Abstract
The aim of this study was to compare the effect of a commercial formulation consisting of natural ingredients and dextrose on the production of salami. We analysed the effect on microbiological, physico-chemical and sensorial properties in the production of nitrite-free swine and roe deer (Capreolus capreolus) salami. Six batches of salami were prepared: four with the addition of starter cultures and diverse substrates (no substrate, skimmed milk, commercial formulation and dextrose) and two without starter cultures as controls (one without substrate and one with skimmed milk). Samples for microbiological and physico-chemical analysis were taken on day 0, day 5 and day 25. A sensory evaluation was performed at the end of the ripening process. The best results regarding the changes in the microbiota were observed in the batch produced with the addition of skimmed milk (final concentration for Pseudomonas spp. was 3.07 ± 1.11 log cfu g−1, for Enterobacteriaceae was 1.57 ± 0.51 log cfu g−1 and the total coliforms were undetectable). As regards the sensory evaluation, the best scores (in particular colour uniformity, fat/lean distribution and mould flavour) were assigned to the salami produced with the addition of dextrose. According to the results obtained in this study, the addition of the commercial formulation to manufacture nitrite and nitrate-free, dry-cured swine and roe deer salami led to no significant effects on their microbiological and sensory characteristics. Overall, the results obtained with the addition of the commercial formulation were very similar to those obtained by adding dextrose, which is the main component of the formulation itself.
The effect of a commercial formulation on microbiological, chemical and sensory characteristics of nitrite-free swine and roe deer (Capreolus capreolus) salami was analysed.
The results obtained with the addition of the commercial formulation were very similar to those obtained by simply adding dextrose or just skimmed milk.
The use of commercial preparation seems therefore not worthwhile.
HIGHLIGHTS
Introduction
The fermentation of raw meat is a well-known method to improve the safety, shelf life and acceptability of certain foods. A particular kind of meat products are those based on hunted game meat. According to Reg. (EC) No. 853/2004, hunted game meat can be placed on the market and used to produce meat preparations and meat products due to the fact that the hunter is considered a primary producer (Anonymus Citation2004). Market and consumer surveys have demonstrated that hunted game meat can be perceived as healthier and obtained in a more ethical way when compared to farm animal meat (Marescotti et al. Citation2019). In fact, wild animals are free-roaming by definition and their meat can be considered organic and grass-fed (Ramanzin et al. Citation2010). Given these characteristics, hunted game meat can also be considered more environmentally friendly than the meat obtained by the intensive livestock farming system, if obtained under strict and regulated hunting practices of course (Ljung et al. Citation2012; Nielsen Citation2016). More specifically, the public appears to find hunting practices more acceptable if they have some purpose (Gamborg and Jensen Citation2017), as in the case of the remarkable demographic increase in Italy of red deer and wild boar, which is often considered more of a problem than a resource (Viganò et al. Citation2019).
Game meat differs from farm animal meat in many ways: it is generally darker, has a stronger taste and is often tougher than meat from farm animals (Demartini et al. Citation2018). Cured, fermented and dried products are made from the hunt, even though their presence is often seasonal (Marescotti et al. Citation2019). The marketing of roe deer (Capreolus capreolus), for instance, depends on the hunting season and culling quotas. Moreover, meat quality is influenced by pasture availability and animal activity: for example, roe has fewer fat reserves after the autumn mating season (Soriano et al. Citation2016). As regards nutritional and sensorial qualities, hunted game meat usually contains a lower fat content and is low in calories and cholesterol. On the other hand, it has a good amount of protein, zinc, iron, vitamin B12 and polyunsaturated fatty acid (Ramanzin et al. Citation2010). Some studies have demonstrated a favourable ɷ6/ɷ3 fatty acid ratio in wild ruminant meat (Valencak et al. Citation2015) and a good, conjugated linoleic acid (CLA) content (Phillip et al. Citation2007). Considering its natural darker colour, hunted game meat products are often marketed as nitrite and nitrate-free, in an attempt to link ethical harvesting to safer production technologies (Zarringhalami et al. Citation2009).
Other than the antimicrobial activity (i.e. absence of Clostridium botulinum toxin), a primary function of nitrites is the production of the characteristic red colour of cured meats (Mossel et al. Citation1995; Adams et al. Citation2015). However, the safety of nitrite to human health has been questioned, as they can cause the formation of carcinogenic N-nitrosamines. Nitrosamines are produced in foods, from nitrites and secondary amines: their formation can occur only under certain conditions, including strongly acidic conditions, such as that of the human stomach (Ozel et al. Citation2010).
The meat industry is often in search of alternatives to reduce the amount of nitrite in cured meat: this interest is further accelerated by the pressure generated from consumer demand for salt- and nitrite-reduced meat products. Natural alternatives are preferred because they are considered to be healthier (Alahakoon et al. Citation2015). For many years, vegetables such as celery, spinach, radish, lettuce and their powder have been used because they contain a considerable amount of nitrates. They have been reported to contain more than 2500 mg of nitrate per kilogram (Santamaria Citation2006): celery powder, for example, contains approximately 3% nitrate (Sindelar et al. Citation2007). Swiss chard (Beta vulgaris), in particular its spray-dried powder, has been used as a natural alternative in meat products (Dong-Min et al. Citation2017). This product is similar to celery powder as regards the content of natural nitrates. The main advantage in comparison to celery is that it does not contain allergens (Gabaza et al. Citation2013). The current Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives, however, requires that nitrites are declared in labelling, regardless the source, therefore they are not a viable choice for producers that want to declare their products as nitrite-free. Citrus spp. and their co-products are also often incorporated in natural formulations for meat products in order to reduce the nitrite residual content. In fact, they contain many functional ingredients and bioactive compounds such as organic acid and polyphenols that have antimicrobial activity (Viuda-Martos et al. Citation2009).
A possible alternative is the use of starter cultures in combination with particular seasoning conditions (hurdle technology) (Barbuti and Parolari Citation2002; Cenci-Goga et al. Citation2018).
The aim of this study was to compare the effect of a commercial formulation for fermented products, consisting of natural ingredients and dextrose, on the production of salami against other batches produced with the addition of just dextrose or just skimmed milk. We, therefore, analysed the effect on microbiological, physical–chemical and sensory properties in the production of nitrite-free swine and roe deer (C. capreolus) salami.
Materials and methods
Salami manufacturing and sampling procedure
The starter culture (SC) of choice is a formulation already successfully used by the authors (Cenci-Goga et al. Citation2012) in the production of swine and venison salami (Dama dama) for its mild fermentation and the ability to mitigate the distinct taste of venison. The characteristics of these strains (Lactococcus lactis ssp. lactis, internal strain ref. 340; L. lactis ssp. lactis, ref. 16; Lactobacillus casei ssp. casei, ref. 208 and Enterococcus faecium ref. 614) have been reported by the authors in previous papers (Cenci-Goga et al. Citation1995; Clementi et al. Citation1998; Cenci-Goga et al. Citation2015; Cenci-Goga et al. Citation2016)
The tested formulation (Naturmix Salagione 1500, Mec import, Rome, Italy, from now on «COMM») is a commercial formulation intended for the production of fermented products, such as salami, sausages and bacon, and replaces antioxidants and preservatives. It is advertised as a replacement of the E300 antioxidant family (vitamin C and its salts) and eliminates preservatives, for example, E250–E252 (nitrites), while giving to the final product its typical red colour. The label prescribes the use of COMM together with starter cultures. It is based on spices, spice extracts and Mediterranean aromatic plants such as Citrus spp., Beta vulgaris (beet) and Piper spp. (pepper). The composition shown on the label indicates also salt, natural flavours, dextrose and sunflower oil as a technological adjuvant. It was added at a concentration of 22 g for each kg of meat, as suggested by the label.
Six batches of salami were prepared: (i) no substrate + SC, (ii) skimmed milk + SC, (iii) COMM + SC, (iv) dextrose + SC, (v) no substrate and no SC, (vi) skimmed milk and no SC; the latter two used as controls. All batches were prepared according to a typical, local recipe by an experienced craftsman at the small-scale processing plant of the Laboratorio di Ispezione degli Alimenti di Origine Animale of the University of Perugia. The meat formulation consisted of lean roe deer meat (28%), pork shoulder and boneless belly (55%) and back fat (16%). Frozen meat was minced and mixed with NaCl (30 g kg−1), pepper (5 g kg−1), garlic (2 g kg−1), white wine (2 mL kg−1) and ascorbic acid (2 g kg−1). SC cultures were added with a lactococci:lactobacilli:enterococci ratio of 2:1:1 at an initial concentration of approximately 107 log cfu g−1. To produce the salami, the meat was chopped in a cutter and mixed in a blender after the addition of the other ingredients.
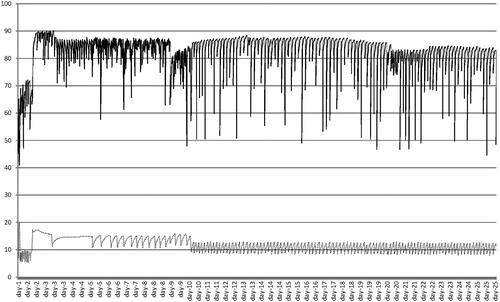
The mixture was stuffed under vacuum into a natural, 30 mm-diameter swine casing and the salami was suspended in a conditioned chamber for fermentation and ripening. The actual temperature and relative humidity during the entire period were recorded by the chamber data-logger (Figure ). Samples for analysis were taken on day 0, day 5 and day 25. At each sampling time, three salami per batch were analysed and three subsamples were taken from each sample. All analyses were conducted within 30 min after sampling.
Microbiological and physical–chemical analysis
For each analysis, 25 g of meat were homogenised in a sterile bag with 225 mL of peptone water (PW, Oxoid, Basingstoke, Hampshire, UK) using a stomacher. 10-fold dilutions were prepared in sterile tubes with 9 mL of Maximum Recovery Diluent (MRD, Oxoid). Dilutions were inoculated in triplicate on different culture media. The total, aerobic, mesophilic microbiota was determined on Plate Count Agar (PCA, Oxoid) at 30 °C for 72 h; Lactococcus spp. on M17 agar (Oxoid) 10% v/v lactose, at 37 °C for 48 h; Lactobacillus spp. on Man, Rogosa and Sharpe Agar (MRS, Oxoid) pH 5.5, at 30 °C for 72 h under anaerobic conditions (Gas generating kit, Oxoid); enterococci on enterococcus agar (ENT, Oxoid), at 37 °C for 48 h; Staphylococcus spp. on Baird Parker agar (BP, Oxoid) containing Egg Yolk Tellurite (Oxoid) at 37 °C for 48 h; Enterobacteriaceae on violet red bile glucose agar (VRBG, Oxoid) at 37 °C for 24 h; total coliforms on violet red bile lactose agar (VRBL, Oxoid) at 37 °C for 24 h; Pseudomonas spp. on pseudomonas agar base (PS103, Oxoid) at 37 °C for 24 h. The colonies were then counted on all the plates, using a colony count viewer (Petri light, PBI, Milan) and colony counter pen (Colony Count, PBI, Milan). To measure the pH, an S40 Seven-Multi digital pH-meter (Mettler-Toledo Italia, Novate Milanese, Italy) was used after mixing 10 g of salami with 90 mL of distilled water. A dew-point hygrometer HygroLab 3 (Rotronic, Huntington, NY, USA) was used to measure water activity (aw).
Sensory evaluation
At the end of the ripening process, a sensory evaluation was performed for the three batches made with substrate and SC addition due to the detection of S. aureus and high coliforms and Enterobacteriaceae counts in the other groups. The panel consisted of 6 assessors selected from among the staff at the Laboratorio di Ispezione Degli Alimenti di Origine Animale, who had previously been trained in descriptive analysis for cured meat products. The tasters were asked to test the salami for the following characteristics: colour uniformity, colour intensity, fat/lean connection, fat/lean distribution, acid flavour, rancid flavour, bitter flavour, salty flavour, mouldy flavour, spicy flavour, flavour intensity, elasticity, hardness, cohesiveness, chewiness, juiciness, fattiness, and overall acceptability. Each assessor was given sheets with a 7-point (unnumbered to avoid biased assessment) scale for each characteristic: 7 = maximum intensity and 1 = minimum intensity. The evaluations were performed in individual booths, built according to the criteria of the International Standards Organisation (International Standards Organisation Citation2003). Samples were taken from the middle of the salami by cutting off 2 cm from each edge. The salami slices were 3 mm thick and were immediately served on a plastic dish, covered with plastic film and coded with random, three-digit numbers. Water and unsalted bread were provided to cleanse the palate between samples. Assessments were carried out under natural light at a room temperature of 20 ± 2 °C. The individual scores for each assessor were then averaged to give a score for the taste panel as a whole. Three evaluations for each different batch of salami were performed. Each evaluation was carried out in different test sessions at the same time of day, between 10 and 12 am.
Colour uniformity was defined as the absence of an external darker halo in the slice due to anomalous drying process; colour intensity as the characteristic red colour of cured meat; fat/lean connection as the degree of adherence of the product’s fat and lean; fat/lean distribution as the uniform distribution of lean and fat on the slice. Acid describes the taste produced by a dilute aqueous solution of most acidic substances; rancid describes the aroma associated with oxidation compounds derived from fat; bitter describes an unpleasantly sharp, harsh taste produced by dilute aqueous solutions of various substances, for example, quinine and caffeine; salty describes the taste produced by a dilute aqueous solution of sodium chloride; mouldy is the characteristic taste associated with the odour of fungi due to the chemical compound 1-octen-3-ol; flavour intensity relates to the overall intensity of the taste; overall acceptability is a subjective parameter added to evaluate palatability. Elasticity is the rapidity of recovery from a deforming force applied with forefinger and thumb; hardness is the force required to compress the sample between the forefinger and thumb to achieve a given deformation; cohesiveness is the resistance of the sample before breaking when under strain; chewiness is the work required to disintegrate the sample before swallowing; juiciness is the perception of the amount of water released by the product during the first bite and fattiness is the perception of the quantity of fat exuded during mastication.
Statistical analysis
For each sampling, the arithmetic means of the three subsamples were calculated and then all the values (converted to log for microbiological analyses) were analysed using GraphPad Prism, version 6.0 h, for Mac OS X (GraphPad, San Diego, CA, United States). Two-way analysis of variance (ANOVA) followed by the Tukey’s multiple comparisons test was performed considering the product type as the treatment. A p-value <.05 was considered to be significant.
Results
Microbiological and physical–chemical analysis
Results are shown in Table . On day 0, in salami prepared without SC addition, lactococci ranged from 1.92 ± 0.41 to 3.81 ± 0.13 log cfu g−1, lactobacilli from 1.16 ± 1.04 to 4.59 ± 0.09 log cfu g−1 and enterococci from 3.10 ± 0.17 to 3.30 ± 0.30 log cfu g−1. In the other four batches of salami prepared with the addition of SC, the count of lactococci, lactobacilli and enterococci was always approximately or above 106 log cfu g−1. At the end of the experiment (day 25), the lactococci and lactobacilli count ranged between 6.60 ± 0.63 log cfu g−1 and 8.81 ± 0.06 log cfu g−1 in all batches. The enterococci count ranged from 2.88 ± 0.02 to 4.20 ± 0.72 log cfu g−1 in salami without SC addition, whereas it was around 106 log cfu g−1 in the batches prepared with the addition of SC. The level of total aerobic mesophilic microbiota counts was similar to the lactobacilli counts. Mean staphylococci counts on day 0 ranged between 4.08 ± 0.14 log cfu g−1 and 4.59 ± 0.18 log cfu g−1. The mean Pseudomonas spp. count ranged between 5.31 ± 0.87 log cfu g−1 and 6.55 ± 0.42 log cfu g−1. The mean Enterobacteriaceae count ranged between 3.86 ± 0.03 log cfu g−1 and 4.96 ± 0.37 log cfu g−1. The coliforms organism means ranged between 3.82 ± 0.06 log cfu g−1 and 5.22 ± 0.42 log cfu g−1. At the end of ripening (day 25), the mean staphylococci counts ranged between 4.30 ± 0.54 log cfu g−1 and 5.19 ± 1.05 log cfu g−1. The mean Pseudomonas spp. counts in the batches produced with substrates and SC were 3.93 ± 0.10 log cfu g−1 in the batch produced with the addition of COMM, 3.07 ± 1.11 log cfu g−1 in the batch produced with the addition of skimmed milk and 4.83 ± 0.05 log cfu g−1 in the batch produced with the addition of dextrose. In control batches Pseudomonas spp. counts were always above 4.93 with a peak of 5.62 ± 0.54 for the batch made without any addition.
Table 1. Microbiota counts (log cfu g−1) for the different batches of salami.
The mean Enterobacteriaceae count was 0.90 ± 0.85 log cfu g−1 in the batch produced with the addition of COMM, 1.57 ± 0.51 log cfu g−1 in the batch produced with the addition of skimmed milk and 1.51 ± 0.45 log cfu g−1 in the batch produced with the addition of dextrose. In control batches, Enterobacteriaceae counts ranged from 2.38 ± 0.16 (skimmed milk without SC) to 4.77 ± 0.33 (no additions of any kind).
The mean of the total coliform organisms was 0.87 ± 0.81 log cfu g−1 in the batch produced with the addition of COMM, not detectable in the batch produced with the addition of skimmed milk and 1.36 ± 0.32 log cfu g−1 in the batch produced with the addition of dextrose. In control batches coliforms counts ranged from 3.26 ± 0.01 (skimmed milk without SC) to 3.65 ± 0.41 (no additions of any kind).
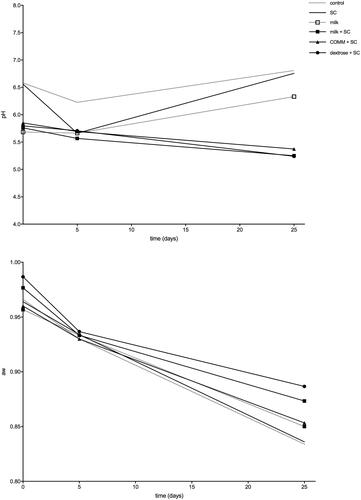
The mean pH values (Figure ) on the day of stuffing ranged between 5.68 (milk and no SC) and 6.58 (no substrate nor SC) and fell to values between 5.56 (milk + SC) and 6.22 (no substrate nor SC) at the end of fermentation (day-5) when the batch made with just SC and the batch added with just milk but no SC reached both 5.66. At the end of ripening (day 25), the pH mean values for the batches produced with the addition of SC were 5.25 in the batch produced with skimmed milk, 5.37 in the batch produced with COMM and 5.24 in the batch produced with the addition of dextrose. pH in the control group made without any addition did not drop and at the end of ripening reached 6.80 while the batch made with just SC and the batch added with just milk but no SC were both above 6 (6.76 for SC and 6.33 for milk). The mean aw values (Figure ) on the day of stuffing were above 0.95 for all batches and dropped to values of 0.93–0.94 at the end of fermentation (day-5) and values of 0.83–0.89 at the end of ripening (day-25), including the control groups.
Sensory evaluation
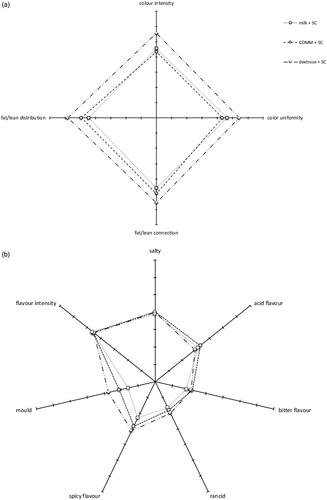
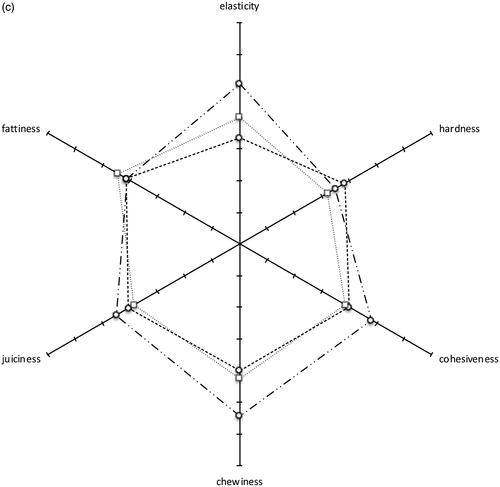
Table and Figure show the results of the descriptive, sensory analysis of the salami at the end of the ripening period. Assessors described the salami as having colour uniformity ranging from 4.33 ± 1.15 to 5.44 ± 1.50, colour intensity ranging from 4.33 ± 1.22 to 5.56 ± 1.26, fat/lean connection from 4.60 ± 1.31 to 5.56 ± 1.31 and fat/lean distribution from 4.45 ± 1.54 to 5.81 ± 1.17. As regards taste, odour and aroma, acidity ranged from 2.94 ± 1.24 to 3.33 ± 1.49, rancidity from 1.65 ± 0.81 to 2.00 ± 1.21, bitterness from 1.85 ± 0.93 to 2.19 ± 1.19, saltiness from 3.90 ± 1.17 to 4.03 ± 1.28, the mouldy flavour from 1.60 ± 0.88 to 2.75 ± 1.69, spicy taste from 2.30 ± 1.17 to 3.13 ± 1.45 and flavour intensity from 4.50 ± 1.46 to 4.60 ± 1.19. As regards texture attributes, elasticity ranged from 3.36 ± 1.17 to 5.06 ± 1.53, hardness from 3.22 ± 1.11 to 3.83 ± 1.20, cohesiveness from 3.85 ± 1.39 to 4.81 ± 1.05, chewiness from 4.00 ± 1.29 to 5.44 ± 1.03, juiciness from 3.85 ± 1.09 to 4.50 ± 0.97 and fattiness 4.13 ± 1.54 to 4.45 ± 1.00. Overall acceptability ranged from 5.11 ± 1.33 for salami produced with the addition of SC and COMM to 5.88 ± 0.96 for salami produced with the addition of SC and dextrose.
Figure 3. (a–c) Sensory descriptive analysis of salami.
Table 2. Sensory descriptive analysis of salami.
Discussion
In this study, the level of SC increased during fermentation and remained constant after fermentation to the end of ripening. By the end of ripening (day 25) Lactococcus spp. and Lactobacillus spp. reached a concentration of 107–108 log cfu g−1 in all batches of salami except the control made without any addition. Enterococci count reached a concentration of 105–106 log cfu g−1 in the batches of salami produced with the addition of SC, whereas it remained at approximately 103–104 log cfu g−1 in the salami produced without SC addition. As regards Staphylococcus spp., the level remained very similar throughout the experiment and no significant difference was observed between the different batches of salami. S. aureus was only detected in the salami produced without SC addition and in the batch produced with SC but without substrate. In these batches, the total coliform count was also high. As a result, the sensory evaluations for these batches were not carried out. A decline in the number of Pseudomonas spp., Enterobacteriaceae and total coliforms was observed at the end of ripening. The reduction of Pseudomonas spp. is of the utmost importance to avoid a bitter taste and slime production or the softening of the salami texture (Cenci-Goga et al. Citation2014; Rossi et al. Citation2018). The reduction rate was higher in the salami produced with skimmed milk + SC, followed by the salami produced with COMM + SC and lower in the salami produced with dextrose + SC and in the controls without SC addition. Paradoxically, the best results regarding the changes in the microbiota were observed in the batches produced with the addition of skimmed milk and SC. The results obtained with the addition of COMM + SC were very similar to those obtained with the addition of dextrose + SC.
The aw endpoints were similar in the salami produced with the addition of skimmed milk + SC, COMM + SC, dextrose + SC and controls, whereas the pH endpoint was higher in the control groups produced without the addition of SC and in the batch made with only SD and no substrate. pH endpoints were similar in the three groups made with the addition of SC and substrate. This is partly directly linked to the levels of lactobacilli. The overall acceptability showed, in general, a preference for the sausages produced with the addition of dextrose.
As regards appearance attributes, a difference was observed in the colour intensity, colour uniformity and fat/lean distribution, with better scores assigned to the salami produced with the addition of SC and dextrose. No statistically significant differences were observed in odours and basic taste parameters, with the exception of a mouldy taste that was lower in the salami produced with the addition of milk. As regards texture attributes, a higher value of elasticity and chewiness was observed in the salami produced with the addition of SC and dextrose.
As mentioned in the introduction, the meat industry is continuously in search of alternatives to reduce the amount of nitrite in cured meat. For many years, vegetables such as celery, spinach, radish, lettuce and their powder have been used because contain a considerable amount of nitrates (Santamaria Citation2006; Sindelar et al. Citation2007). However, the indirect addition of nitrates to foods via nitrate-rich extracts of vegetables such as spinach or celery should be considered an additive use, and not a food use. In such cases, the extract is being added for preservation as it contains a standardised level of nitrate and consequently, such use would not be permitted by Regulation 1333/2008 as these extracts have not been approved as preservatives. This is the position of many institutions and bodies such as the Food standards agency (Food Standards Agency Citation2015), the Italian health ministry and the Bundesverwaltungsgericht in Germany (De Stefani 2019).
Conclusions
Considering the development of the microbiota during fermentation and ripening and the reduction of undesirable groups of bacteria, unexpectedly, the best results were obtained with the addition of skimmed milk, followed by the addition of COMM and dextrose. If we look at the sensory characteristics, salami produced with the addition of dextrose takes the lead, especially for colour intensity, colour uniformity and fat/lean distribution. Overall, the results obtained with the addition of COMM were very similar to those obtained with the addition of dextrose, which is actually the main component of the formulation.
Ethical approval
This article does not contain any studies with human or animal subjects.
Authors’ contributions
All authors contributed in equal parts to the manuscript.
Acknowledgements
The authors wish to express sincere appreciation to members of Polyglot, Perugia for careful reading and comments on the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adams MR, Moss MO, McClure P. 2015. Food microbiology. 4 ed. Cambridge (UK): Royal Society of Chemistry.
- Alahakoon AU, Jayasena DD, Ramachandra S, Jo C. 2015. Alternatives to nitrite in processed meat: up to date. Trends Food Sci Technol. 45(1):37–49.
- Anonymus. 2004. Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for on the hygiene of foodstuffs. Official Journal of the European Union, L139, 30.4.2004
- Barbuti S, Parolari G. 2002. Validation of manufacturing process to control pathogenic bacteria in typical dry fermented products. Meat Sci. 62(3):323–329.
- Cenci-Goga BT, Clementi F, Di Antonio E. 1995. Behaviour of lactic and non lactic microflora during production and ripening of farm manufactured Pecorino cheese. Ann Microbiol. 45(2):219–236.
- Cenci-Goga BT, Karama M, Sechi P, Iulietto MF, Grispoldi L, Selvaggini R, Ceccarelli M, Barbera S. 2018. Fate of selected pathogens in spiked «SALAME NOSTRANO» produced without added nitrates following the application of NONIT™ technology. Meat Sci. 139:247–254.
- Cenci-Goga BT, Karama M, Sechi P, Iulietto MF, Novelli S, Mattei S. 2014. Evolution under different storage conditions of anomalous blue coloration of Mozzarella cheese intentionally contaminated with a pigment-producing strain of Pseudomonas fluorescens. J Dairy Sci. 97(11):6708–6718.
- Cenci-Goga BT, Karama M, Sechi P, Iulietto MF, Novelli S, Selvaggini R, Barbera S. 2016. Effect of a novel starter culture and specific ripening conditions on microbiological characteristics of nitrate-free dry-cured pork sausages. Ital J Anim Sci. 15(3):358–374.
- Cenci-Goga BT, Karama M, Sechi P, Iulietto MF, Novelli S, Selvaggini R, Mattei S. 2015. Growth inhibition of selected microorganisms by an association of dairy starter cultures and probiotics. Ital J Anim Sci. 14(2):3250–3745.
- Cenci-Goga BT, Rossitto PV, Sechi P, Parmegiani S, Cambiotti V, Cullor JS. 2012. Effect of selected dairy starter cultures on microbiological, chemical and sensory characteristics of swine and venison (Dama dama) nitrite-free dry-cured sausages. Meat Sci. 90(3):599–606.
- Clementi F, Cenci-Goga BT, Trabalza-Marinucci MD, Antonio E. 1998. Use of selected starter cultures in the production of farm manufactured goat cheese from thermized milk. Ital J Food Sci. 10(1):41–56.
- De Stefani F. 2019. Prosciutti e salumi “senza conservanti e senza nitriti artificiali”? Quando l’etichetta è ingannevole. La risposta dell’esperto. [accessed 2020 September 2020]. https://ilfattoalimentare.it/nitriti-vegetali-etichette.html
- Demartini E, Vecchiato D, Tempesta T, Gaviglio A, Viganò R. 2018. Consumer preferences for red deer meat: a discrete choice analysis considering attitudes towards wild game meat and hunting. Meat Sci. 146:168–179.
- Dong-Min S, Ko-Eun H, Cheol-Won L, Tae-Kyung K, Yoo-Sun P, Sung Gu H. 2017. Effect of swiss chard (Beta vulgaris var. cicla) as nitrite replacement on color stability and shelf-life of cooked pork patties during refrigerated storage. Korean J Food Sci Anim Resour. 37(3):418–428.
- Food Standards Agency. 2015. Food additives legislation guidance to compliance. London (UK): Food Standards Agency.
- Gabaza M, Claeys E, Smet SD, Raes K. 2013. Potential of fermented spinach extracts as a nitrite source for meat curing. Paper presented at: Proceedings of the 59th International Congress of Meat Science and Technology (ICOMST); Aug 18–23; Izmir, Turkey.
- Gamborg C, Jensen FS. 2017. Attitudes towards recreational hunting: a quantitative survey of the general public in Denmark. J Outdoor Recreat Tour. 17:20–28.
- International Standards Organisation. 2003. Sensory analysis — guidelines for the use of quantitative response scales. ISO 4121:2003. Geneva (Switzerland): International Organization for Standardization.
- Ljung PE, Riley SJ, Heberlein TA, Ericsson G. 2012. Eat prey and love: game-meat consumption and attitudes toward hunting. Wildl Soc Bull. 36(4):669–675.
- Marescotti ME, Caputo V, Demartini E, Gaviglio A. 2019. Discovering market segments for hunted wild game meat. Meat Sci. 149:163–176.
- Mossel DAA, Corry JEL, Struijk CB, Baird RM. 1995. Essentials of the micromicrobiology of food. Chichester (UK): John Wiley & Sons Ltd.
- Nielsen. 2016 October 24. Weighing consumers’ growing appetite for ‘clean’ meat labeling. Insight News. Fresh facts historical data, 2011-2015:[about 3 screens]. Accessed January 2021. https://www.nielsen.com/us/en/insights/article/2016/weighing-consumers-growing-appetite-for-clean-meat-labeling/
- Ozel MZ, Gogus F, Yagci S, Hamilton JF, Lewis AC. 2010. Determination of volatile nitrosamines in various meat products using comprehensive gas chromatography-nitrogen chemiluminescence detection. Food Chem Toxicol. 48(11):3268–3273.
- Phillip LE, Oresanya TF, Jacques JS. 2007. Fatty acid profile, carcass traits and growth rate of red deer fed diets varyng in the ratio of concentrate: dried and pelletted roughage, and raised for venison production. Small Rumin Res. 71(1–3):215–221.
- Ramanzin M, Amici A, Casoli C, Esposito L, Lupi P, Marsico G, Mattiello S, Olivieri O, Ponzetta MP, Russo C. 2010. Meat from wild ungulates: ensuring quality and hygiene of an increasing resource. Ital J Anim Sci. 9:318–331.
- Rossi C, Serio A, Chaves-López C, Anniballi F, Auricchio B, Goffredo E, Cenci-Goga BT, Lista F, Fillo S, Paparella A. 2018. Biofilm formation, pigment production and motility in Pseudomonas spp. isolated from the dairy industry. Food Control. 86:241–248.
- Santamaria P. 2006. Nitrate in vegetables: toxicity, content, intake and EC regulation. J Sci Food Agric. 86(1):10–17.
- Sindelar JJ, Cordray JC, Sebranek JG, Love JA, Ahn DU. 2007. Effects of varying levels of vegetable juice powder and incubation time on color, residual nitrate and nitrite, pigment, pH, and trained sensory attributes of ready-to-eat uncured ham. J Food Science. 72(6):S388–S395.
- Soriano A, Montoro V, Vicente J, Sànchez-Migallòn BF, Benìtez S, Utrilla MC, Garcìa Ruiz A. 2016. Influence of evisceration time and carcass ageing conditions on wild venison quality. Preliminary study. Meat Sci. 114:130–136.
- Valencak TG, Gamsjager L, Ohrnberger S, Culbert N, Ruf T. 2015. Healthy n-6/n-3 fatty acid composition from five European game meat species remains after cooking. BMC Res Notes. 8(1):273.
- Viganò R, Demartini E, Riccardi F, Corradini A, Besozzi M, Lanfranchi P, Chiappini PL, Cottini A, Gaviglio A. 2019. Quality parameters of hunted game meat: sensory analysis and pH monitoring. Ital J Food Safety. 8(1):7724.
- Viuda-Martos M, Fernández-López J, Sayas-Barbera E, Sendra E, Navarro C, Pérez-Álvarez JA. 2009. Citrus co-products as technological strategy to reduce residual nitrite content in meat products. J Food Sci. 74(8):R93–R100.
- Zarringhalami S, Sahari MA, Hamidi-Esfehani Z. 2009. Partial replacement of nitrite by annatto as a colour additive in sausage. Meat Sci. 81(1):281–284.