Abstract
To evaluate the protective effects of dietary resveratrol (RES) supplementation against oxidative stress in piglets, a total of 30 weaned piglets (aged 28 + 2 d) were randomly divided into five treatments (n = 6): C group (basal diet), NC group (basal diet + diquat challenge), and basal diet containing either 10, 30, or 90 mg/kg of RES administered to piglets injected with diquat. The trail lasted 21 d. On day 15 after the initiation of treatment, piglets were intraperitoneally injected with 10 mg/kg body weight (BW) diquat or the same amount of saline. The feed intake and faecal consistency were recorded daily. Blood samples were collected for antioxidative, immune parameters and cytokines analysis. RES addition showed a negative tendency (p = .052) on faecal score during the pre-challenge period. Diquat challenge reduced the growth performance and induced oxidative stress and inflammatory responses of piglets (p < .05). However, the growth performance was improved, and faecal score was decreased in 90 mg/kg RES groups (p < .05). Compared with the NC group, addition of 90 mg/kg RES increased activities of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and total antioxidant capacity (T-AOC), and reduced malonaldehyde (MDA), cortisol and adrenocorticotropic hormone (ATCH) concentration (p < .05). Moreover, supplementation with 90 mg/kg RES increased serum concentration of interleukin-4 (IL-4) (p < .05) but decreased serum tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) (p < .05). In conclusion, the study indicated that RES showed overwhelming advantage in reducing diarrhoea and attenuating the oxidative stress of piglets challenged with diquat.
Dietary resveratrol supplementation improved growth performance and reduce diarrhoea of piglets challenged with diquat.
The protective effects of dietary resveratrol supplementation against oxidative stress and inflammation in diquat-challenged piglets were confirmed.
The experimental results provide theoretical and practical value for further research on the antioxidant mechanisms of resveratrol in weaned piglets.
Highlights
Introduction
Piglets weaning stress can lead to disturbance of free radical metabolism and antioxidant system, thereby causing the occurrence of oxidative stress and changes in the antioxidant defense capacity, including decreased activity of antioxidant enzymes (Han et al. Citation2009; Han et al. Citation2011; Yin et al. Citation2014). Oxidative stress refers to the imbalance between the pro-oxidant and antioxidant systems, which often lead to excessive production of reactive oxygen species (ROS) (Bhat et al. Citation2015). ROS overproduction contributes to damage of cellular lipids, proteins, and nucleic acids, thus compromising cell function and triggering cell death (Zorov et al. Citation2014). Oxidative stress may be involved in the development of many kinds of chronic diseases, such as inflammatory bowel and gastrointestinal mucosa diseases in human and animals (Miller et al. Citation1993; Bhattacharyya et al. Citation2014). For piglets, oxidative stress may induce growth retardation, intestinal dysfunction, and even death, which resulted in serious economic losses (Zhu et al. Citation2012). Diquat, a bipyridyl herbicide, can stimulate the cellular free radical species produce by action of molecular oxygen, which cause oxidative stress (Yin et al. Citation2015b). Diquat challenge has been widely used in vivo model of oxidative stress. Diquat has been reported to affect growth performance and nutritional metabolism (Lv et al. Citation2012; Cao et al. Citation2018). Thus, we utilised a well-established oxidative stress model by injecting diquat in this study.
Intake of dietary antioxidant nutrients, including vitamin E, vitamin C, selenium, and polyphenols, can improve antioxidant defense capacity, which has been considered a feasible way to attenuate oxidative stress (Lykkesfeldt and Svendsen Citation2007; Kim et al. Citation2012; Chen et al. Citation2016). Polyphenols naturally occurring in plants have been reported to exhibit powerful antioxidant activities in multiple in vitro and in vivo studies (Pandey and Rizvi Citation2009). Resveratrol (trans-3,4,5-trihydroxystilbene, RES) is a natural polyphenolic compound isolated from grapes, berries, peanuts and herbal medicines. Many studies have reported that RES exerted efficient antioxidative (Cheng et al. Citation2015; Cao et al. Citation2019) and anti-inflammatory capacity (Cullberg et al. Citation2014; Gan et al. Citation2019). RES can attenuate mitochondrial dysfunction in the liver of intrauterine growth retarded piglets by improving mitochondrial biogenesis, ATP production, and redox status (Zhang et al. Citation2017). Administration of RES during pregnancy and lactation could improve the antioxidant status of sows and piglets (Meng et al. Citation2018). In addition, Gan et al. (Citation2019) reported that RES can alleviate intestinal inflammation by inhibiting expression of pro-inflammatory cytokine and increasing the secretion of immunoglobulin. Moreover, RES has been reported to attenuate intestinal oxidative stress and improve growth performance of piglets induced by diquat (Cao et al. Citation2019).
Oxidative stress is complex and tissue-dependent, but it is considered useful to measure oxidative stress biomarkers and antioxidant enzymes to assess the body's ‘oxidation state’ (Bernabucci et al. Citation2002). To our knowledge, there are no available data assessing the causal link between resveratrol and the improvement in piglets’ health status. Therefore, the objective of this study was to determine whether dietary RES supplementation can attenuate oxidative stress and inflammation of piglets challenged with diquat.
Materials and methods
Animal, diets, and experimental design
The study was approved by the Animal Care and Use Committee of Hainan University (Haikou China). A total of 30 healthy piglets (Duroc × Landrace × Large Yorkshire, 7.25 ± 0.13 kg), weaned at 28 ± 2 d, were randomly divided into five treatments with 6 replication pens per treatment and 1piglets per pen. The treatments were: control group (basal diet), NC group (basal diet + diquat challenge), and RES group (basal diet containing either 10, 30 or 90 mg/kg of RES + diquat challenge. RES was purchased from Shanghai Yuanye Co. Ltd. (Shanghai, China) and thoroughly well-mixed with the basal diet. Diquat was purchased from Sigma-Aldrich (St. Louis, MO, USA) and given at a dose of 10 mg/kg BW referring to previous studies (Xu et al. Citation2018). Diquat was dissolved in 0.9% NaCl solution and filter-sterilized. The concentration of the diquat solution was 10 mg/ml. The basal diets were formulated according to the criteria recommended by NRC (NRC Citation2012). Piglets were housed individually in nursery room with the environment temperature maintained at 25–29 °C. All animals had free access to water and diets. The ingredient and chemical compositions of diets are shown in . The experiment lasted for 21 d. At 08:00 on day 15, the piglets in NC and RES groups were intraperitoneally injected with 10 mg/kg BW of diquat, while the piglets in control group were intraperitoneally injected with the same amount of sterile saline (0.9%).
Growth performance and diarrhoea rate determination
Piglets were weighed before the morning feeding on day 1, 15 and 22. Feed intake was recorded daily. Average daily gain (ADG), average daily feed intake (ADFI) and feed/gain ratio (F/G) were determined for 1–14 d and 15–21 d, respectively. The data of ADG, F/G and faecal score was calculated according to the following formula. ADG= (final BW – initial BW)/(experiment days × amounts); F/G = Average feed intake/average daily gain; Faecal score = the total scores of diarrhoea piglets in each group/(experiment days × total number of piglets in each group). Faecal consistency of each pig was daily observed and scored according to the study reported by He et al. (Citation2017). The criterion of diarrhoea was scored as below: 1, hard faeces; 2, normal consistency of faeces formed; 3, soft faeces; 4, very soft and containing a small amount of water-like faeces; 5, semisolid containing more than half water-like faeces; 6, watery faeces.
Blood sample collection and analysis
Blood samples were collected from the anterior vena cava into nonheparinized tubes at 5 h after diquat injection and at 08:00 am on the day 7 after injection. After 20 min standing at room temperature, serum samples were obtained, centrifuged at 3000 × g for 15 min at 4°C and then stored at −20°C until assay. The parameters of antioxidant, including superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), total antioxidant capacity(T-AOC) and malonaldehyde (MDA) in serum were assayed by using assay kits according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). In brief, SOD, T-AOC, and GSH-Px were analysed by xanthine oxidase-xanthine reaction method, ferric-reducing/antioxidant power reaction method, and reduced glutathione method, respectively. MDA capacity was assayed by 2-thiobarbituric acid method.
Serum concentration of cortisol, adrenocorticotropic hormone (ATCH), tumour necrosis factor-α (TNF-α), interferon-γ (INF-γ), interleukin-2(IL-2), interleukin-4 (IL-4) interleukin-6 (IL-6), interleukin-1β (IL-1β), immunoglobulin G (IgG), immunoglobulin A (IgA), and immunoglobulin M (IgM) were tested by using porcine ELISA Kits (Shanghai Fankel Industrial Co., Ltd, Shanghai, China) according to the manufacturer’s instructions. In brief, 50 μL of standard and sample diluent was added to antibody-coated 96-well plates, covered with an adhesive strip and then incubated for 30 min at 37 °C. The samples were decanted and washed 5 times with wash buffers. and then 50 μl of horseradish peroxidase (HRP)-conjugate reagent were added to each well, then incubated for 30 min at 37 °C. After being washed manually 5 times, 50 μL of chromogen solution A and chromogen solution B were added to each well, mixed gently and incubated in a dark room for 10 min at 37 °C. After then, 50 μL of Stop solution was added, and within 15 min, the optical density was read at 450 nm using a microtiter plate reader (ReadMax-1900, Shanghai Shanpu Biological Technology Co., Ltd, Shanghai, China). Standard curves were prepared and were used to quantify corresponding indexes levels of samples.
Statistical analysis
Statistical analysis was performed by the one-way ANOVA procedure of SPSS 17.0. Differences among treatments were compared using Duncan’s multiple range tests. A value of p < .05 was considered statistically significant. .05 < p < .10 was considered a tendency. Results were expressed as mean ± standard error
Table 1. Composition and nutrient levels of the basal diet (air-dry basis) (%).
Results
Growth performance and faecal score in weaned piglets
The experiment consisted of pre-challenge period (1–14 d) and post-challenge period (15–21 d). summarises the growth performance of the piglets. During the pre-challenge period, RES supplementations had no influence on the ADG, ADFI, and F/G ratio (p > .05). However, RES supplementation showed a linear negative tendency (p = .052) on faecal score compared with the C group.
Table 2. Effect of dietary resveratrol on growth performance of weaned piglets.
During the post-challenge period (15–21 d), compared with the piglets in control group, diquat challenge reduced ADG, ADFI, and increased F/G and faecal score (p < .05). However, supplementation with 30 mg/kg or 90 mg/kg RES increased ADG and ADFI compared with the NC group (p < .05). The F/G ratio and faecal score in 90 mg/kg RES group were lower than that in NC group (p < .05). There was no difference of F/G ratio and faecal score between C and RES-90 treatments (p > .05).
Serum ACTH and cortisol
At 5 h post-injection, piglets in NC group had higher levels of serum ACTH than that of the C group (p < .05). However, dietary RES supplementation decreased serum ACTH levels in comparison with NC group. At 7 d post-injection, piglets in NC group had higher serum ACTH levels than C and RES groups. No differences (p > .05) of ACTH were observed among the C, RES-30 and RES-90 groups (Figure ).
Figure 1. Effect of dietary resveratrol supplementation and diquat challenge on levels of ACTH (A) and Cortisol (B) in piglets. 1C group: basal diet without diquat; NC group: basal diet + diquat; RES-10 group: basal diet +10 mg/kg resveratrol + diquat; RES-30 group: basal diet +30 mg/kg resveratrol + diquat; RES-90 group: basal diet +90 mg/kg resveratrol + diquat. Values are expressed as means ± SE (n = 6). a,b,cMeans values with different letters indicate significantly difference (p < .05).
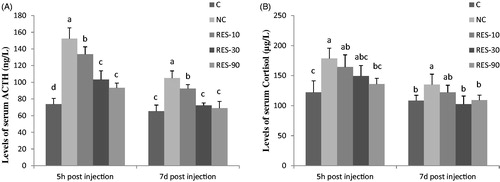
At 5 h and 7 d post-injection, piglets in NC group increased the serum cortisol levels compared with the C group (p < .05), but supplementation with 90 mg/kg RES at 5 h post-injection (p < .05) and supplementation with 30 mg/kg or 90 mg/kg RES at 7 d post-injection (p < .05) significantly decreased serum cortisol levels compared with the NC group. Moreover, there were no differences (p > .05) of cortisol among the C, RES-30 and RES-90 groups (Figure ).
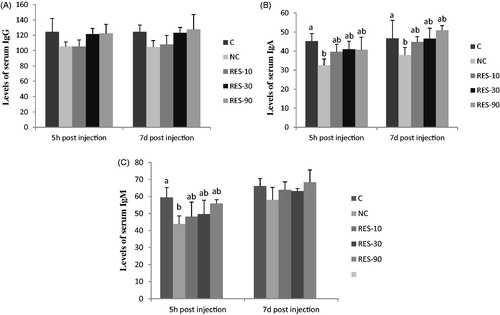
Serum immune parameters
As shown in Figure , the levels of serum IgG at 5 h and 7d post-injection and serum IgM at 7d post-injection were not affected by treatments (p > .05). But piglets in NC group had lower levels of serum IgM at 5 h post-injection than that of C group (p < .05). At 5 h and 7d post-injection, the levels of serum IgA in NC group were lower than C group (p < .05). No significant differences were observed in Ig A and IgM among the C and RES groups (p > .05) (Figure ).
Figure 2. Effect of dietary resveratrol supplementation and diquat challenge on levels of serum IgG(A), IgA(B) and IgM (C) in piglets. 1C group: basal diet without diquat; NC group: basal diet + diquat; RES-10 group: basal diet +10 mg/kg resveratrol + diquat; RES-30 group: basal diet +30 mg/kg resveratrol + diquat; RES-90 group: basal diet +90 mg/kg resveratrol + diquat. Values are expressed as means ± SE (n = 6). a,b,cMeans values with different letters indicate significantly difference (p < .05).
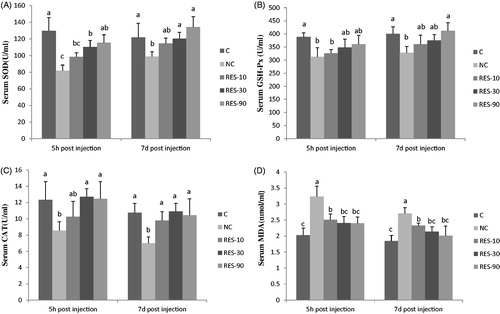
Serum antioxidant parameters
As shown in Figure , piglets in NC treatment had lower serum activity of SOD, GSH-Px, and CAT at 5 h and 7d post-injection than that of the C group (p < .05). No significant differences were observed in serum SOD, GSH-Px, and CAT among the C, RES-30 and RES-90 groups (p > .05), except that serum SOD in RES-30 group was lower than C group at 5 h post-injection(p > .05).The concentrations of serum MDA in NC group was higher than C and RES groups, but no significant differences were observed in serum MDA among the C, RES-30 and RES-90 groups (p > .05).
Figure 3. Effect of dietary resveratrol supplementation and diquat challenge on serum antioxidant enzymes activities and MDA content in piglets. 1C group: basal diet without diquat; NC group: basal diet + diquat; RES-10 group: basal diet +10 mg/kg resveratrol + diquat; RES-30 group: basal diet +30 mg/kg resveratrol + diquat; RES-90 group: basal diet +90 mg/kg resveratrol + diquat. Values are expressed as means ± SE (n = 6). a,b,cMeans values with different letters indicate significantly difference (p < .05).
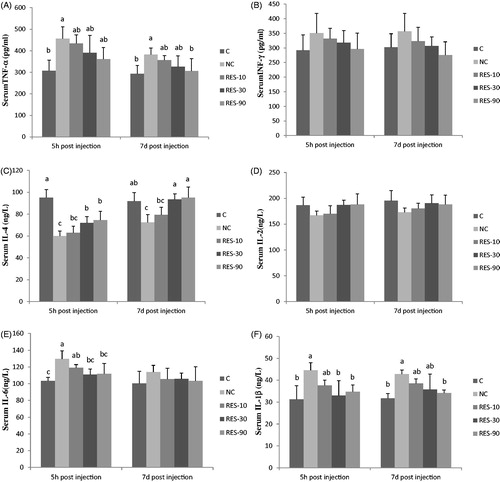
Serum profiles of cytokines
As shown in Figure. , piglets in NC treatment had higher serum concentration of TNF-α, IL-1ß at 5 h and 7d post-injection, and IL-6 at 5 h post-injection than those in C group (p < .05). No significant differences were observed in serum TNF-α, IL-1ß and IL-6 among the C and RES groups (p > .05), except that serum IL-6 in RES-10 group was higher than C group at 5 h post-injection (p > .05). The serum concentration of IL-4 in NC group was lower than that in C group, but no differences were observed in serum IL-4 among the C, RES-30 and RES-90 groups (p > .05). The serum concentration of INF-γ, IL-2, at 5 h and 7 d post-injection were not affected by treatments (p > .05).
Figure 4. Effect of dietary resveratrol supplementation and diquat challenge on serum content of cytokines in piglets. 1C group: basal diet without diquat; NC group: basal diet + diquat; RES-10 group: basal diet +10 mg/kg resveratrol + diquat; RES-30 group: basal diet +30 mg/kg resveratrol + diquat; RES-90 group: basal diet +90 mg/kg resveratrol + diquat. Values are expressed as means ± SE (n = 6). a,b,cMeans values with different letters indicate significantly difference (p < .05). TNF-α, tumour necrosis factor-α; IFN-γ, interferon-γ; IL-6, interleukin-6; IL-1β, interleukin-1β; IL-2, interleukin-2; IL-4, interleukin-4.
Discussion
In the current experiment, oxidative stress piglet models induced by diquat were used to evaluate the effects of RES supplementation on attenuating oxidative stress. Diquat challenge reduced growth performance and increased faecal score, which agreed with published report (Xu et al. Citation2018; Guo et al. Citation2020). It probably due to diquat-induced oxidative stress disrupted intestinal barrier and caused mitochondrial dysfunction in pigs (Cao et al. Citation2018). RES supplementations had no effect on growth performance during the pre-challenged period, except for a linear negative tendency on faecal score was observed. Similar to our study, the results in Zhang et al. (Citation2019) indicated that dietary supplementation with 600 mg/kg RES had no effect on growth performance of growing-finishing pigs. However, the effect of RES on growth performance and faecal score became evident after diquat injection. Addition of 90 mg/kg RES significantly decreased F/G ratio and faecal score. Previous study has reported that RES supplementation improved the feed efficiency in suckling piglets (Zhang et al; Citation2017). Cao et al. (Citation2019) also reported that RES addition reversed the decline of growth performance and attenuated intestinal damage in diquat challenged piglets. Moreover, studies have confirmed RES can protect the intestinal barrier function due to its antioxidant capacity (Ozkan et al. Citation2009). Thus, the result could be explained that RES supplementation improving the antioxidant defense capacity, thus conducive to maintaining the intestinal health after diquat challenge, and finally improving growth performance. The result of decreasing faecal score by RES supplementation is similar with another study reported by Cui et al. (Citation2018), who reported that resveratrol could alleviate diarrhoea in piglets induced by rotavirus infection.
The hypothalamic–pituitary–adrenal (HPA) axis is acted as the major system for stress regulation (Fazio and Ferlazzo Citation2003). Under the circumstance of stress, the hypothalamus secretes corticotrophin-releasing factor, resulting in a rapid release of ACTH from hypophysis. Elevated ACTH stimulated the secretion of glucocorticoids from the adrenal cortex into the circulation of animals (Kudielka et al. Citation2005). Cortisol, the main glucocorticoid in HPA axis, is an effective stress indicator in animals. The elevation of cortisol may suppress some types of immune functions (Puppe et al. Citation1997; Mormède et al. Citation2007). The present study indicated that diquat challenge significantly increased the concentration of ACTH and cortisol at 5 h and 7d post-injection. However, this up-regulation condition was suppressed markedly by RES supplementation. The dose of 90 mg/kg RES showed the best results, suggesting that the proper dose of RES could alleviate stress. Previous studies have shown that supplementation with RES can reduce the level of ACTH and beneficially protect broilers against heat stress (Zhang et al. Citation2017; He et al. Citation2019). Liang et al. (Citation2019) demonstrated that RES inhibited the production and secretion of cortisol in LPS challenged lambs. Studies in rodents also illustrated that RES alleviated heat stress by decreasing serum cortisol level (Cheng et al. Citation2019), which suggests that RES was not species specific in its functions and could be most likely to be applied to all animals.
Immunoglobulin, including IgG, IgM and IgA, are important components of the of the body's immune system in animals. Immunoglobulin participate in the regulation of immunity and defense of infection in organisms (Liu and Zhang Citation1989). In the present study, diquat challenge significantly reduced the serum IgA content at 5 h and 7d post-injection, and the IgM content at 5 h post-injection compared with control group. The similar results were reported by Wen et al. (Citation2020), who found that diquat-induced oxidative stress triggers immune response by decreasing the content of IgM and IgG. However, no significant changes in the serum IgG, IgA, and IgM were observed among the RES and diquat challenge groups, in contrast to the reports showing that the serum IgG content was significantly increased in piglets fed with 150 mg/kg or 300 mg/kg resveratrol (Chen et al. Citation2019). Thus, it can be suggested that different humoral immune response to RES addition may depend on the supplemental dose of RES and duration of the experiment, as well as animal health status and management conditions.
Oxidative stress caused by overproduction of endogenous ROS, could lead to the oxidation of biomolecules (e.g. proteins, lipids, and DNA), (Bachowski et al. Citation1997; Mayne Citation2003). There are antioxidant enzymes, such as SOD, GSH-Px, and CAT, which contribute to the antioxidant capacity in the body (Matés et al. Citation1999). Decreased activity of these enzymes can produce excessive free radicals and peroxides, and finally lead to cellular membrane damage (Valavanidis et al. Citation2009). MDA, one of the final products of lipid peroxidation as well as an excellent oxidative stress marker, is equipped with cellular toxicity and increased MDA can be explained as the result of cellular membrane damage (Nielsen et al. Citation1997; Niedernhofer et al. Citation2003). According to the previous studies, diquat could stimulate a rapid increase in ROS production and disrupt antioxidative enzyme system (; Cao et al. Citation2018). In the present study, piglets in NC group had significantly lower activities of serum SOD, GSH-Px, and CAT, and higher concentration of MDA than those in the control group at 5 h and 7 d post-injection, indicating that the antioxidative capacity of piglets was damaged. RES supplementation was proved to greatly improve the antioxidant capacity of diquat challenged piglets. The antioxidase activities (i.e. SOD and CAT) were increased, while levels of MDA were decreased by dietary supplementation with 30 mg/kg or 90 mg/kg RES at 5 h and 7 d post-injection. The serum activity of GSH-Px was also increased in piglets fed with 90 mg/kg RES diet than those piglets fed with the NC diet at 7 d post-injection. These results are analogous to those reported previously in sows (Meng et al. Citation2018), broilers (He et al. Citation2019), and rats (Cheng et al. Citation2019). The observed rise in antioxidant indices induced by RES supplementation may be explained as an important reason for the improvement in growth performance of piglets challenged by diquat.
Many researchers have demonstrated that oxidative stress is closely related to inflammatory (Yuan et al. Citation2017). Inflammatory cytokines, such as IL-1β, IL-6, IL-4, INF-γ and TNF-α, are vital agents in the inflammation. In fact, TNF-α can produce free radicals, leading to oxidative stress (Starke et al. Citation2014). Besides, TNF-α, serves as the first signal to induce other pro-inflammatory cytokines production, including IL-1β. IL-1β contributes to the control of several inflammatory actions, mainly by increasing pro-inflammatory genes expression and the secondary cytokines production (Bry and Lappalainen Citation2006). In contrast, IL-4 is thought to suppress or decrease the inflammatory responses by inhibiting the secretion of pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α (Rubinstein and Rubinstein Citation1990). Previous studies have demonstrated that RES supplementation, under stress conditions, displays beneficial activity against inflammatory responses in piglets (Gan et al. Citation2019), rats (Cheng et al. Citation2019) and lambs (Liang et al. Citation2019) by modulating the production of cytokines. In the present study, injecting diquat significantly increased the levels of serum IL-1β, IL-6, and TNF-α, whereas decreased the levels of serum IL-4 in piglets. The administration of RES at 30 and 90 mg/kg led to an evident reduction in the levels of IL-1β, IL-6, and TNF-α, which indicated that RES alleviated diquat-induced inflammation and regulated immune abnormalities in weaned piglets.
Conclusions
Our results indicated that daily administration of diets with 30-90 mg/kg resveratrol are able to counterbalance the adverse effects resulting from an administration of diquat at a dosage of 10 mg/kg BW. It demonstrated that RES might serve as an effective additive to alleviate acute oxidative stress and inflammatory response in piglets. Further studies will be conducted to explore long-term effects of RES in pigs and elucidate its molecular mechanisms of the antioxidant property.
Acknowledgements
The assistance of all staff at Animal Nutrition Laboratory in Tropical Crops Genetic Resources Institute of CATAS, and Laboratory of Tropical Animal Breeding, Reproduction, and Nutrition, at Hainan University are highly appreciated.
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Bachowski S, Kolaja KL, Xu Y, Ketcham CA, Stevenson DE, Walborg EF, Klaunig JE. 1997. Role of oxidative stress in the mechanism of dieldrin's hepatotoxicity. Ann Clin Lab Sci. 27(3):196–209.
- Bernabucci U, Ronchi B, Lacetera N, Nardone A. 2002. Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season. J Dairy Sci. 85(9):2173–2179.
- Bhat AH, Dar KB, Anees S, Zargar MA, Masood A, Sofi MA, Ganie SA. 2015. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed Pharmacother. 74:101–110.
- Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. 2014. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 94(2):329–354.
- Bry K, Lappalainen U. 2006. Pathogenesis of bronchopulmonary dysplasia: the role of interleukin 1beta in the regulation of inflammation-mediated pulmonary retinoic acid pathways in transgenic mice. Semin Perinatol. 30(3):121–128.
- Cao S, Shen Z, Wang C, Zhang Q, Hong Q, He Y, Hu C. 2019. Resveratrol improves intestinal barrier function, alleviates mitochondrial dysfunction and induces mitophagy in diquat challenged piglets. Food Funct. 10(1):344–354.
- Cao S, Wu H, Wang C, Zhang Q, Jiao L, Lin F, Hu C. 2018. Diquat-induced oxidative stress increases intestinal permeability, impairs mitochondrial function, and triggers mitophagy in piglets. J Anim Sci. 96(5):1795–1805.
- Chen J, Han JH, Guan WT, Chen F, Wang CX, Zhang YZ, Lv YT, Lin G. 2016. Selenium and vitamin E in sow diets: effect on antioxidant status and reproductive performance in multiparous sows. Anim Feed Sci Tech. 221:111–123.
- Chen X, Zeng Z, Huang Z, Chen D, He J, Chen H, Yu B, Yu J, Luo J, Luo Y, Zheng P. 2019. Effects of dietary resveratrol supplementation on immunity, antioxidative capacity and intestinal barrier function in weaning piglets. Anim Biotechnol. 2019:1–6.
- Cheng L, Jin Z, Zhao R, Ren K, Deng C, Yu S. 2015. Resveratrol attenuates inflammation and oxidative stress induced by myocardial ischemia-reperfusion injury: role of Nrf2/ARE pathway. Int J Clin Exp Med. 8(7):10420–10428.
- Cheng K, Song Z, Li S, Yan E, Zhang H, Zhang L, Wang C, Wang T. 2019. Effects of resveratrol on intestinal oxidative status and inflammation in heat-stressed rats. J Therm Biol. 85:102415.
- Cullberg KB, Foldager CB, Lind M, Richelsen B, Pedersen SB. 2014. Inhibitory effects of resveratrol on hypoxia‐induced inflammation in 3T3‐L1 adipocytes and macrophages. J Funct Foods. 7(1):171–179.
- Cui Q, Fu Q, Zhao X, Song X, Yu J, Yang Y, Sun K, Bai L, Tian Y, Chen S, et al. 2018. Protective effects and immunomodulation on piglets infected with rotavirus following resveratrol supplementation. PLoS One. 13(2):e0192692.
- Fazio E, Ferlazzo A. 2003. Evaluation of stress during transport. Vet Res Commun. 27:519–524.
- Gan Z, Wei W, Li Y, Wu J, Zhao Y, Zhang L, Wang T, Zhong X. 2019. Curcumin and resveratrol regulate intestinal bacteria and alleviate intestinal inflammation in weaned piglets. Molecules. 24(7):1220.
- Guo J, He L, Li T, Yin J, Yin Y, Guan G. 2020. Antioxidant and anti-inflammatory effects of different zinc sources on diquat-induced oxidant stress in a piglet model. Biomed Res Int. 2020:3464068.
- Han J, Shuvaev VV, Muzykantov VR. 2011. Catalase and superoxide dismutase conjugated with platelet-endothelial cell adhesion molecule antibody distinctly alleviate abnormal endothelial permeability caused by exogenous reactive oxygen species and vascular endothelial growth factor. J Pharmacol Exp Ther. 338(1):82–91.
- Han YH, Moon HJ, Br Y, Kim SZ, Kim SH, Park WH. 2009. The effect of MAPK inhibitors on arsenic trioxide-treated Calu-6 lung cells in relation to cell death. ROS and GSH levels. Anticancer Res. 29:3837–3844.
- He L, Huang N, Li H, Tian J, Zhou X, Li T, Yao K, Wu G, Yin Y. 2017. AMPK/α-ketoglutarate axis regulates intestinal water and ion homeostasis in young pigs. J Agric Food Chem. 65(11):2287–2298.
- He S, Li S, Arowolo MA, Yu Q, Chen F, Hu R, He J. 2019. Effect of resveratrol on growth performance, rectal temperature and serum parameters of yellow-feather broilers under heat stress. Anim Sci J. 90(3):401–411.
- Kim JC, Hansen CF, Mullan BP, Pluske JR. 2012. Nutrition and pathology of weaned pigs: nutritional strategies to support barrier function in the gastrointestinal tract. Anim Feed Sci Technol. 173(1–2):3–16.
- Kudielka BM, Kirschbaum CK, Kirschbaum C. 2005. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 69(1):113–132.
- Liang Y, Zhou J, Ji K, Liu H, Degen A, Zhai M, Jiao D, Guo J, Zhao Z, Yang G. 2019. Protective effect of resveratrol improves systemic inflammation responses in LPS-injected lambs. Animals (Basel). 9(11):872.
- Liu WJ, Zhang YY. 1989. Studies on immunopathology of porcine epizootic diarrheaIV. Observations on IgA, IgM and IgG -producting cell in lamina propria of gastrointestinal tract and titers of IgA IgM and IgG in gastrointestinal secretions and serum. Bull Vet Coll PLA. 3 (9):259–264.
- Lv M, Yu B, Mao XB, Zheng P, He J, Chen D. 2012. Responses of growth performance and tryptophan metabolism to oxidative stress induced by diquat in weaned pigs. Animal. 6(6):928–934.
- Lykkesfeldt J, Svendsen O. 2007. Oxidants and antioxidants in disease: oxidative stress in farm animals. Vet J. 173(3):502–511.
- Matés JM, Pérez-Gómez C, Núñez de Castro I. 1999. Antioxidant enzymes and human diseases. Clin Biochem. 32(8):595–603.
- Mayne ST. 2003. Antioxidant nutrients and chronic disease: use of biomarkers of exposure and oxidative stress status in epidemiologic research. J Nutr. 133:933–940.
- Meng Q, Guo T, Li G, Sun S, He S, Cheng B, Shi B, Shan A. 2018. Dietary resveratrol improves antioxidant status of sows and piglets and regulates antioxidant gene expression in placenta by Keap1-Nrf2 pathway and Sirt1. J Anim Sci Biotechnol. 9:34.
- Miller JK, Brzezinska-Slebodzinska E, Madsen FC. 1993. Oxidative stress, antioxidants, and animal function. J Dairy Sci. 76(9):2812–2823.
- Mormède P, Andanson S, Aupérin B, Beerda B, Guémené D, Malmkvist J, Manteca X, Manteuffel G, Prunet P, van Reenen CG, et al. 2007. Exploration of the hypothalamic-pituitary-adrenal function as a tool to evaluate animal welfare. Physiol Behav. 92(3):317–339.
- Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. 1997. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem. 43(7):1209–1214.
- Niedernhofer LJ, Daniels JS, Rouzer CA, Greene RE, Marnett LJ. 2003. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J Biol Chem. 278(33):31426–31433.
- NRC. 2012. Nutrient requirements of swine. 11th rev. ed. Washington (DC): National Academic Press.
- Ozkan OV, Yuzbasioglu MF, Ciralik H, Kurutas EB, Yonden Z, Aydin M, Bulbuloglu E, Semerci E, Goksu M, Atli Y, et al. 2009. Resveratrol, a natural antioxidant, attenuates intestinal ischemia/reperfusion injury in rats. Tohoku J Exp Med. 218(3):251–258.
- Pandey KB, Rizvi SI. 2009. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2(5):270–278.
- Puppe B, Tuchscherer M, Tuchscherer A. 1997. The effect of housing conditions and social environment immediately after weaning on the agonistic behaviour, neutrophil/lymphocyte ratio, and plasma glucose level in pigs. Livest Prod Sci. 48(2):157–164.
- Rubinstein AI, Rubinstein DB. 1990. Interleukin-4 (IL-4) inhibits secretion of IL-1 beta, tumor necrosis factor alpha, and IL-6 by human monocytes. Blood. 76:1392–1397.
- Starke RM, Raper DM, Ding D, Chalouhi N, Owens GK, Hasan DM, Medel R, Dumont AS. 2014. Tumor necrosis factor-α modulates cerebral aneurysm formation and rupture. Transl Stroke Res. 5(2):269–277.
- Valavanidis A, Vlachogianni T, Fiotakis C. 2009. 8-hydroxy-2' -deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 27(2):120–139.
- Wen C, Guo Q, Wang W, Duan Y, Zhang L, Li J, He S, Chen W, Li F. 2020. Taurine alleviates intestinal injury by mediating tight junction barriers in diquat-challenged piglet models. Front Physiol. 11:449.
- Xu YQ, Xing YY, Wang ZQ, Yan SM, Shi BL. 2018. Pre-protective effects of dietary chitosan supplementation against oxidative stress induced by diquat in weaned piglets. Cell Stress Chaperones. 23(4):703–710.
- Yin J, Wu MM, Xiao H, Ren WK, Duan JL, Yang G, Li TJ, Yin YL. 2014. Development of an antioxidant system after early weaning in piglets. J Anim Sci. 92(2):612–619.
- Yin J, Liu MF, Ren WK, Duan JL, Yang G, Zhao YR, Fang RJ, Chen XL, Li TJ, Yin YL. 2015. Effects of dietary supplementation with glutamate and aspartate on diquat-induced oxidative stress in piglets. PLoS One. 10:0122893.
- Yuan D, Hussain T, Tan B, Liu Y, Ji P, Yin Y. 2017. The evaluation of antioxidant and anti-inflammatory effects of eucommia ulmoides flavones using diquat-challenged piglet models. Oxid Med Cell Longev. 2017:8140962.
- Zhang C, Zhao X, Wang L, Yang L, Chen X, Geng Z. 2017. Resveratrol beneficially affects meat quality of heat-stressed broilers which is associated with changes in muscle antioxidant status. Anim Sci J. 88(10):1569–1574.
- Zhang HZ, Chen DW, He J, Zheng P, Yu J, Mao XB, Huang ZQ, Luo YH, Luo JQ, Yu B. 2019. Long-term dietary resveratrol supplementation decreased serum lipids levels, improved intramuscular fat content, and changed the expression of several lipid metabolism-related miRNAs and genes in growing-finishing pigs. J Anim Sci. 97(4):1745–1756.
- Zorov DB, Juhaszova M, Sollott S. 2014. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 94(3):909–950.
- Zhu LH, Zhao KL, Chen XL, Xu JX. 2012. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J Anim Sci. 90(8):2581–2589.
