. No difference in average haemolytic E. coli count was found between the two groups (233 vs 183 CFU, group T and C respectively; p = .209). Considering all the observations made the average prevalence of Clostridium spp. was similar between the two groups (31.6 vs 41.7%, group T and C respectively; p > .05). Concerning symbiotic positive bacteria, no significant difference was detected in the number of Lactobacillus spp. (179 vs 191 CFU, group T and C respectively; p = .292).
Figure 1. Regression between the average count of haemolytic Escherichia coli in faeces during the whole trial, expressed as Colony Forming Unit (CFU) and the relative final body weight (% of initial body weight - BW) of the Control (C–white circles, dotted line) and Treated (T–black circles, continuous line) group of male calves (from 16 to 72 days of age). The T group received 11 g/day of short and medium chain fatty acids (SMCFA) dissolved in milk.
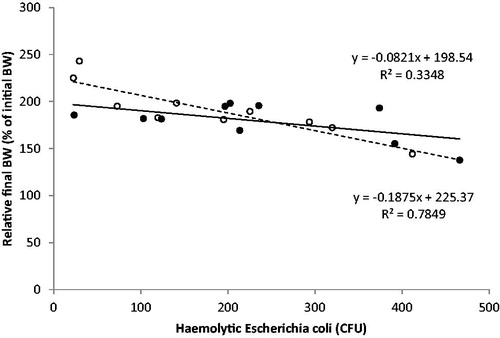
Figure 2. Regression between the average individual prevalence of Clostridium spp. in faeces during the whole trial (%), and the relative final body weight (% of initial body weight - BW) of the Control (C–white circles, dotted line) and Treated (T–black circles, continuous line) groups of male calves (from 16 days to 72 of age). The T group received 11 g/day of short and medium chain fatty acids (SMCFA) dissolved in milk.
Discussion
The present study was performed to investigate the effect of the administration of a SMCFA on health status and growth of preweaned calves.
The preliminary in vitro antibacterial activity test performed on the SMCFA showed efficacy especially against E. coli ATCC25922 reference strain at 6.3% of MIC, which is representative of one of the main microorganisms responsible for diarrhoea in calves (Kolenda et al. Citation2015). This suggests the potential inclusion of SMCFA in calf diets to reduce future occurrence of diarrhoea. A similar product was previously tested by Foresti et al. (Citation2014) on weaning and fattening swine at the doses of 0.4 mL/Kg of BW, reporting positive results against Brachyspira hyodysenteriae infection which is responsible for swine dysentery. Additionally, Gutierrez Del Alamo et al. (Citation2006) tested a similar product on broilers at the concentrations of 0.1, 0.15 and 0.20% in the feedstuff. Results showed improved daily gain reflecting positive gut function and health status when malabsorption syndrome was experimentally induced in broilers. In the present trial, the product concentration was around 1.5% of milk powder and was gradually diluted by the increased consumption of starter fed to calves. The average concentration throughout the duration of the study was 0.17% in the diet, which are similar to the doses tested in the study performed by Gutierrez Del Alamo et al. (Citation2006).
The treatment showed a positive impact on total respiratory score, mainly influenced by cough score, which had a lower occurrence in the group T. The lower scores of group C for nasal discharges, ear bearing and faecal consistency would suggest that the calves were healthier compared to those of group T. However, there was a higher need for AB therapy administration in group C calves, therefore there was an inconsistency amongst the health status evaluations. This is potentially due to higher cough scores recorded in group C, which suggest a greater morbidity of calves respiratory diseases.
The absence of Ab therapy required in group T calves, confirms the potential bactericidal/bacteriostatic activity of the SMCFA which was preliminary demonstrated in vitro. A study using an essential oil mixture (94 mg/day) as an Ab alternative in calves found similar results in which the requirement for Ab treatments against digestive and respiratory diseases were reduced (Soltan Citation2009). Similarly, Agazzi et al. (Citation2014) demonstrated the use of species-specific probiotics included in milk replacer to potentially reduce diarrhoea occurrence in calves.
The health status of calves was confirmed to be in normal ranges through haematological data (Mohri et al. Citation2007; Šoltésová et al. Citation2015) and showed no differences between groups. The EOS, BASO and HCT trends throughout the study were similar to those reported by Mohri et al. (Citation2007) and (Johnston et al., Citation2016) in Holstein calves. These differences may be due to the colostrum intake, short life time of erythrocyte, decreased foetal haemoglobin concentration, and the feeding and rearing system. Thus, physiological changes in the haematological parameter can vary during the first weeks of life which was demonstrated by (Klinkon and Ježek Citation2012).
The BHBA has been utilised as a marker for the evolution of rumen mucosae absorption capacity (Ślusarczyk et al. Citation2010). This was observed in the present study by the increasing levels of BHBA throughout the duration of the trial for both groups. However, group T showed a significantly higher absorption capacity compared to group C at 44 days of age which is close to the theoretical standard weaning time (42 days). The BHBA levels for both groups were lower compared to those reported by Ślusarczyk et al. (Citation2010), in which supplementation of sodium butyrate in weaning calves at the same age was evaluated. The NEFA was an indicator for lipid mobilisation by body reserves in calves resulting from a negative energy balance; however this parameter was within normal ranges (Sasaki et al. Citation2002) and there were no differences between the groups. Heinrichs (Citation2005) summarised that the presence and absorption of volatile fatty acids stimulate rumen epithelial metabolism and therefore could be a stimulus that induces rumen epithelial development. Moreover, according to Baldwin et al. (Citation2004), among fatty acids butyrate tends to exert the maximum stimulatory effect and based on Gorka et al. (Citation2009) the development of ruminal epithelium increases when butyrate and propionate salts are fed. The hystomorphometrical analysis results obtained by the present experiment have been already published by Ragionieri et al. (Citation2016) and suggested a greater trend towards parakeratosis of the ruminal mucosae of the group C calves. This process can be exacerbated by the lack of effective fibre (Sherwood et al., Citation2006) leading to a depression in nutrient adsorption and metabolic activity per weight unit (Nocek and Kesler Citation1980). The T animals, instead showed a lower amount of degenerative tissue accumulation on the exposed horn surfaces, that could lead to a higher efficiency of absorption. This is consistent with the results on BHBA at 44 d of age. These calves are expected to improve their FCR, albeit over times longer than that of the experimentation, because SMCFA supplementation seems to better regulate cell proliferation (Sakata and Tamate Citation1978; Citation1979) and cause an ‘emollient effect’ (Garret and Grisham Citation1998) leading to an easier ‘peeling’ of the stratum corneum of the mucosae avoiding hyperkeratosis.
As observed by Gutierrez Del Alamo et al. (Citation2006), a majority of positive effects for alternatives to Ab can be better observed when the health status of the animals is impaired and the integrity of the intestine is compromised. In the present study, calves were maintained in a healthy environment, so probably the gut microflora was properly balanced resulting in potentially masked advantages of SMCFA. The development of the small intestine is affected by the type of liquid feed supplemented to the animal (Górka et al., Citation2011) which impacts the digestion and absorption of nutrients, and consequently calf growth and health status. However, in the present trial, body development measurements as well as FE and FCR indicated no differences between groups even if a significant interaction was found between the group and interval for the SBW indicating a different trends of growth of the two groups. In general, the growth parameters were consistent with those reported by Marchi et al. (Citation2020) on preweaning calves raised under similar conditions.
The negative relationships observed between haemolytic E. coli count and Clostridium spp. prevalence with relative final BW throughout the experiment indicated a greater negative impact from these bacteria on group C. The supplementation of SMCFA could have potentially reduced the effects of pathogenic bacteria on the intestinal mucosa, which was supported by a slightly lower prevalence of Clostridium spp. resulting in enhanced growth for group T. Moreover, it should be noted that although there were no differences between groups for the mean count of Lactobacillus spp., it was numerically higher for group T. All these observations should be considered in future studies.
Conclusions
The SMCFA inclusion in calf diets at the dose of 11 g/day, tends to exert beneficial effects on calf health status and rumen function, leading to a reduction in the need for Ab treatments.
Ethical approval
The animals were intended for a zootecnical evaluation, the blood analysis were performed only as a routinely clinical check by the Staff of the veterinary hospital.
Acknowledgements
The authors acknowledge Dr. Irene Enfiomusi, Mr. Giuliano Alba and Mr. Andrea Rossi for their technical assistance.
Disclosure statement
None of the authors have any financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of this paper.
Additional information
Funding
References
- Agazzi A, Tirloni E, Stella S, Maroccolo S, Ripamonti B, Bersani C, Caputo JM, Dell’Orto V, Rota N, Savoini G. 2014. Effects of species-specific probiotic addition to milk replacer on calf health and performance during the first month of life. Ann Animal Sci. 14(1):101–115.
- Baldwin RL, McLeod KR, Klotz JL, Heitmann RN. 2004. Rumen development, intestinal growth and hepatic metabolism in the pre- and postweaning ruminant. J Dairy Sci. 87:E55–E65.
- Batovska DI, Todorova IT, Tsvetkova IV, Najdenski HM. 2009. Antibacterial study of the medium chain fatty acids and their 1-monoglycerides: individual effects and synergistic relationships. Pol J Microbiol. 58(1):43–47.
- Blanco J, Blanco M, Alonso MP, Blanco JE, González EA, Garabal JI. 1992. Characteristics of haemolytic Escherichia coli with particular reference to production of cytotoxic necrotizing factor type 1 (CNF1). Res. Microbiol. 143(9):869–878.
- Bresciani C, Sabbioni A, Ciampoli R, Bertocchi M, Saleri R, Cabassi SC, Bigliardi E, Di Ianni F, Parmigiani E. 2016. An innovative hyperimmune bovine plasma for prophylaxis and therapy of neonatal dairy calf diarrhea - a clinical trial. Large Animal Rev. 22:115–119.
- Cho Y-I, Yoon K-J. 2014. An overview of calf diarrhea - infectious etiology, diagnosis, and intervention. J Vet Sci. 15(1):1–17.
- Clinical and Laboratory Standards Institute. 2008. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved Standard—Seventh Edition, in Document M7-A7. Wayne (PA): CLSI.
- Foresti F, Pussi A, Giacomini E, Lazzaro M, Migliori M, Ruggeri J, Zanoni M, Parini E, Coceva G, Pasquali P. 2014. Effect of specific mix of monoglycerides and diglycerides of short and medium chain fatty acids in fattening pigs diets to control swine dysentery. Cancun, Mexico: IPVS.
- Garret RH, Grisham CM, 1998. Lipids. In: Biochemistry. 2nd ed. Glen Allen (VA): College Publishing; p. 238–258.
- Gorka P, Kowalski ZM, Pietrzak P, Kotunia A, Kiljanczyk R, Flaga J, Holst JJ. 2009. Effect of sodium butyrate supplementation in milk replacer. J Physiol Pharmocol. 60:47–53.
- Górka P, Kowalski ZM, Pietrzak P, Kotunia A, Jagusiak W, Zabielski R. 2011. Is rumen development in newborn calves affected by different liquid feeds and small intestine development?. J. Dairy Sci. 94(6):3002–3013.
- Grandi G, Kramer LH, Quarantelli A, Righi F. 2016. Influence of oregano essential oil (OEO) on prevalence and oocyst shedding dynamics of naturally acquired Eimeria spp. infection in replacement dairy heifers. Ann Animal Sci. 16(1):171–179.
- Gutierrez Del Alamo A, De Los Mozos J, Van Dam JTP, Perez de Ayala P. 2006. The use of short and medium chain fatty acids as an alternative to antibiotic growth promoters in broilers infected with malabsorption syndrome. Paper presented at the 16th European Symposium on Poultry Nutrition, August 26–30; Strasbourg, France.
- Heinrichs J. 2005. Rumen development in the dairy calf rudimentary reticulo-rumen changes in rumen epithelium. Technology. 17:179–187.
- Johnston D, Kenny DA, Kelly AK, Mccabe MS, Mcgee M, Waters SM, Earley B. 2016. Characterisation of haematological profiles and whole blood relative gene expression levels in Holstein-Friesian and Jersey bull calves undergoing gradual weaning. Animal. 10(9):1547–1556.
- Kabara JJ, Marshall DL. 2010. Medium-chain fatty acids and esters. Antimicrobials in Food, Chapter 11:327–360.
- Kolenda R, Burdukiewicz M, Schierack P. 2015. A systematic review and meta-analysis of the epidemiology of pathogenic Escherichia coli of calves and the role of calves as reservoirs for human pathogenic E. coli. Front Cell Infect Microbiol. 5:23.
- Marchi L, Degola F, Rosario G, Quarantelli A, Simoni M. 2020. Textured vs pelletted feed impact on dairy heifers pre-weaning. Paper presented at the 28th International Symposium Animal Science Days-“Future Perspectives in Animal Production.” September 23–25, virtual conference.
- Marseglia A, Pitino R, Bresciani C, Quarantelli A, Righi F. 2020. Measurement of transfer of colostral passive immunity in dairy calves. Paper presented at the 28th International Symposium Animal Science Days-“Future Perspectives in Animal Production.” September 23–25, virtual conference.
- McGuirk SM. 2009. Critical calf care. Am Assoc Bovine Practictioner Proc. 42:163–167.
- McGuirk SM, Peek SF. 2014. Timely diagnosis of dairy calf respiratory disease using a standardized scoring system. Anim Health Res Rev. 15(2):145–147.
- Mohri M, Sharifi K, Eidi S. 2007. Hematology and serum biochemistry of Holstein dairy calves: age related changes and comparison with blood composition in adults. Res Vet Sci . 83(1):30–39.
- NAHMS, 2014. National Animal Health Monitoring System. Dairy cattle management practices in the United States. USDA Animal and Plant Health Inspection Service: Veterinary Services: Centers for Disease Epidemiology and Animal Health (USDA: APHIS: VS: CEAH), Fort Collins, CO.
- Nocek JE, Kesler EM. 1980. Growth and rumen characteristics of Holstein steers fed pelleted or conventional diets. J Dairy Sci . 63(2):249–254.
- Quinn PJ, Carter ME, Markey B, Carter GR. 1994. Bacterial pathogens: microscopy, culture and identification. Clinical veterinary microbiology, Mosby-Year Book Europe, 20:60
- Ragionieri L, Cacchioli A, Ravanetti F, Botti M, Ivanovska A, Panu R, Righi F, Quarantelli A, Gazza F. 2016. Effect of the supplementation with a blend containing short and medium chain fatty acid monoglycerides in milk replacer on rumen papillae development in weaning calves. Ann Anat. 207:97–108.
- Sakata T, Tamate H. 1978. Rumen epithelial cell proliferation accelerated by rapid increase in intraruminal butyrate. J Dairy Sci. 61(8):1109–1113.
- Sakata T, Tamate H. 1979. Rumen epithelium cell proliferation accelerated by propionate and acetate. J Dairy Sci. 62(1):49–52.
- Sasaki O, Yamamoto N, Togashi K, Minezawa M. 2002. Effects of age, environments and sex on plasma metabolite levels in young Holstein calves. Asian Australas J Anim Sci. 15(5):637–642.
- Sherwood L, Klandorf H, Yancey P.2006. Fisiologia degli Animali- dai geni agli organismi, Zanichelli, Bologna.
- Simoni M, Baldrighi N, Degola F, Marchi L, Marseglia A, Righi F. 2020. Low doses of lactoferrin supplementation in weaning calves. Paper presented at the 28th International Symposium Animal Science Days-“Future Perspectives in Animal Production.” September 23–25, virtual conference.
- Ślusarczyk K, Strzetelski J, Furgał-Dierżuk I. 2010. The effect of sodium butyrate on calf growth and serum level of ß-hydroxybutyric acid. J Anim Feed Sci. 19(3):348–357.
- Soltan MA. 2009. Effect of essential oils supplementation on growth performance, nutrient digestibility, health condition of Holstein male calves during pre- and post-weaning periods. Pakistan J Nutr. 8(5):642–652.
- Šoltésová H, Nagyová V, Tóthová C, Nagy O. 2015. Haematological and blood biochemical alterations associated with respiratory disease in calves. Acta Vet Brno. 84(3):249–256.
- Klinkon M, Ježek, J. 2012. Values of blood variables in calves. A bird’s-eye view of veterinary medicine’. (Ed. CC Perez-Marin): 301–320.
- Tyler JW, Hancock DD, Parish SM, Rea DE, Besser TE, Sanders SG, Wilson LK. 1996. Evaluation of 3 assays for failure of passive transfer in calves. Journal of Veterinary Internal Medicine, 10(5): 304–307.
- van der Hoeven RS, Steffens JC. 2000. Biosynthesis and elongation of short- and medium-chain-length fatty acids. Plant Physiol. 122(1):275–282.
