Abstract
This study was to investigate physicochemical characteristics, fatty acids, minerals and hematological alterations in broiler chickens artificially infected with 1 × 104 sporulated oocysts of Eimeria tenella. Results revealed that pH values were decreased (p ˂ .05) in the infected group (IG) at 7 and 9 days post infection (dpi) than those at 5 dpi, although no significant differences between the non-infected group (NIG) and IG. Infection with E. tenella did not affect meat colour parameters except that redness was lower (p ˂ .05) than that in the NIG. Cooking loss was decreased (p ˂ .05) in NIG and IG at 7 and 9 dpi than that at 5 dpi. Thiobarbituric acid reactive substance (TBARS) value was increased (p ˂ .05) in IG at 7 dpi than 5 or 9 dpi and NIG (p ˂ .01). Total protein (TP) and total cholesterol (TC) were significantly lowered in IG at 5 dpi. Other blood parameter results showed decreases of glucose (GLU), triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL) in infected chicken at 7 dpi compared to those in the NIG without significance. However, albumin (ALB) level was decreased (p ˂ .01) in the IG compared with NIG at 5 dpi. Levels of aspartate aminotransferase (AST) were lowered (p < .05) in the IG than NIG at 7 dpi. Mineral contents of Ca, Mg, Cu, and Zn were lowered (p ˂ .01) in the IG than those in the NIG at 7 dpi. Based on these results indicate that a low dose of E. tenella has no impact on meat quality traits, fatty acids although it can slightly affect blood parameters and mineral contents.
The low dose of Eimeria tenella has no impact on meat quality traits and fatty acids of broiler meat.
A significant increase in some blood parameter is observed in infected broiler chickens at 9 dpi.
Mineral contents of Ca, Mg, Cu and Zn decreases in infected broiler meat at 7 dpi.
HIGHLIGHTS
Introduction
Avian coccidiosis is caused by apicomplexan parasites of the genus Eimeria (Pastor-Fernández et al. Citation2018) and one of the most important diseases which affect the poultry industry worldwide (Güven et al. Citation2013). There are specifically recognised seven different species of Eimeria causing haemorrhagic enteritis (E. tenella, E. brunetti, E. necatrix) with species-specific sites within the intestinal tract and malabsorptive (E. maxima, E. acervulina, E. mitis, and E. praecox) to the domestic chicken (Shirley et al. Citation2005). Earlier studies also reported that E. tenella, E. maxima, E. acervulina, and E. necatrix being the most prevalent and economically significant species (Awais et al. Citation2012; Thenmozhi et al. Citation2014). Each of these strains causes different symptoms with different invasion sites in the avian intestine. Eimeria tenella invades to the caecum. It is highly pathogenic, causing bloody caecal coccidiosis (Kaufmann Citation1996) which is a severe disease characterised by bleeding, high mortality, loss of weight gain, emaciation, and lost skin pigmentation. Its diagnosis is dependent upon the finding of intestinal lesions with prominent blood and often firm bloody cores accompanying clusters of large schizonts and oocysts. Exclusively in the broiler industry, coccidiosis causes an estimated global loss of more than US$3 billion annually (Dalloul and Lillehoj Citation2006). Therefore, coccidiosis is a very important disease affecting poultry production. However, many poultry farms in the world overlook slight contamination by Eimeria because low-level infection does not show a particular problem. Coccidiosis causes tissue damage in the intestinal tract, subclinical enteric infection, blood loss and subacute mortality (Güven et al. Citation2013). The infection also causes severe enteritis with haemorrhages, tissue damage, and some metabolic disturbances (Dede et al. Citation2002). However, Eimeria infected chickens are sold in the market despite 10 or more weeks of age and also reported that older birds would likely have acquired immunity to any Eimeria species found and unexpectedly high prevalence of oocysts observed in market-age meat chickens (Joseph et al. Citation2011). Although coccidiosis is an intestinal parasitic disease, most chickens have the potential to be infected by Eimeria. Since chicken is an important part of the human diet, the effects of coccidiosis on physicochemical characteristics of broiler chicken meat is very important. Blood serum analysis of Eimeria-infected chickens has shown a significant increase in alanine aminotransferase level and changes in total protein and cholesterol levels in the sera (Patra et al. Citation2009). Eimeria acervulina is known to affect intestinal nutrient absorption regarded as required for the broiler growth and metabolism (Allen Citation1988). Another earlier study by Witlock and Ruff (Citation1977) has demonstrated that this parasite also interrupts nutrient absorption, generating changes in protein, carbohydrate, lipid, and macro and micro mineral metabolism. Chickens with coccidiosis often show decreased body weight gain and less muscle mass compared to uninfected controls (Fetterer and Allen Citation2001). However, physiological and biochemical processes causing these alterations remain unclear. Poultry meat (chicken) quality, sanitation, and food stability are very important for consumers. Although broiler meats are main source of protein worldwide for human, limited researches have been done on the effects of coccidiosis on meat quality attributes of broilers. Therefore, the aim of this study was to investigate physicochemical characteristics, fatty acids, minerals and hematological alterations in broilers artificially infected with sporulated E. tenella oocysts.
Materials and methods
Ethics statement
The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Jeonbuk National University (CBNU 2018-097), Republic of Korea with the project number 318022-04-2-CG000. Animal care and handling are in compliance with the regulations of the IAEC Guidelines for the Euthanasia of Animals: 2015 Edition. The sampling procedures complied with the ‘Guidelines on Ethical Treatment of Experimental Animals’ (2015).
Experimental design and bird husbandry
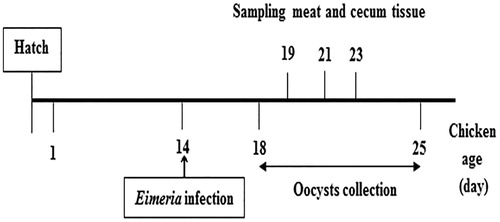
This study was divided into two parts. First, we tried to elucidate normal oocysts shedding patterns (Cha et al. Citation2018) of E. tenella in faeces until 11 days post-infection (dpi) and identify the Eimeria strain. Second, quality traits, fatty acids, minerals and hematological alterations were conducted at 5, 7 and 9 dpi (Figure ). The number of treatment, replication, pen (experimental unit), and broiler birds per pen in the research were 2, 4, 8, and 10, respectively. A total of one hundred commercial broilers male chicks (breed: Arbour acre) at one day of age with an average initial body weight 42.0 ± 0.20 g were purchased from a local hatchery (Bukeum, South Korea) and placed in 10 pens (4 pens for NIG, non-infected group, 4 pens for IG, infected group and 2 pens for oocysts shedding count) each containing 10 birds. The pens (0.90 × 0.60 × 0.60 m3) were equipped with feeder, nipple drinker, and birds in NIG and IG raised in two separate rooms provided the same environmentally controlled facilities to avoid cross-infection. They were reared in a coccidia-free environment under routine laboratory conditions. In this study, E. tenella (GenBank: FJ447468.1) oocysts were propagated and sporulated using standard procedures (Holdsworth et al. Citation2004). Birds of IG were infected at 14 days of age with 1 × 104 E. tenella oocysts (in 0.2 mL) by oral zonde. All the birds were fed the anticoccidial drug-free commercial diets (Daehan Feed Co., Ltd., Korea; Table ). The feed and water were provided ad libitum intake throughout the experimental period. The commercial feed was supplied at 9 AM and 6 PM.
Table 1. Chemical composition of commercial basal diet.
Confirmation of identity of Eimeria using PCR
The species of E. tenella was identified and confirmed by PCR using an assay directed towards the internal transcribed spacer (ITS-1) as described previously (Haug et al. Citation2007). All primers used in this study were designed from the ITS-1 region of ribosomal DNA. Primer sequences, amplicon sizes, and annealing temperatures are listed in Table . Purified oocysts were washed in PBS and disrupted using 1 mm glass beads with a vortexing speed of 3000 xg for 5 min (Cha et al. Citation2014) to ensure the maximum crushing of oocysts. DNA was extracted from the lysate using GeneAll DNA extraction kit (GeneAll, Seoul, South Korea) according to the manufacturer’s instructions. Amplification of species-specific ITS-1 sequences from genomic DNA was carried out in 20 µL reaction volume containing 2 µL 10x PCR Buffer, 2 µL deoxynucleotide triphosphate (dNTP), and 0.25 µL iMAX II DNA Polymerase (iNtRON Biotechnology). The amplification was performed in a Takara PCR Thermal Cycler (Takara Biotechnology Co. Ltd.) with the following cycling condition: an initial denaturation step at 95 °C for 5 min followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 65 °C for 30 s, and extension at 72 °C for 1 min. Finally, a prolonged extension step at 72 °C for 10 min was added to complete the amplification process. Each amplicon (5 µL) was mixed with 1 µL loading buffer and analysed by electrophoresis in 1.5% agarose gel stained with ethidium bromide. PCR products (amplicons) were identified by size using a 100 bp DNA ladder.
Table 2. PCR primers targeting the ITS-1 of E. tenella.
Histopathology
Intestinal sections (middle part of caecum for E. tenella) were collected and fixed in 10% formalin after slaughtering of chickens from each group. Collected tissues were then dehydrated in absolute alcohol, cleared in xylene, embedded in paraffin to prepare fine tissue blocks. Paraffin tissues were cut into sections with thickness of 5 µm. These sections were then subjected to routine haematoxylin and eosin staining (Lillie Citation1965).
Monitoring of oocysts shedding into faeces
Faeces of infected chickens were collected every day at 9 AM from 4 dpi to 11 dpi. Afterward, oocyst counting was conducted using a McMaster egg counting technique (Haug et al. Citation2006).
Sampling of meat
After Eimeria infection, 10 birds were randomly selected at 5, 7, and 9 dpi and were killed by exsanguination, de-feathered. These chickens were manually eviscerated and tissue samples (breast muscles) were dissected, collected, and freeze-dried for fatty acids and minerals analysis. A part muscle samples were stored at −20 °C for meat quality parameters and freeze-dried samples were stored at −70 °C until further analysis.
Meat colour, pH, cooking loss and 2-thiobarbituric acid reactive substances (TBARS) analyses
Three-colour (L*, a* and b*) coordinate measurements per sample were taken at three different locations on bloomed cut surfaces of meat sample blocks using a Minolta chromameter (CR-300, Minolta Camera Co., Ltd., Osaka, Japan), with a 1 cm aperture, illuminant D65 and a 2° viewing angle. According to the Commission International de l’Eclairage (CIE) system, the colour was expressed as CIE L*, a*, and b* (lightness, redness, and yellowness).
At 24 h post-mortem, pH values of breast muscles were measured in triplicates using a portable pH metre (pK21 pH metre, NWK-Binar GmbH, Landsberg, Germany). Afterwards, breast muscles were excised for further analyses.
Cooking loss was assessed by the gravimetric method of Honikel (Citation1998). Samples were weighed, put into plastic bags, and placed in an 80 °C water bath. When the internal temperature of 75 °C was reached, samples were cooled and weighed again. The difference in weight before and after boiling was expressed as the ‘percentage cooking loss’.
TBARS as a lipid oxidation value was measured by a modified method of Buege and Aust (Citation1978). Briefly, a meat sample of 2.5 g was filled into a 50 mL falcon test tube and homogenised with 7.5 mL deionised distilled water as well as 10 mL of thiobarbituric acid (TBA)/trichloroacetic acid (TCA) solution using a Homogeniser (T 25 digital ULTRA-TURRAX®, Germany) at 12,000 rpm for 20 s. To prevent fat oxidation, 25 μL of butylated hydroxyanisole (BHA) was added to each homogenised sample. The homogenate was filled up to 30 mL with distilled water (DW), vortexed, and heated in a 90 °C preheated water bath for 15 min to develop colour. These heated homogenised samples were cooled in ice-cold water for 15 min and centrifuged at 3000 × g for 15 min at 4 °C using a Hanil Supra 21 K Centrifuge Machine (Hanil Science Industrial Co., Ltd., Korea). After centrifugation, the absorbance of the resultant supernatant solution was taken in duplicates and averaged using a UV spectrophotometer (Multiskan Go, Thermo Scientific, USA) at 531 nm compared with a blank containing distilled water and a TBA/TCA solution. TBARS values were calculated by multiplying the average absorbance value with 5.88. Results are expressed as mg malondialdehyde (MDA)/kg of meat.
Blood of sampling and measurement
Blood was collected from the jugular vein just before slaughter. A published procedure (Ben Rayana et al. Citation2008) was used for blood collection and storage. We used Nova Stat Profile 8 CRT (NOVA Biomedical Corp, Waltham, MA) to measure glucose (GLU) levels. Serum was separated by centrifugation at 3000 xg for 10 min and stored at −20 °C until further biochemical analysis using a Hitachi 7020 (Hitachi, Tokyo, Japan) to measure total protein (TP), albumin (ALB), aspartate aminotransferase (AST), alanine aminotransferase (ALT), total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), uric acid (UA), creatinine (CRE), and blood urea nitrogen (BUN) levels.
Measurement of fatty acids
Fatty acids were measured by gas chromatography (GC). Freeze-dried meat samples (0.5 g) were added to 2 mL of boron-trifluoride in methanol and 2 mL of methanol in glass tubes. These tubes were capped with Teflon-lined caps to prevent loss of volume. Samples were then placed on a heating block at 80 °C. After 10 min, these tubes were vortexed individually every 5 min for 2 h. After 2 h of repeated vortex mixing, samples were allowed to cool at room temperature. After adding 3 mL of distilled water and 3 mL of hexane, tubes were capped again and mixed by vortexing for 15 s. After centrifugation (2,000 xg, 5 min) to separate phases, the supernatant was transferred to GC vial for analysis. GC was performed for 1 μL sample on a Shimadzu GC-2014 instrument (Shimadzu Co., Columbia, MD) using a FAMEWAX column (30 m × 0.32 mm i. d., 0.25 μm; column temperature, 250 °C) with nitrogen/air as carrier gas at a flow rate of 53.8 mL/min (split ratio 30:1). The initial temperature was 150 °C. It was increased to 250 °C with an equilibration time of 3 min.
Measurement of minerals
Minerals were measured using inductively coupled plasma mass spectrometry (ICP-MS). A 0.05 g sample in 600 μL of 70% nitric acid was added into a 15 mL conical tube and reacted in a fume hood. After reaction for 2 d, sample tubes were incubated at 80 °C for 5 h. DW was added to adjust the volume to 10 mL and the sample was diluted with 2% nitric acid from 101 to 104 for analysis. Mg, K, Ca, Fe, and Zn levels were analysed by ICP-MS (Agilent 7500a, Santa Clara, CA).
Statistical analysis
Data were analysed with ANOVA procedure followed by Duncan’s multiple range test using SAS software (SAS Version 9.3). Student’s t-test was used to analyse the statistical difference between non-infected and infected groups. All values are expressed as mean ± SE. Statistical significance was considered at p < .05 and p < .01.
Results and discussion
Eimeria amplicons identification by gel electrophoresis
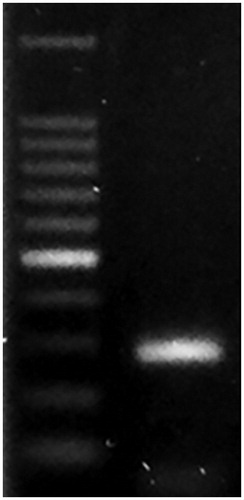
Specific identification of individual Eimeria species was confirmed by positive amplification of DNA from E. tenella using species-specific primers (Figure ).
Faecal oocysts shedding pattern
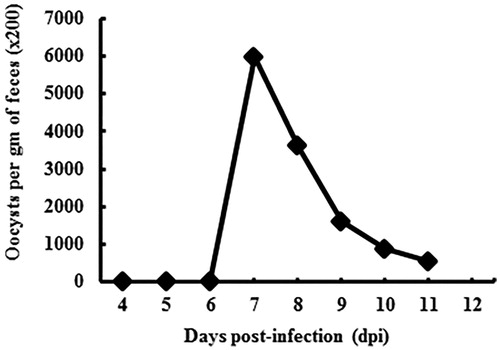
The minimum prepatent period of E. tenella in chickens has been reported to be 115 h (Long and Reid Citation1982). However, there is no precise information on the general faecal oocysts shedding pattern of Eimeria species. As shown in Figure , the peak faecal oocyst shedding point of E. tenella was at 7 dpi. Numbers of shedding oocysts continuously decreased after the peak-point, consistent with the results of previous studies (Cha et al. Citation2018).
Histological observation
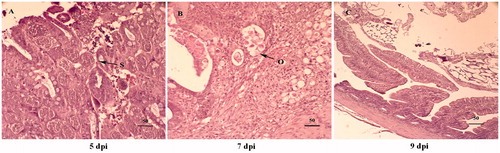
Based on histopathological observation, lesions in the caecum of chickens infected with E. tenella showed extensive oocyst infiltration, haemorrhages, and developmental stages of schizonts at 5 dpi (Figure ). Intracellular oocysts were observed at 7 dpi (Figure ). At 9 dpi, almost intact caecum tissues were observed (Figure ).
Physicochemical parameters of meat
The pH value was significantly (p ˂ .05) decreased in the IG as time went by during the experiment period, although no significant differences were observed between the NIG and IG. However, it was not significantly changed in the NIG during the experiment period (Table ). Eimeria tenella infection did not result in any significant differences in meat colour parameters in the IG at different time points, although the redness value was significantly decreased compared to that in the NIG. This result was consistent with the results of Chodová et al. (Citation2018). Rajput et al. Citation2014 reported that the coccidiosis reduced redness (a*) of meat. In a previous study, Mancini and Hunt Citation2005 showed that a decreased a* value, might be linked to the lowered mineral content in muscle tissue. Shaw et al. Citation2012 demonstrate that coccidiosis increased blood loss and decreased mineral absorption in the muscle which may lower a* value. Our study also revealed that decreased mineral contents in muscle tissue. In another study, Chodová et al. (Citation2018) also found that a* minus value in subclinical E. tenella infection by different concentration oocysts. However, limited information was available concerning the meat colour in chicken meat in relation to E. tenella infection. Cooking loss in the IG was significantly (p < .05) lower than other dpi, however it rapidly recovered at 9 dpi. Because infected chicken showed bleeding of caecum, moisture in chick meat might be decreased. Thiobarbituric acid reactive substance (TBARS) values (p < .01) were different between NIG and IG at 7 dpi. This means that infected chick meat at this time point is susceptible to lipid oxidation. At this time point, a large amount of Eimeria oocyst shedding and bloody faeces were also observed (Figure ). Abd El-Maksoud et al. (Citation2014) have reported that MDA level is significantly elevated in coccidia chicken. They also stated that coccidiosis could lead to oxidative stress of infected chicks expressed by alterations of antioxidant enzymes and increased MDA. The elevation in blood MDA concentration in infected chickens could be attributed to increased reactive oxygen species (ROS) production, resulting in lipid peroxidation. Another earlier study has demonstrated that blood MDA level is increased in infected birds probably due to oxidative stress after infection (Koinarski et al. Citation2005).
Table 3. Meat quality parameters and TBARS values of breast muscles of broiler chickens artificially infected with 1 × 104 sporulated oocysts of E. tenella.
Blood parameters
Blood parameter results are summarised in Table . Total protein and TC were significantly lowered in IG at 5 dpi. Levels of GLU, TG, HDL, and LDL in Eimeria infected chicken were decreased at 7 dpi compared to those in the NIG, showing no significant differences between the two groups. However, albumin level (p < .01) was decreased in the IG compared to that in the NIG at 5 dpi. Freeman (Citation1970) has suggested that decreased serum glucose levels could be attributed to anorexia because anorexia and intestinal tract inflammation can inhibit glucose absorption, resulting in the use of liver glycogen reserves. In severe infections, hypoglycaemia can occur due to inhibition of liver glycogenolysis. These results are almost similar to some previous studies showing that hypoglycaemia can occur in coccidiosis infected birds (Freitas et al. Citation2008; Patra et al. Citation2009). Marked reductions in protein and albumin levels after Eimeria infection have been reported by Patra et al. (Citation2009). They might be responsible for nutrient malabsorption, liver abnormalities, and marked haemorrhagic enteritis. Low level of cholesterol in Eimeria infected birds due to failure in its synthesis by the liver and absorbed in the intestine has also been reported (Machado Citation2002). Cholesterol is an important component of other molecules (Attia et al. Citation2017). Its reduction caused by parasitism can affect the synthesis of steroid hormones, Vit-D, and bile salts. Furthermore, ALT and AST levels were lowered in infected birds than those in the control group without significant differences. However, the AST level was significantly lowered (p < .05) in the IG than NIG at 7 dpi. These results are similar to results of Mondal et al. (Citation2011) showing that ALT levels are decreased in broilers infected with a field isolate of E. tenella. Another study has also indicated that ALT and AST levels are decreased in E. tenella and E. brunetti infected broilers (Adamu and Chaiwat Citation2013). On the other hand, these results were inconsistent with some previous studies demonstrating that coccidiosis produces a significant increase in ALT level (Biu et al. Citation2006; Patra et al. Citation2009), an indicator of hepatocellular damage. A similar response has been reported by Akpavie (Citation1998). Mondal et al. (Citation2011) have also revealed that plasma AST activity is increased in infected broilers infected with a lower dose of E. tenella. AST and ALT are enzymes found in erythrocytes. Thus, decrease in activities of serum ALT and AST reported in the current study might be associated with a high reduction of erythrocytes due to loss of blood in the gastrointestinal tract. Other blood parameters such as uric acid, creatine, or blood urea nitrogen did not show significant differences after infection. Furthermore, these inconsistent results appearing in the previous literature concerning the responsiveness to E. tenella infection might be resulted many factors such as different strains and doses in broiler.
Table 4. Blood parameters of broiler chickens artificially infected with 1 × 104 sporulated oocysts of E. tenella.
Fatty acid compositions
The results of fatty acid contents are presented in Table . Content of myristoleic acid in breast meat of the IG was lower than that of the NIG at 9 dpi (p < .05) and eicosapentaenoate was decreased significantly in the IG at 5 dpi (Table ). However, arachidonic acid content of the IG was also lower than that of the NIG at 5 dpi. Although medium-chain fatty acids have anticoccidial activity (Tan and Long Citation2012), fatty acids in breast meat showed no change overall. Up to date, literature related to fatty acid compositions to Eimeria infection in broiler is very rare. Results suggest that infection with E. tenella at a low dose cannot influence the fatty acid changes in broiler meat.
Table 5. Fatty acid compositions in breast muscles of broiler chickens artificially infected with 1 × 104 sporulated oocysts of E. tenella.
Mineral contents
The results of mineral contents are presented in Table . Levels of Ca, Cu, Zn (p ˂ .01) were decreased in the IG than those in the NIG at 7 dpi. Levels of Mg (p < .05) were also decreased in the IG than those in the NIG at 7 dpi. There were no significant differences in levels of Na, K, or Fe between infected and non-infected birds at 7 dpi. Changes in trace element metabolism during acute coccidiosis probably involve at least several events occurring simultaneously in parasitised-birds. Species of Eimeria and the dose of infective oocysts administered to birds are apparently important factors in determining the nature of the response of trace element metabolism to infection. The level of Cu in birds infected with E. tenella were markedly (p < .01) lower than those in the non-infected control. However, they are higher in chickens infected with E. acervulina without showing any toxic sign (Georgieva et al. Citation2011). This undoubtedly reflects the specific region of intestine parasitised in each case and the severity of the infection. Species of coccidia such as E. acervuline that invade the duodenum have been shown to depress the absorption of zinc and iron, although they increase the absorption of copper (Turk Citation1981; Southern and Baker Citation1983). Burke and Fenton (Citation1985) have established that the increase in lipid peroxidation in the liver is due to Zn deficiency. In our current study, lipid peroxidation was also increased in the muscle at 7 dpi. Since species of coccidia such as E. tenella can invade more distal regions of the intestine, they have been found to have only minor effects on iron absorption (Turk 1981). Thus, reduced absorption does not explain the lower iron status in chicks infected with E. tenella. Our results suggest that minerals like Ca, Mg, Cu, and Zn declined due to E. tenella infection; however previous literature reported that minerals changed in broiler meat infected by other species of Eimeria.
Table 6. Mineral contents in breast muscles of broiler chickens artificially infected with 1 × 104 sporulated oocysts of E. tenella.
Conclusions
In conclusion, infection with E. tenella at a low dose does not induce significant changes in physicochemical characteristics of broilers meat. Besides, cannot influence fatty acid changes in broiler meat. However, small doses of E. tenella caused significant reductions in some blood parameters among infected chickens at 9 dpi. Infected broiler chickens also showed significant decreases in the contents of Ca, Mg, Cu, and Zn at 7 dpi. Further researches are suggested with different doses of Eimeria infection up to market-age and continuous infection of Eimeria such as farm infection pattern on the tested parameters.
Ethical approval
All experiments were performed in accordance with guidelines approved by the Institutional Animal Care and Use Committee (IACUC) of Jeonbuk National University.
Disclosure statement
The authors declare that they have no conflict of interests.
Additional information
Funding
References
- Abd El-Maksoud HA, Afaf DAM, El-Badry MA. 2014. Biochemical effect of coccidia infestation in laying hen. Benha Vet Med J. 26(1):127–133.
- Adamu M, Chaiwat B. 2013. Protective effects of Moringastenopetala leaf supplemented diets on Eimeria tenella infected broiler chickens in DebreZeit, Central, Ethiopia. J Kasetsart. 47:398–406.
- Akpavie SO. 1998. The biology, field presentation and approach to enhanced diagnosis of coccidiosis. Paper presented at the Animal Care Konsult Services/NVMA Seminar, (ACKSNS’98); Abuja, Nigeria.
- Allen PC. 1988. The effect of Eimeria acervulina infection on plasma lipids and lipoproteins in young broiler chicks. Vet Parasitol. 30(1):17–30.
- Attia YA, Al-Harthi MA, Korish MM, Shiboob MM. 2017. Fatty acid and cholesterol profiles, hypocholesterolemic, atherogenic, and thrombogenic indices of broiler meat in the retail market. Lipids Health Disease. 16(40):1–11.
- Awais MM, Akhtar M, Iqbal Z, Muhammad F, Anwar MI. 2012. Seasonal prevalence of coccidiosis in industrial broiler chickens in Faisalabad, Punjab, Pakistan. Trop Anim Health Prod. 44(2):323–328.
- Ben Rayana MC, Burnett RW, Covington AK, D’Orazio P, Fogh-Andersen N, Jacobs E, Külpmann WR, Kuwa K, Larsson L, Lewenstam A, et al. 2008. IFCC guideline for sampling, measuring and reporting ionized magnesium in plasma. Clin Chem Lab Med. 46(1):21–26.
- Biu AA, Yusuf SD, Rabo JS. 2006. Use of neem (Azadirachtaindica) aqueous extract as a treatment for poultry coccidiosis in Borno state. Nigeria Afr Sci. 7:3.
- Buege JA, Aust SD. 1978. Microsomal lipid peroxidation. Methods Enzymol. 52:302–310.
- Burke JP, Fenton MR. 1985. Effect of a zinc-deficient diet on lipid peroxidation in liver and tumor subcellular membranes. Proc Soc Exp Biol Med. 179(2):187–191.
- Cha JO, Talha AFSM, Lim CW, Kim B. 2014. Effects of glass bead size, vortexing speed and duration on Eimeria acervulina oocyst excystation. Exp Parasitol. 138:18–24.
- Cha JO, Zhao J, Yang MS, Kim WI, Cho HS, Lim CW, Kim B. 2018. Oocyst-shedding patterns of three eimeria species in chickens and shedding pattern variation depending on the storage period of Eimeria tenella Oocysts. J Parasitol. 104(1):18–22.
- Chodová D, Tůmová E, Sládková K, Langrová I, Jankovská I, Vadlejch J, Čadková Z, Krejčířová R. 2018. Effects of subclinical Eimeria tenella infection on Pectoralis major muscle in broiler chickens. Italian J Anim Sci. 17(1):18–21.
- Dalloul RA, Lillehoj HS. 2006. Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Rev Vaccines. 5(1):143–163.
- Dede S, Deger Y, Alkan M, Cemek M. 2002. Oxidation products of nitric oxide and the concentration of antioxidant vitamins in parasitized goats. Acta Vet Br. 71:1–5.
- Fetterer RH, Allen PC. 2001. Eimeria tenella infection in chickens: effect on plasma and muscle 3-methylhistidine. Poult Sci. 80(11):1549–1553.
- Freeman BM. 1970. Carbohydrate stores in chickens infected with Eimeria tenella. Parasitology. 61(2):245–251.
- Freitas FLC, Almeida KS, Machado RZ, Machado CR. 2008. Lipid and glucose metabolism of broilers (Gallus gallus domesticus) experiment infected with Eimeria acervulina. Rev Bras Cienc Avic. 10(3):157–162.
- Georgieva NV, Gabrashanska M, Koinarski V, Yaneva Z. 2011. Zinc supplementation against Eimeria acervulina induced oxidative damage in broiler chickens. Vet Med Inter. 2011:1–7.
- Güven E, Beckstead RB, Kar S, Vatansever Z, Karaer Z. 2013. Molecular identification of Eimeria species of broiler chickens in Turkey. Ankara Üniv Vet Fak Derg. 60:245–250.
- Haug A, Thebo P, Mattsson JG. 2007. A simplified protocol for molecular identification of Eimeria species in field samples. Vet Parasitol. 146(1–2):35–45.
- Haug A, Williams RB, Larsen S. 2006. Counting coccidial oocysts in chicken faeces: a comparative study of a standard McMaster technique and a new rapid method. Vet Parasitol. 136(3–4):233–242.
- Holdsworth PA, Conway DP, McKenzie ME, Dayton AD, Chapman HD, Mathis GF, Skinner JT, Mundt HC, Williams RB. 2004. World Association for the Advancement of Veterinary Parasitology (WAAVP) guidelines for evaluating the efficacy of anticoccidial drugs in chickens and turkeys. Vet Parasitol. 121(3–4):189–212.
- Honikel KO. 1998. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 49(4):447–457.
- Joseph DO, Hunter DB, Barta JR. 2011. Molecular identification of Eimeria species infecting market-age meat chickens in commercial flocks in Ontario. Vet Parasitol. 178(3–4):350–354.
- Kaufmann J. 1996. Parasitic infections of domestic animals: a diagnostic manual. Basel (Swizerland): Birkhauser Verlag; p. 341–348.
- Koinarski V, Georgieva N, Gadjeva V, Petkov P. 2005. Antioxidant status of broiler chickens, infected with Eimeria acervulina. Revue de Medecine Veterinaire. 156(10):498–502.
- Lillie RD. 1965. General staining and mounting procedures. In: Histopathologic technic and practical istochemistry. 3rd ed. New York (NY): McGraw-Hill; p. 84–106.
- Long PL, Reid WM. 1982. A guide for the diagnosis of coccidiosis in chickens. Athens (GA): University of Georgia; p. 17.
- Machado CM. 2002. Crescimento do tecidoadiposo. In: Macari M, Furlan RL, Gonzales E, editors. Fisioligiaaviaraaplicada a frangos de corte. Jaboticabal (Brazil): FUNEP-UNESP. p. 375.
- Mancini RA, Hunt MC. 2005. Current research in meat color. Meat Sci. 71(1):100–121.
- Mondal DK, Chattopadhyay S, Batabyal S, Bera AK, Bhattacharya D. 2011. Plasma biochemical indices at various stages of infection with a field isolate of Eimeria tenella in broiler chicken. Vet World. 4:404–409.
- Pastor-Fernández I, Kim S, Billington K, Bumstead J, Marugán-Hernández V, Küster T, Ferguson DJP, Vervelde L, Blake DP, Tomley FM. 2018. Development of cross-protective Eimeria-vectored vaccines based on apical membrane antigens . Int J Parasitol. 48(7):505–518.
- Patra G, Rajkhowa TK, Ali MA, Tiwary JG, Sailo L. 2009. Studies on clinical, gross, histopathological and biochemical parameters in broiler birds suffered from Eimeri anecatrix infection in Aizawl District of Mizoram. Int J Poult Sci. 8 (11):1104–1106.
- Rajput N, Ali S, Naeem M, Khan MA, Wang T. 2014. The effect of dietary supplementation with the natural carotenoids curcumin and lutein on pigmentation, oxidative stability and quality of meat from broiler chickens affected by a coccidiosis challenge. Br Poult Sci. 55(4):501–509.
- Shaw AL, Macklin KS, Blake JP. 2012. Phytase supplementation in a reduced calcium and phosphorus diet fed to broilers undergoing an Eimeria challenge. J Poult Sci. 49:178–182.
- Shirley MW, Smith AL, Tomley FM. 2005. The biology of avian Eimeria with an emphasis on their control by vaccination. Adv Parasit. 60:285–330.
- Southern LL, Baker DH. 1983. Eimeria acervulina infection and the zinc-copper interrelationship in the chick. Poult Sci. 62(2):401–404.
- Tan GH, Long K. 2012. Preliminary study of anticoccidial activity of medium chain fatty acids (MCFA) and their corresponding monoglycerides on broiler chicken coccidiosis. Int J Biotechnol Wellness Industr. 1:134–141.
- Thenmozhi V, Veerakumari L, Raman M. 2014. Preliminary genetic diversity study on different isolates of Eimeria tenella from South India. Int J Adv Vet Sci Technol. 3(1):114–118.
- Turk DE. 1981. Coccidial infections and iron absorption. Poult Sci. 60(2):323–326.
- Witlock DR, Ruff MD. 1977. Comparison of the intestinal surface damage caused by Eimeria mivati, E. necatrix, E. maxima, E. brunetti and E. acervulina by scanning electron microscopy. J Parasitol. 63(2):193–199.