Abstract
The purpose of this study was to investigate the effects of sodium butyrate as a substitute for antibiotics on the production performance and intestinal barrier of meat rabbits. Six hundred healthy weaned rabbits were randomly divided into three groups (40 replicates per group): fed a basal diet (control), fed a basal diet containing 0.5% sodium butyrate (sodium butyrate), or fed a basal diet containing 0.004% antibiotics (antibiotic). The trial included 7 days of adaptation and 28 days of testing. After adding sodium butyrate to the feed, the mortality and diarrhoea rates of the weaned meat rabbits were reduced, and the composition of their intestinal flora was improved. Treatment significantly increased the villus height of the ileum (p < .05), decreased the crypt depth of the jejunum, and increased the V/C ratio of intestinal tissue. The contents of IL-4, IFN-γ and SIgA in the intestinal tissue were significantly increased, indicating improvement of the intestinal immune barrier. The expression of occludin in the duodenum also increased significantly. To sum up, sodium butyrate can be added to the diet of growing meat rabbits instead of antibiotics. The physical barrier, immune barrier and microbial barrier of rabbit intestine were improved. Rabbit farmers can use sodium butyrate to prepare a new type of eco-friendly feed.
Antibiotics in rabbit feed can be replaced by sodium butyrate.
The addition of sodium butyrate to feed can reduce the mortality and diarrhoea rate of rabbits.
Sodium butyrate can improve the intestinal barrier of rabbits.
Highlights
Introduction
In rabbit breeding, intestinal problems are common, and the death of meat rabbits caused by digestive tract stress after weaning is a major problem (Liu et al. Citation2019). Intestinal disorders are also one of the main causes of rabbit deaths. Therefore, in order to reduce the mortality of rabbits, antibiotics are often added to their feed. However, the abuse of antibiotics has led to many serious problems, resulting in adverse consequences such as multidrug-resistant bacteria. Consequently, researchers are looking for alternatives to antibiotics. At the same time, maintaining intestinal health without antibiotics is also an important challenge for the aquaculture (Cervantes Citation2015).
Short-chain fatty acids (SCFAs) are organic fatty acids containing 1–6 carbon atoms (Cook and Sellin Citation1998). They mainly contain acetic acid, propionic acid and butyric acid. In the gut, bacteria produce SCFAs by fermenting undigested carbohydrates (Koh et al. Citation2016) As a SCFA, sodium butyrate can reduce gastric emptying, improve feed digestibility and stimulate the maturation of the intestinal mucosa (Mazzoni et al. Citation2008) Butyric acid is a major short-chain fatty acid (SCFA) in the body, which may have anticancer and anti-inflammatory effects, and it has a positive effect on the intestinal barrier, intestinal flora, and satiety (Peng et al. Citation2009). At present, butyric acid is a well-known feed additive that can promote intestinal development and enhance intestinal function. It has been found that the addition of sodium butyrate to the diet of broilers has a positive effect on the intestinal epithelial cells of broilers (Qaisrani et al. Citation2015).
As a feed additive, it can improve production performance, reduce mortality, improve intestinal integrity and inhibit the reproduction of harmful bacteria in the gut (Fernández-Rubio et al. Citation2009; Timbermont et al. Citation2010; Sunkara et al. Citation2011). The main mechanism is that free butyric acid can penetrate the bacterial cell wall and change the intracellular pH, thus affecting the reproduction of pathogenic bacteria (van Der Wielen et al. Citation2000). Wang et al. (Citation2016) found that sodium butyrate changed the diversity and relative abundance of microorganisms in the intestinal tract and reduced the content of ammonia in the caecal contents of laying hens, indicating that sodium butyrate helps to reduce ammonia emissions.
The present experiment was conducted to investigate the effect of the addition of sodium butyrate to the diet on the performance and intestinal barrier of rabbits, to identify applicative effects of sodium butyrate as an antibiotic substitute in rabbit production.
Materials and methods
Animal welfare statement
All study procedures were approved by the Shandong Agriculture University Animal Care and Use Committee (SDAUA-2015-011) and were conducted in accordance with the Guidelines for Experimental Animals established by the Ministry of Science and Technology (Beijing, China).
Animal diets, management, and experimental design
A pre-experiment was done in the early stage of the experiment. 0.1%, 0.5% and 1.0% sodium butyrate were added to the feed of weaned meat rabbits, respectively. At the end of the experiment, the production performance of rabbits fed with 0.5% sodium butyrate feed was the best.
In this experiment, 600 weaned healthy meat rabbits (300 males, 300 females) with an average weight of 1192.2 g were divided into 3 groups (Each group had 100 males and 100 females, 40 replicates per group, 5 rabbits per replicate, each replicate was randomly distributed according to the average weight of male and female). Each group was fed with different feeds. Among them, the sodium butyrate group’s feed was supplemented with 0.5% sodium butyrate (sodium butyrate from the Beijing Challenge Animal Husbandry Technology Co., Ltd.), the antibiotic group’s feed was supplemented with 0.004% antibiotic (bacitracin zinc), and the control group was fed the basal diet.
There were 40 cages in each experimental group, 5 experimental rabbits per cage. They were fed twice a day, once at 8:00 and once at 16:00. The pre-test period was 7 days, and the trial period was 28 days. Before the experiment, the experimental environment was thoroughly cleaned and disinfected, and natural lighting and disinfection were maintained throughout the experiment. All diets met the nutritional needs of growing rabbits. The feed intake and body weight gain were recorded during the test period. This study was conducted in the rabbit nutrition laboratory of Shandong Agricultural University. The compositions and chemical analysis of the feeds are shown in Table .
Table 1. Composition and nutrient levels of basal diets (air-dry basis).
The rabbits in the antibiotic group were only used in the experiment. At the end of the experiment, all of the rabbits in the antibiotic group were euthanised and their meat did not enter the market.
Sample collection and preparation
During the experiment, daily feed and surplus feed were weighed, and the bodyweight of rabbits was recorded every week. At the end of the trial, 8 rabbits (4 males and 4 females) from each group were randomly selected for sample collection after a 12-hour fast. Four pieces of the jejunum, ileum and duodenum were taken from each rabbit, two of which were fixed with 4% paraformaldehyde fixed solution for 24 hours, replaced with fresh 4% paraformaldehyde 24 hours later, and then replaced with fresh 4% paraformaldehyde every 7 days. The fixed tissue was used to create intestinal tissue sections to observe intestinal morphological changes. The other two pieces were collected in tubes and placed in liquid nitrogen, and then stored at −80 °C until the analysis was performed. Two samples of the caecal contents were collected from each rabbit into 5 mL cryopreservation tubes, placed in liquid nitrogen, and then preserved at −80 °C until the intestinal flora analysis was performed.
Analysis of production performance
At the beginning of the experiment, the initial body weight, daily feed intake and the number of rabbits with diarrhoea were recorded. At the end of the experiment, the terminal weight was recorded, and the average daily feed intake (ADFI), average daily gain (ADG) and feed gain ratio (F/G) were calculated (Du et al. Citation2013; Li et al. Citation2013). The numbers of rabbits with diarrhoea or that died were recorded daily throughout the study. The death and diarrhoea rates of the rabbits were calculated.
ADFI = Total feed intake per rabbit during normal feeding/Test days
ADG= {Terminal weight (FBW) − Initial weight (IBW)}/Test days
Death rate = Number of dead rabbits/Total number of rabbits in each group × 100%
Diarrhoea rate = Number of rabbits with diarrhoea/Total number of rabbits in each group × 100%
F/G = ADFI/ADG
Analysis of intestinal morphology
After the rabbits were slaughtered, the tissues of the duodenum, jejunum and ileum were collected from each rabbit. The samples were processed, embedded, and stained according to the procedures of Liu et al. (Citation2019a, Citation2019b). In brief, 2 pieces were fixed with 4% paraformaldehyde, which was replaced with fresh 4% paraformaldehyde every 7 days. From each group we randomly selected 8 samples, and each sample consisted of 2 tissue sections, for a total of 144 sections. The sections were stained with haematoxylin-eosin. The tissue sections were prepared and stained by Wuhan Saiwei Biotechnology Co., Ltd.
Analysis of intestinal flora
After the test rabbits were slaughtered, two samples of their caecum contents were collected from each test rabbit, rapidly frozen in liquid nitrogen, and then stored at −80 °C. Six samples (18 samples in total) were randomly selected from each group for 16S rDNA sequencing (Illumina Novaseq Sequencer was the sequencing platform, 250 PE) (Wu et al. Citation2017). The sequencing process and instruments were provided by Hangzhou Lianchuan Biological Co., Ltd.
Analysis of intestinal immune barrier
After the rabbits were slaughtered, the jejunum, ileum and duodenum were placed in 1.5 mL cryopreservation tubes, flash-frozen in liquid nitrogen, and then preserved at −80 °C until analysis. The contents of interleukin-4 (IL-4), interferon-gamma (IFN-γ) and secretory immunoglobulin A (SIgA) in the jejunum, ileum and duodenum were measured in samples from each group.
Five samples were randomly selected from each group, and approximately 1 g tissue was used for each sample. The tissue was homogenised, and then PBS was added to the homogenate at 1:9, then it was serially diluted into five concentrations. Each sample was assayed in duplicate. The tests were carried out according to the instructions of the corresponding kit, and the OD value was determined by absorbance at 450 nm. The contents of IL-4, IFN- γ and SIgA were determined by enzyme linked immunosorbent assays (ELISA) (kits provided by Shanghai Hengyuan Biological Co., Ltd. Shanghai, China).
The methods for determining IL-4, IFN-γ and SIgA are almost the same. Taking IL-4 as an example, the method was as follows: The level of rabbit IL-4 in the sample was determined by the double-antibody sandwich method. The purified rabbit IL-4 antibody was bound to a microwell plate to prepare the solid-phase antibody. The sample was added, and any IL-4 present bound to the antibody attached to the plate, and then it was combined with an HRP labelled IL-4 antibody to form an antibody-antigen-enzyme labelled antibody complex. After thorough washing, the substrate TMB was added for colouration. TMB is converted to blue under the catalytic activity of the HRP enzyme and then to yellow after the addition of acid. The depth of the colour is positively correlated with the amount of IL-4 in the sample. The absorbance (OD) was measured by a microplate reader at 450 nm, and the concentration of rabbit IL-4 in the sample was calculated by the use of a standard curve. At the end of the experiment, Excel was used to calculate the concentration (ng/L). We used SAS (Version 8e, SAS Institute, Cary, NC, USA) to process the data and we used GraphPad (GraphPad softioare Inc. CA, USA) to graph the data.
Analysis of genes related to the intestinal barrier
Total RNA extraction and qRT-PCR were carried out according to the previously described methods (Fu et al. Citation2017, Citation2018; Liu et al. Citation2017). The RNA was quantified with a biophotometer (Roche, Basel, Switzerland). The PCR data were analysed using the 2−ΔΔCT method. The mRNA levels of the target genes were normalised to β-actin (ΔCT) (Wang et al. Citation2017; Zhu et al. Citation2017). The primer sequences are shown in Table .
Table 2. Gene-specific primers used for the analysis of rabbit gene expression.
Statistical analysis
Data were analysed with the generalised linear model (GLM) one-way analysis of variance test using the SAS statistical software package, followed by Duncan’s multiple range test to compare differences among treatments when significant differences (p < .05) were observed.
Results
Effect of sodium butyrate on production performance of rabbits
The effect of dietary sodium butyrate on rabbit performance is shown in Table . Among them, sodium butyrate group had no significant effect on average daily gain, average feed intake or feed-weight ratio (p > .05), but sodium butyrate group had an increasing trend for weight gain and feed intake.
Table 3. Effect of Sodium butyrate on production performance of meat rabbit (n = 40).
Effect of sodium butyrate on intestinal morphology
There were differences in the intestinal morphological indicators among the groups (Table ). For the jejunum, there was a significant difference in crypt depth (p < .05). The crypt depths of the sodium butyrate and antibiotic groups were significantly less than that of the control group. Additionally, the V/C value had a very significant difference (p < .01). The V/C values of the sodium butyrate and antibiotic groups were significantly greater than that of the control group. For the ileum, the villi heights and V/C values were significantly different (p < .05). The villi heights and V/C values of the sodium butyrate and antibiotic groups were greater than that of the control group. For the duodenum, the V/C values were significantly different (p < .05). There were no significant differences in the other indicators.
Table 4. Effect of sodium butyrate on intestinal morphology of meat rabbits (n = 8).
Effect of sodium butyrate on the intestinal flora
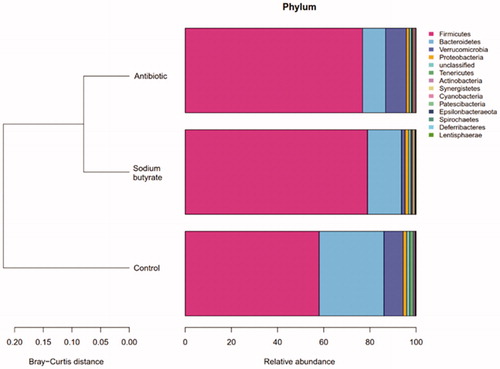
From the cluster diagram, a shorter left branch means a more similar species composition. It can be seen that the species compositions of the sodium butyrate group and the antibiotic group are similar, and they are different from the control group (Figure ).
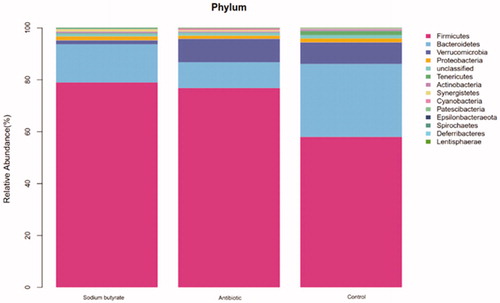
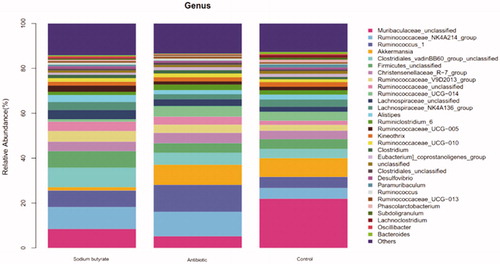
According to the OTU classification and the classification status recognition of the results, the specific bacterial composition of each sample at the phylum, class, order, family and genus level can be obtained. The display at the phylum level is shown in Figure , and the display at the genus level is shown in Figure .
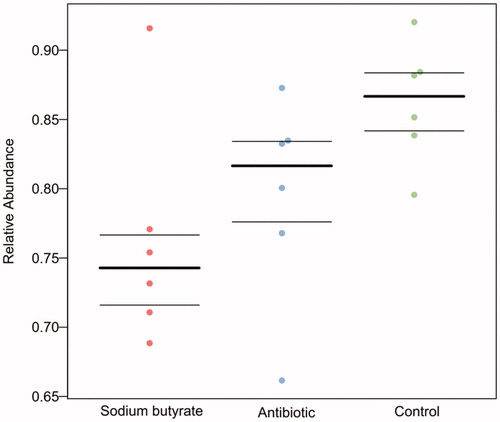
According to the prediction of potential pathogenic bacteria, the sodium butyrate group had the least potential pathogenic bacteria, the antibiotic group was the second, and the control group had the most potential pathogenic bacteria (Figure ).
Effect of sodium butyrate on intestinal immune barrier
There were significant differences in the contents of IL-4 in the jejunum, ileum and duodenum among the groups. There was a very significant difference in the content between the sodium butyrate group and the control group (p < .01, Figure ), and between the antibiotic group and the control group (p < .01). There was no significant difference between the sodium butyrate group and the antibiotic group (p > .05). The content of IL-4 in the intestinal tissues of the sodium butyrate and antibiotic groups was greater than that of the control group.
Figure 5. Effect of Sodium butyrate on the contents of IL-4, IFN- γ and SIgA. The same letter means that there is no significant difference (p>.05), while different letters indicate that there is a significant difference (p<.05). IL-4: interleukin-4; IFN-γ: interferon-γ; SIgA: secretory immunoglobulin A.
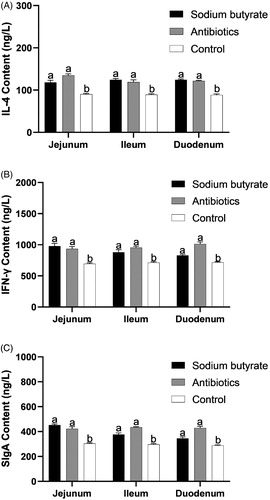
For IFN-γ, there were significant differences in the jejunum, ileum and duodenum among the groups. There was a very significant difference in the content between the sodium butyrate group and the control group (p < .01), and between the antibiotic group and the control group (p < .01). There was no significant difference between the sodium butyrate group and the antibiotic group (p > .05). The content of IFN-γ in the intestinal tissue of the sodium butyrate and antibiotic groups was greater than that of the control group.
There were significant differences in the content of SIgA in the jejunum, ileum and duodenum among the groups. There was a very significant difference in the content between the sodium butyrate group and the control group (p < .01), and between the antibiotic group and the control group (p < .01). There was no significant difference between the sodium butyrate group and the antibiotic group (p > .05). The content of SIgA in the intestinal tissue of the sodium butyrate and antibiotic groups was greater than that of the control group.
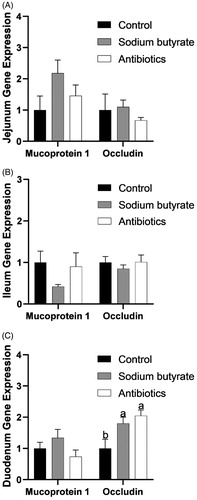
Effect of sodium butyrate on the intestinal barrier related genes
The expression of mucoprotein 1 (Muc 1) and occludin in the jejunum, ileum and duodenum are shown in Figure . Among them, there was a significant difference in the expression of occludin in the duodenum (p < .05), but there was no significant difference in the expression of the other genes (p > .05).
Discussion
Sodium butyrate reduces the mortality and diarrhoea rates of the weaned rabbits
There have been many reports about the effect of butyrate on animal production performance. When sodium butyrate 0.8 g/kg was added to their feed, the ADG of piglets increased by 19.91% (Piva et al. Citation2002). However, the addition of sodium butyrate to the diet did not significantly affect rabbit growth performance in our present experiment. These results imply that the effect of sodium butyrate on growth performance in different species is different. It is also possible that the addition of sodium butyrate in this experiment did not reach the optimum amount for growing meat rabbits. However, the present results showed that the addition of sodium butyrate significantly reduced the mortality and diarrhoea rates of meat rabbits, indicating that sodium butyrate could improve the rabbits’ health. To examine its mechanisms, we studied the effect of sodium butyrate on the intestinal barrier.
Sodium butyrate improves the intestinal physical barrier
The small intestine is the main place for the digestion and absorption of nutrients. The intestinal epithelium covering the gastrointestinal tract consists of a monolayer of enterocytes covered by a mucus gel layer. Weaning leading to oxidative stress causes intestinal mucosal damage, characterised by decreased villus height and deepening of the crypts (Hu et al. Citation2013).
Chen et al. (Citation2018) showed that Clostridium butyricum could increase the villus height and V/C ratio of the duodenum, jejunum and ileum. In this experiment, the results were the same as those of previous studies. When sodium butyrate was added to the diet, the villus height of the jejunum increased by 2.24% compared with the control group, the crypt depth decreased by 8.39%, and it had a very significant effect on the V/C ratio of the jejunum (p < .01). After adding sodium butyrate, the villus height increased by 9.31%, the crypt depth decreased by 14.6%, and the V/C ratio was increased significantly in the ileum (p < .05). For the duodenum, the V/C ratio increased significantly after the addition of sodium butyrate.
The above results show that the addition of sodium butyrate to the feed can increase the inner surface area of the small intestine, which can promote the absorption of nutrients in the small intestine.
Gastrointestinal epithelial cells act as a physical barrier and a defence against the diffusion of pathogens, toxins, and allergens from the external environment into the tissues. The major determinant of intestinal barrier function is the intercellular tight junctions (Li et al. Citation2018). Occludin is considered to be the most important tight junction protein. The regulating effect of sodium butyrate on occludin gene expression is related to the specific intestinal segment. Sodium butyrate could increase occludin expression in the duodenum but not in the ileum or jejunum (Zong et al. Citation2019). These results suggest that sodium butyrate could improve the intestinal barrier and that occludin may be a pivotal target associated with this process.
Mucin is a high molecular weight glycosylated protein secreted by epithelial cells, which has a protective and lubricating effect on the cells. In this experiment, the addition of sodium butyrate had no significant effect on the gene expression of mucin, indicating mucin may not be the major target of sodium butyrate in improving the intestinal barrier.
Sodium butyrate improves the intestinal flora
As an organic part of the intestinal microecological environment, the intestinal microflora are not only affected by the intestinal environment but also by a series of physiological and biochemical reactions. The intestinal flora of healthy animals mainly includes lactic acid bacteria, anaerobes and aerobic bacteria, among which the lactic acid bacteria mainly include lactic acid bacteria, bifidobacteria and streptococci; the anaerobes mainly include Bacteroides and Peptococcus; and the aerobic bacteria mainly include Enterobacteriaceae (Shanahan Citation2001). These microbes are combined in a certain proportion, which restrict and depend on each other to maintain a balance (Kimura et al. Citation1997). As seen from Figure , the compositions of the intestinal flora in the sodium butyrate and antibiotic groups are similar.
At the phylum level, the top 10 flora in relative abundance are Firmicutes, Bacteroidetes, Verrucomicrobia, Proteobacteria, Tenericutes, Actinobacteria, Synergistetes, Cyanobacteria, Patescibacteria and Epsilonbacteraeota in turn. According to Figure , we can see that the abscissa represents different groups, the ordinate represents the grouping, and the different colours represent different flora. We can see the differences between the different groups of samples at the phylum level. Normally, there are mainly Firmicutes (50%–75%), Bacteroides (10%–50%), Actinobacteria (1%–10%) and Proteobacteria (less than 1%) in the intestinal tract (Manichanh et al. Citation2012). Na-Ri et al. (Citation2015) believed that Proteus can be used as a criterion for the identification of intestinal flora disorders and underlying diseases, and a good intestinal environment can be considered when its content is low. When the proportion of Proteus fluctuates, it will become the cause of intestinal inflammation. In this experiment, the contents of Proteobacteria in the three groups were all in the normal range. After the addition of sodium butyrate to the feed, the Firmicutes became the dominant flora, and the Actinobacteria and Bacteroides were still in the normal range. Based on this, we can speculate that the addition of sodium butyrate to the diet can improve the composition of the intestinal flora and increase the content of thick-walled bacteria in meat rabbits, to promote intestinal development and maintain intestinal health.
From the point of view of Figure , the species with the top 30 abundances were selected and a relative abundance horizontal bar chart of the species was drawn. The top 10 species were as follows: Muribaculaceae_unclassified, Ruminococcaceae_NK4A214_group, Ruminococcus_1, Akkermansia, Clostridiales_vadinBB60_group_unclassified, Firmicutes_unclassified, Christensenellaceae_R-7_group, Ruminococcaceae_V9D2013_group, Ruminococcaceae_unclassified, and Ruminococcaceae _UCG-014, comparing the differences of the intestinal flora among the groups.
After adding sodium butyrate to the feed, Ruminococcaceae became the dominant flora in the rabbit intestine. Ruminococcaceae belongs to the Firmicutes, which is a Gram-positive bacteria. The main fermentation product of Ruminococcaceae in the intestinal tract is short-chain fatty acids. Compared with the control group, the content of Ruminococcaceae was increased by approximately 10%.
Lachnospiraceae is a gram-positive bacteria found in the digestive tract of many mammals and it is associated with the prevention of colon cancer (Meehan and Beiko Citation2014). Lachnospiraceae can ferment glucose in animals to produce formic acid, lactic acid, acetic acid, ethanol, CO2 and H2. In this experiment, when sodium butyrate was added to the diet, the content of Lachnospiraceae in the caecal content of rabbits increased compared with the antibiotic group and the control group.
Microbial communities in different host positions are involved in many aspects of mammalian health, including metabolism, immune response, disease status, and even behaviour. As the focus of the microbial community field changes from the detection of disease correlations to the identification of disease mechanisms, it is very important to understand the functional ability of microorganisms in the microbial community. For example, a better understanding of the ratio of Gram-negative to Gram-positive bacteria in the gastrointestinal tract will contribute to more targeted antibiotic treatment, while determining the content of anaerobes and pathogens in donor faecal samples will help in more accurately predicting the effectiveness and safety of faecal microbiome transplantation.
RobKnight Lab (California American University, CA, USA) has developed a software called BugBase, which is a tool that can predict the phenotype of microbiome samples. As shown in Figure , BugBase was used to predict the potential pathogenicity, and different phenotypes of different groups were predicted using scatter plots. The three lines in the figure represent the upper quartile, the average value and the lower quartile from top to bottom, respectively. From the scatter plots, the content of potential pathogenic bacteria in the sodium butyrate group was significantly lower than that in the control group. These results showed that the addition of sodium butyrate to the feed improved the intestinal flora and inhibited the reproduction and growth of harmful bacteria.
Sodium butyrate improves the intestinal immune barrier
Interleukin-4 (IL-4) is a multipotent immunoreactive regulatory molecule and a recognised anti-inflammatory cytokine, which plays an important role in immune regulation and anti-tumour effects (Schüler et al. Citation1999). IL-4 is a cytokine with multiple biological functions produced by Th2 cell subsets, which can enhance humoral immunity mediated by immunoglobulin E (IgE) and have the ability to kill cells. According to the results of this experiment, it was found that the IL-4 content of the jejunum, ileum and duodenum of meat rabbits fed with sodium butyrate was significantly greater than that of the control group, and there was no significant difference in IL-4 content between the sodium butyrate group and the antibiotic group. Through the analysis of the experimental results, it can be concluded that sodium butyrate can not only promote the proliferation of T cells in intestinal tissue but also promote the secretion of IL-4 and enhance intestinal immune function by activating T cells in intestinal tissue.
Interferon-γ (IFN-γ), also known as immune interferon, is not only a highly effective antiviral bioactive substance but also a lymphokine with extensive immunomodulatory effects. In vivo, Th1 cells mainly secrete IL-2 and IFN-γ and participate in cellular immune responses, while Th2 cells mainly secrete IL-4 and IL-10, which participate in humoral immune responses (Shanshan et al. Citation2015). In this experiment, the content of IFN-γ in the intestinal tissue of the experimental group supplemented with sodium butyrate and antibiotics increased significantly, which indicated that the antiviral and anticancer activity in the intestinal tract of meat rabbits was improved after adding sodium butyrate.
IFN-γ can also regulate the production of IL-4. It was found that the gene expression of Th2 cytokines was completely inhibited after endogenous IFN-γ was neutralised with the corresponding antibodies, indicating that IFN-γ plays an essential role in the early expression of the IL-4 gene (Torres et al. Citation2004). Therefore, the increase of IL-4 content in the sodium butyrate group may be because butyric acid promotes the proliferation of intestinal activated T cells to promote the secretion of IL-4, or it may promote the secretion of IFN-γ by Th1 cells. An increase of the content of IFN-γ promotes the secretion of IL-4.
Another molecule that plays an important role in the intestinal immune response is SIgA. SIgA is secreted by plasma mother cells, and it can effectively bind to antigens and prevent viruses, bacteria and other harmful antigens from adhering to the intestinal epithelium, promoting humoral and cellular immunity in the intestinal tract, and finally effectively reject or remove harmful antigens. SIgA is an antibody secreted by B lymphocytes in the lamina propria of the respiratory tract and digestive tract, which is present on the surface of the mucosa to form an immune protective layer (Zhang et al. Citation2014). In this experiment, the addition of sodium butyrate to the diet significantly increased the content of SIgA in the intestinal tissue, which was the same as the results of previous experiments. It can be seen that the addition of sodium butyrate to feed can stimulate the secretion of intestinal mucosal antibodies, enhance the immune ability of the intestine, and improve its disease resistance.
Conclusions
The dietary addition of sodium butyrate reduced the mortality and diarrhoea rates of weaned meat rabbits and improved their intestinal health by regulating the physical barrier, the immune barrier, and the microflora.
Therefore, we believe that sodium butyrate can be used in meat rabbit breeding as a substitute for antibiotics.
Ethical approval
The protocol used in this experiment approved by the Institutional Animal Care and Use Committee of Institute of Animal Science and Veterinary Medicine, Shandong Agricultural University (Taian, Shandong, China).
Acknowledgments
The author thanks the staff of Liaocheng Huifu Agriculture and Animal Husbandry Co., Ltd. The author also thank American Journal Experts (AJE, www.aje.cn) for English language editing.
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Cervantes HM. 2015. Antibiotic-free poultry production: is it sustainable? J Appl Poultry Res. 24:91–97.
- Chen L, Li S, Zheng J, Li W, Jiang X, Zhao X, Li J, Che L, Lin Y, Xu S, et al. 2018. Effects of dietary Clostridium butyricum supplementation on growth performance, intestinal development, and immune response of weaned piglets challenged with lipopolysaccharide. J Anim Sci Biotechno. 9:957–970.
- Cook SI, Sellin JH. 1998. Review article: short chain fatty acids in health and disease. Aliment Pharmacol Ther. 12:499–507.
- Du HT, Wang CY, Wang XP, Ma MW, Li FC. 2013. The effects of dietary α-linolenic acid on growth performance, meat quality, fatty acid composition, and liver relative enzyme mRNA expression of growing meat rabbits. J Anim Feed Sci. 22:122–129.
- Fernández-Rubio C, Ordóñez C, Abad-González J, Garcia-Gallego A, Honrubia MP, Mallo JJ, Balaña-Fouce R. 2009. Butyric acid-based feed additives help protect broiler chickens from Salmonella enteritidis infection. Poultry Sci. 88:943–948.
- Fu C, Liu L, Li F. 2018. Acetate alters the process of lipid metabolism in rabbits. Animal. 12:1895–1902.
- Fu CY, Liu L, Gao Q, Sui XY, Li FC. 2017. Cloning, molecular characterization, and spatial and developmental expression analysis of GPR41 and GPR43 genes in New Zealand rabbits. Animal. 11:1798–1806.
- Hu CH, Xiao K, Luan ZS, Song J. 2013. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J Anim Sci. 91:1094–1101.
- Kimura K, McCartney AL, McConnell MA, Tannock GW. 1997. Analysis of fecal populations of bifidobacteria and lactobacilli and investigation of the immunological responses of their human hosts to the predominant strains. Appl Environ Microbiol. 63:3394–3398.
- Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. 2016. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 165:1332–1345. 2016,
- Li HH, Li YP, Zhu Q, Qiao JY, Wang WJ. 2018. Dietary supplementation with Clostridium butyricum helps to improve the intestinal barrier function of weaned piglets challenged with enterotoxigenic Escherichia coli K88. J Appl Microbiol. 125 :964–975.
- Li JW, Wang XP, Wang CY, Zhu YL, Li FC. 2013. Effects of dietary electrolyte balance on growth performance, nitrogen metabolism and some blood biochemical parameters of growing rabbits. Asian Australas J Anim Sci. 26:1726–1731.
- Liu L, Liu H, Fu C, Li C, Li F. 2017. Acetate induces anorexia via up-regulating the hypothalamic pro-opiomelanocortin (POMC) gene expression in rabbits. J Anim Feed Sci. 26:266–273.
- Liu L, Zhao X, Liu Y, Zhao H, Li F. 2019a. Dietary addition of garlic straw improved the intestinal barrier in rabbits. J Anim Sci. 97:4248–4255.
- Liu L, Zuo W, Li F. 2019b. Dietary addition of Artemisia argyi reduces diarrhea and modulates the gut immune function without affecting growth performances of rabbits after weaning. J Anim Sci. 97:1693–1700.
- Manichanh C, Borruel N, Casellas F, Guarner F. 2012. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 9:599–608.
- Mazzoni M, Le Gall M, De Filippi S, Minieri L, Trevisi P, Wolinski J, Lalatta-Costerbosa G, Lallès JP, Guilloteau P, Bosi P. 2008. Supplemental sodium butyrate stimulates different gastric cells in weaned pigs. J Nutr. 138:1426–1431.
- Meehan CJ, Beiko RG. 2014. A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol Evol. 6:703–713.
- Na-Ri ST, Woong Whon JW, Jin-Woo B. 2015. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 33:496–503.
- Peng L, Li ZR, Green RS, Holzman IR, Lin J. 2009. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 139 :1619–1625.
- Piva A, Morlacchini M, Casadei G, Gatta PP, Biagi G, Prandini A. 2002. Sodium butyrate improves growth performance of weaned piglets during the first period after weaning. Ital J Anim Sci. 1:35–41.
- Qaisrani SN, van Krimpen MM, Kwakkel RP, Verstegen MWA, Hendriks WH. 2015. Diet structure, butyric acid, and fermentable carbohydrates influence growth performance, gut morphology, and cecal fermentation characteristics in broilers. Poultry Sci. 94:2152–2164.
- Schüler T, Qin Z, Ibe S, Noben-Trauth N, Blankenstein T. 1999. T helper cell type 1-associated and cytotoxic T lymphocyte-mediated tumor immunity is impaired in interleukin 4-deficient mice. J Exp Med. 189:803–810.
- Shanahan F. 2001. Inflammatory bowel disease: immunodiagnostics, immunotherapeutics, and ecotherapeutics. Gastroenterology. 120:622–635.
- Shanshan C, Qihui L, Wen Z. 2015. Expression of Th1/Th2 cytokines in partial target organs of type 2 diabetes mellitus rhesus monkey. J Zhejiang Univ-sc A. 41:302–308.
- Sunkara LT, Achanta M, Schreiber NB, Bommineni YR, Dai G, Jiang W, Lamont S, Lillehoj HS, Beker A, Teeter RG, et al. 2011. Butyrate enhances disease resistance of chickens by inducing antimicrobial host defense peptide gene expression. PLoS One. 6:e27225.
- Timbermont L, Lanckriet A, Dewulf J, Nollet N, Schwarzer K, Haesebrouck F, Ducatelle R, Van Immerseel F. 2010. Control of Clostridium perfringens-induced necrotic enteritis in broilers by target-released butyric acid, fatty acids and essential oils. Avian Pathol. 39:117–121.
- Torres KC, Dutra WO, Gollob KJ. 2004. Endogenous IL-4 and IFN-gamma are essential for expression of Th2, but not Th1 cytokine message during the early differentiation of human CD4+ T helper cells. Hum Immunol. 65:1328–1335.
- van Der Wielen PW, Biesterveld S, Notermans S, Hofstra H, Urlings BA, van Knapen F. 2000. Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Appl Environ Microbiol. 66:2536–2540.
- Wang A, Wang Y, Di Liao X. 2016. Sodium butyrate mitigates in vitro ammonia generation in cecal content of laying hens. Environ Sci Pollut R. 23:1–8.
- Wang C, Zhu Y, Li F, Huang L. 2017. The Effect of Lactobacillus isolates on growth performance, immune response, intestinal bacterial community composition of growing Rex Rabbits. J Anim Physiol Anim Nutr. 101:e1–e13.
- Wu SJ, Liu L, Zhu YL, Wang CY, Li FC. 2017. Effect of varying the energy density on growth performance, meat quality, caecum fermentation and microbiota of growing Rex Rabbits. Anim Prod Sci. 57:90.
- Zhang L, Cao GT, Zeng XF, Zhou L, Ferket PR, Xiao YP, Chen AG, Yang CM. 2014. Effects of Clostridium butyricum on growth performance, immune function, and cecal microflora in broiler chickens challenged with Escherichia coli K88. Poultry Sci. 93:46–53.
- Zhu Y, Sun Y, Wang C, Li F. 2017. Impact of dietary fibre:starch ratio in shaping caecal archaea revealed in rabbits. J Anim Physiol Anim Nutr (Berl). 101:635–640.
- Zong X, Wang TH, Lu ZQ, Song DG, Zhao J, Wang YZ. 2019. Effects of Clostridium butyricum or in combination with Bacillus licheniformis on the growth performance, blood indexes, and intestinal barrier function of weanling piglets. Livest Sci. 220:137–142.