Abstract
Disrupting mutations affecting the tyrosinase (TYR) gene cause different forms of albinism in mice, humans and several other mammals. Classical genetic studies have already reported five alleles at the European rabbit Albino locus, indicated to be part of the C series, each of them with different actions on pheomelanin and eumelanin production, as well as on the eye colour. A few of these alleles have been already characterised at the DNA level by sequencing the coding region of the TYR gene in few rabbit breeds or strains with specific alleles at this locus. In this study, we further characterised the TYR gene by sequencing all coding and flanking regions in a total of 25 rabbits from 11 domestic breeds (Belgian Hare, Burgundy Fawn, Californian, Champagne d’Argent, Giant Chinchilla, Giant Grey, Havana, Leprino di Viterbo, New Zealand White, Silver and White Vienna) and in 11 wild rabbits from Sardinia. Sequencing data identified a total of 15 polymorphisms. We confirmed five missense mutations already detected by other authors, three of which associated with different coat colour phenotypes: p.T373K determining the albino allele; p.E294G causing the Himalayan and the chinchilla alleles; p.T358I observed only in Chinchilla rabbits. In addition to seven other synonymous mutations and one polymorphism in the 3’-untranslated region, two novel missense mutations were identified (p.T144S and p.K224T, the latest was detected only in wild rabbits). This study further contributed to disclose variability in the rabbit TYR gene and confirmed the effects on coat colours of missense mutations.
Mutations in the tyrosinase (TYR) gene cause several coat colour alleles at the Albino locus in mammals.
The TYR gene was sequenced in 11 domestic rabbit breeds and in a wild rabbit population.
Fifteen single nucleotide polymorphisms (SNPs) were identified: seven were missense mutations.
In silico analyses and information already available predicted the effects of some SNPs on coat colour.
This study further contributed to disclose variability in the rabbit TYR gene.
Highlights
Introduction
Coat colour genetics has been the subject of many studies that have led to the identification of more than 300 loci that affect pigmentation in mice (Montoliu et al. Citation2009; Lamoreux et al. Citation2010). Recently, several coat colour loci in the European rabbit (Oryctolagus cuniculus; thereafter indicated as rabbit) have been genetically characterised by sequencing genes involved in coat colour variability in different domestic breeds (Fontanesi et al. Citation2006, Citation2010a, Citation2010b, Citation2014a, Citation2014b; Utzeri et al. Citation2014; Demars et al. Citation2018).
Coat colours largely depends on the amount and distribution of two types of melanin, pheomelanin (yellow-red pigment) and eumelanin (black-brown pigment) located in the skin, in the hair and in the pigmented cell layers of the eyes (e.g. Searle Citation1990; Lin and Fisher Citation2007; Lamoreux et al. Citation2010).
Tyrosinase (TYR) is a key enzyme of the melanogenetic pathway. TYR is involved in the initial step, in which tyrosine is transformed in a metabolic intermediate both shared by eumelanin and pheomelanin production routes (Hearing Citation2011). The mammalian TYR gene coding region is about 1.6 kb long and it is composed of five exons. TYR encoded protein has two active sites, known as CuA and CuB, each binding a copper ion, that are derived from the first and the third exon, respectively (Goldfeder et al. Citation2014; Kanteev et al. Citation2015).
Mutations in the TYR gene are associated with several coat colour variations in mammals. Mutations leading to a loss of protein function cause different forms of albinism in mice, humans, cattle, cat, donkey and several other species (Oetting and King Citation1999; Berson et al. Citation2000; Beermann et al. Citation2004; Schmutz et al. Citation2004; Imes et al. Citation2006; Anistoroaei et al. Citation2008; Rees Citation2011; Utzeri et al. Citation2016). Lack of tyrosinase activity results in the absence of melanin formation due to the inability to form the melanin precursor; in humans, oculocutaneous albinism type 1 (OCA1) is a direct result of this inactivity (Berson et al. Citation2000).
Classical genetic studies have already reported five alleles in the rabbit Albino locus, indicated to be part of the C series, each of them with different actions on pheomelanin and eumelanin production, as well as on the eyes (Searle Citation1968): C, wild colour (with normal melanin production and dark eye-colour); cchd, dark chinchilla, with reduced pheomelanin production, normal eumelanin production and dark eye colour); cchm, medium chinchilla (without any pheomelanin production, slightly reduced eumelanin production and reddish-black eye-colour); cchl, light chinchilla (no pheomelanin production, reduced eumelanin production and red eye-colour); ch, Himalayan (no pheomelanin production, reduced eumelanin production and located only at the extremities and pink eye-colour); c, albino (without any melanin production and pink eye). The rabbit TYR gene is located on chromosome 1 and encodes a 530 amino acid (aa) protein, in which the first 18 aa constitute the signal peptide (Aigner et al. Citation2000; Carneiro et al. Citation2014).
The coding region of the rabbit TYR gene has been sequenced in a few rabbits with different alleles at the Albino locus (Aigner et al. Citation2000). A missense mutation causing the p.T373K amino acid substitution, identified in New Zealand White rabbits, has been indicated to cause the recessive c albino allele (Aigner et al. Citation2000). The same mutation in humans causes a type I oculocutaneous albinism (OCA1; Sanabria et al. Citation2012). Genome edited rabbits in which this mutation has been artificially introduced confirmed the disrupting effect of this amino acid change (Song et al. Citation2018). Other two missense mutations in the rabbit TYR coding region have been associated to different coat colour phenotypes: p.E294G has been identified in Californian rabbits showing the Himalayan phenotype: the same p.E294G together with p.T358I amino acid change has been identified in Chinchilla rabbits (Aigner et al. Citation2000). Few other missense mutations at this locus shared by several rabbits of different strains were also identified (Aigner et al. Citation2000). The preliminary work of Aigner et al. (Citation2000) did not characterise all alleles of the C series that would be expected to segregate at the Albino locus in Oryctolagus cuniculus, according to what was reported by the classical genetic investigations (Searle Citation1968).
In this study we further characterised the variability of the rabbit TYR gene by sequencing all coding regions and portions of non-coding regions in 11 domestic breeds carrying different putative alleles at the Albino locus and in wild rabbits having wild type coat colour.
Materials and methods
Animals and samples
A total of 25 domestic rabbits from 11 different breeds (2 Belgian Hare, 2 Burgundy Fawn, 3 Californian, 3 Champagne d’Argent, 2 Giant Chinchilla, 1 Giant Grey, 1 Havana, 2 Leprino di Viterbo, 4 New Zealand White, 2 Silver and 3 Vienna White) and 11 wild rabbits hunted in Sardinia were included in this study. All domestic animals had standard breed coat colours and were registered to the corresponding breed herd book, managed by the Italian Rabbit Breeders Association (ANCI, 2020). Wild rabbits had the wild type coat colour. Hairs were collected from the domestic rabbits and liver samples were provided by hunters from the hunted animals that were obtained during a regular hunting season. All animals were not raised or hunted for the purpose of this study and were not treated in any way. Therefore, no ethical issues were involved in this study.
PCR and DNA sequencing
Genomic DNA was extracted from hair roots or from liver using a standard phenol-chloroform-isoamyl alcohol extraction protocol (Sambrook et al. Citation1989) and the Wizard® Genomic DNA Purification Kit (Promega Corp., Madison, Wisconsin, USA). Seven primer pairs were designed using PRIMER3 (Untergasser et al. Citation2012) on the sequence of the TYR gene annotated in the OryCun2.0 rabbit genome (Carneiro et al. Citation2014), including flanking or intronic regions to amplify all five exons and parts of the introns (Supplementary Table S1). PCR were carried out on a 2700 Thermal Cycler (Life Technologies, Waltham, MA, USA) in a total volume of 20 μL including 10 pmol of each primer, 50 mg of isolated DNA and the KAPA HiFi HotStart Mastermix (Kapa Biosystems, Wilmington, MA, USA). PCR cycles were: initial denaturation step at 95 °C for 5 min; 35 cycles at 95 °C for 30 s, 30 s at the primer pair annealing temperature (50–60 °C; Supplementary Table S1), 72 °C for 30 s: final extension step at 72 °C for 5 min. After purification with 2 U of ExoSAP-IT (USB Corporation, Cleveland, OH, USA) for 15 min at 37 °C, PCR fragments were sequenced using the BrightDye® Terminator Cycle Sequencing Kit (NIMAGEN, Nijmegen, The Netherlands). Sequencing reactions were then loaded on an ABI3100 Avant capillary sequencer (Applied Biosystems, Foster City, California, USA).
Table 1. Polymorphisms identified in the rabbit TYR gene.
Sequence data analyses
Obtained sequences were visually inspected, assembled and aligned using MEGA 6 software (Tamura et al. Citation2013). The effect of each single nucleotide polymorphisms (SNPs) was evaluated using the Variant Effect Predictor (VEP) (McLaren et al. Citation2016) implemented in Ensembl Genome Browser (https://www.ensembl.org/info/docs/tools/vep/index.html), and the effect of all missense mutations was analysed using SIFT algorithm (Kumar et al. Citation2009). Haplotype sequences were defined with the help of the PHASE v. 2.1.1 software (Stephens et al. Citation2001) and their phylogenetic relationships based on the TYR coding region was inferred using the UPGMA method implemented in MEGA 6 (Tamura et al. Citation2013). The phylogenetic tree was rooted with the TYR sequence of Lepus capensis (EMBL accession number ERZ1612838) and was run with 1000 bootstraps.
Results and discussion
A total of 2027 bp, including the complete coding region of the TYR gene (1593 bp) and non-coding regions (434 bp of 3′-untranslated regions and 5′-flanking regions) were sequenced in all investigated rabbits. New sequence data have been submitted to the EMBL European Nucleotide Archive with accession numbers ERZ1610238 to ERZ1610254, ERZ1611434 to ERZ1611437, ERZ1610162 to ERZ1610169 and ERZ1612835 to ERZ1612837.
Table summarises all mutations that were identified in this study that included 20 single nucleotide polymorphisms (SNPs) and one insertion/deletion (indel). A total of 15 SNPs was identified in the coding regions, seven of which were missense mutations that caused amino acid changes in several domains of the encoded protein (Figure ).
Figure 1. Schematic representation of the rabbit tyrosinase protein domains (SP: signal peptide; EGF: epidermal growth factor-like domain; CuA, CuB: copper binding; TM: transmembrane domain) with reported the amino acid substitutions identified in this study with the rabbit breeds and population that carried the indicated variants: (1) Giant Chinchilla, (2) Silver, (3) Belgian Hare, (4) Leprino di Viterbo, (5) Havana, (6) Burgundy Fawn, (7) Giant Grey, (8) Californian, (9) New Zealand White, (10) Wild rabbit from Sardinia.
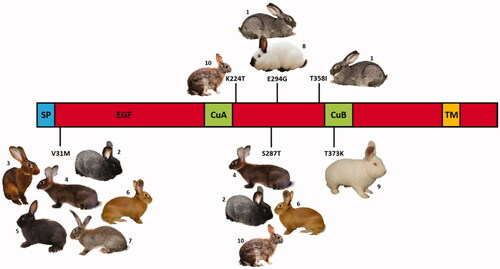
Among them, five missense mutations (p.V31M; p.S287T; p.E294G; p.T358I; p.T373K) were already detected by Aigner et al. (Citation2000): three of them are strain-specific and associated with different coat colour phenotypes, confirming what was suggested by a previous study (Aigner et al. Citation2000). The p.T373K amino acid change was homozygous in all New Zealand White rabbits, which are albino animals, indicating that it causes the c allele at this locus. The p.E294G substitution was identified in homozygous state in all Californian rabbits (which have a temperature sensitive albinism as they carry the Himalayan or ch allele at the Albino locus) and in all Giant Chinchilla rabbits whereas the p.T358I amino acid change was homozygous only in Chinchilla rabbits. It is however still unclear how the two missense mutations observed in Chinchilla rabbits by our study and Aigner et al. (Citation2000) could be associated with the three Chinchilla alleles (cchd, dark chinchilla; cchm, medium chinchilla; or cchl, light chinchilla) described by Searle (Citation1968).
The p.E294G and p.T373K substitutions identified in two forms of albinisms in rabbits are the same mutations causing different OCA1 forms in humans (e.g. Sanabria et al. Citation2012). The two other missense mutations (p.V31M and p.S287T), already detected in a few rabbit breeds by Aigner et al. (Citation2000), i.e. German Giant, Belgian Hare and Vienna White for the p.V31M substitution and Belgian Hare for the p.S287T amino acid change, were also present in homozygous or heterozygous conditions in several other rabbits of different breeds included in our study (p.V31M: Belgian Hare, Burgundy Fawn, Champagne d’Argent, Giant Grey, Havana, Leprino di Viterbo, New Zealand White, Silver, Vienna White and wild rabbits; pS287T: Belgian Hare, Burgundy Fawn, Champagne d’Argent, Silver and wild rabbits). That means that these two missense mutations are not strain or breed-specific and are present in breeds with different coat colours, confirming that they might not impact on the function of the tyrosinase protein and that they should not be associated with any coat colour variants in Oryctolagus cuniculus. Therefore, they could be considered as alleles that can have normal melanin production. Thus, they could be different forms of the wild type C allele defined by classical genetic studies (Searle Citation1968).
In addition to these missense mutations already described, two novel missense mutations occurring in exon 1 were detected (Table ): p.T144S that was found in a Burgundy Fawn rabbit in heterozygous condition; p.K224T that was detected in six wild rabbits, three were homozygous and three were heterozygous for the mutated allele. All these wild rabbits had the wild grey/brown coat colour.
In wild mammals, the TYR gene is known to be under natural selective pressure due to its relevance in producing pigments for mimicry (Hubbard et al. Citation2010). In wild rabbits, the need to preserve a functional tyrosinase enzyme is crucial for the fitness of the animals. The new missense mutation (p.K224T) detected in wild rabbits is located between the two cupper binding domains (CuA and CuB) of the TYR enzyme but, according to the in silico SIFT results, this amino acid substitution could be tolerated (p = .13) and it might be neutral. The in silico prediction of the other missense mutation (p.T144S) (p = .25) and the fact that both novel missense mutations were not strain or breed-specific may suggest that they do not play any relevant role in altering the function of the TYR protein and, in turn, they should not have any major effect on the coat colour of rabbits. Based on these evidences, they could be considered additional alleles at the Albino locus which give full colour (with normal melanin production and dark eye-colour) and could be considered different forms of C (or wild type alleles).
A total of seven novel synonymous polymorphisms located in exon 1 and exon 2, six novel polymorphisms located in intronic regions (two in intron 2, two intron 3 and two in intron 4) and one novel SNP in the 3′-untranslated region (UTR) were identified in some domestic breeds and in wild rabbits (Table ). Wild rabbits carried most of these variants, a few of which were identified only in this population (Table ).
Analysing the obtained sequence data for the coding region of the rabbit TYR gene, a total of 31 different haplotypes could be inferred. Figure S1 reports the phylogenetic relationships that was obtained using these haplotypes. Some haplotypes were shared by more than one breed and several breeds had more than one haplotype. For example, it was interesting to note that two haplotypes were detected in the albino New Zealand White rabbits if we consider only the coding region (three were the haplotypes if we consider also intron variants), all carrying the mutated nucleotide at the p.T373K polymorphic site. Two haplotypes were identified in Californian and two also in Giant Chinchilla rabbits that are the two breeds that have a characteristic coat colour determined by alleles of the C series and that differed by only one polymorphic position (p.T358I) that is present in Chinchilla rabbits but not in Californian rabbits. Three haplotypes were identified in Vienna White rabbits that, despite their white colour, are not affected by any albinism. Two different haplotypes were identified in Burgundy Fawn and in Belgian Hare breeds whereas nine haplotypes were identified in wild rabbits.
Conclusions
Coat colour is considered one of the first traits on which domestication operated. The complete characterisation of all coat colour genes in rabbit might provide additional hints on the domestication processes and on the constitution of the large number of fancy breeds. Coat colour genes might be also relevant in the adaptation of wild and feral rabbit populations to different environments. This study further contributed to disclose the variability of the TYR gene in different rabbit breeds and confirmed the presence of a few mutations already described to cause different coat colours in Oryctolagus cuniculus.
From all these data, it seems however that we still miss some information that could help to clarify the presence (or alternatively) the absence of a few other alleles of the C series that have been predicted by classical genetic studies, as it seems that some chinchilla alleles have not been characterised at the molecular level yet (Searle Citation1968). The TYR gene should be analysed in other breeds or in rabbits with precise grades of chinchilla patterns to further evaluate this question. It could be also possible that coat colour variants attributed to alleles of this locus could be actually derived by the action of modifier genes and not by the effect of other mutations at the rabbit TYR gene.
Ethical approval
Wild rabbits were provided by hunters who hunted the animals under the hunting rules of Sardinia. Animals were not treated in any way or killed for the purpose of this study. All authors declare that this study follows the principles of the Declaration of Helsinki.
tjas_a_1877574_sm1226.docx
Download MS Word (107.1 KB)Acknowledgements
The authors thank rabbit breeders and hunters for the collaboration in this study, in particular the self-managed groups of Compagnia Lecca di San Basilio and Mr. Giuliano Pesce (Campu Chervaggiu group).
Disclosure statement
None of the authors has a financial or personal relationship with other people or organisations that could inappropriately influence this publication.
Additional information
Funding
References
- Aigner B, Besenfelder U, Müller M, Brem G. 2000. Tyrosinase gene variants in different rabbit strains. Mamm Genome. 11(8):700–702.
- Anistoroaei R, Fredholm M, Christensen K, Leeb T. 2008. Albinism in the American mink (Neovison vison) is associated with a tyrosinase nonsense mutation. Anim Genet. 39(6):645–648.
- Beermann F, Orlow SJ, Lamoreux ML. 2004. The Tyr (albino) locus of the laboratory mouse. Mamm Genome. 15(10):749–758.
- Berson JF, Frank DW, Calvo PA, Bieler BM, Marks MS. 2000. A common temperature-sensitive allelic form of human tyrosinase is retained in the endoplasmic reticulum at the nonpermissive temperature. J Biol Chem. 275(16):12281–12289.
- Carneiro M, Rubin CJ, Di Palma F, Albert FW, Alföldi J, Martinez Barrio A, Pielberg G, Rafati N, Sayyab S, Turner-Maier J, et al. 2014. Rabbit genome analysis reveals a polygenic basis for phenotypic change during domestication. Science. 345(6200):1074–1079.
- Demars J, Iannuccelli N, Utzeri VJ, Auvinet G, Riquet J, Fontanesi L, Allain D. 2018. New insights into the melanophilin (MLPH) gene affecting coat color dilution in rabbits. Genes (Basel). 9(9):430.
- Fontanesi L, Forestier L, Allain D, Scotti E, Beretti F, Deretz-Picoulet S, Pecchioli E, Vernesi C, Robinson TJ, Malaney JL, et al. 2010a. Characterization of the rabbit agouti signaling protein (ASIP) gene: transcripts and phylogenetic analyses and identification of the causative mutation of the nonagouti black coat colour. Genomics. 95(3):166–175.
- Fontanesi L, Scotti E, Allain D, Dall’Olio S. 2014a. A frameshift mutation in the melanophilin gene causes the dilute coat colour in rabbit (Oryctolagus cuniculus) breeds. Anim Genet. 45(2):248–255.
- Fontanesi L, Scotti E, Colombo M, Beretti F, Forestier L, Dall'Olio S, Deretz S, Russo V, Allain D, Oulmouden A. 2010b. A composite six bp in-frame deletion in the melanocortin 1 receptor (MC1R) gene is associated with the Japanese brindling coat colour in rabbits (Oryctolagus cuniculus). BMC Genet. 11:59.
- Fontanesi L, Tazzoli M, Beretti F, Russo V. 2006. Mutations in the melanocortin 1 receptor (MC1R) gene are associated with coat colours in the domestic rabbit (Oryctolagus cuniculus). Anim Genet. 37(5):489–493.
- Fontanesi L, Vargiolu M, Scotti E, Latorre R, Faussone Pellegrini MS, Mazzoni M, Asti M, Chiocchetti R, Romeo G, Clavenzani P, et al. 2014b. The KIT gene is associated with the English spotting coat color locus and congenital megacolon in Checkered Giant rabbits (Oryctolagus cuniculus. PLoS One. 9(4):e93750.
- Goldfeder M, Kanteev M, Isaschar-Ovdat S, Adir N, Fishman A. 2014. Determination of tyrosinase substrate-binding modes reveals mechanistic differences between type-3 copper proteins. Nat Commun. 5(1):1–5.
- Hearing VJ. 2011. Determination of melanin synthetic pathways. J Invest Dermatol. 131(E1):E8–E11.
- Hubbard JK, Uy JAC, Hauber ME, Hoekstra HE, Safran RJ. 2010. Vertebrate pigmentation: from underlying genes to adaptive function. Trends Genet. 26(5):231–239.
- Imes DL, Geary LA, Grahn RA, Lyons LA. 2006. Albinism in the domestic cat (Felis catus) is associated with a tyrosinase (TYR) mutation. Anim Genet. 37(2):175–178.
- Kanteev M, Goldfeder M, Fishman A. 2015. Structure-function correlations in tyrosinases. Protein Sci. 24(9):1360–1369.
- Kumar P, Henikoff S, Ng PC. 2009. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 4(7):1073–1081.
- Lamoreux ML, Delmas V, Larue L, Bennett DC. 2010. The colors of mice. A model genetic network. West Sussex, UK: Wiley-Blackwell, John Wiley & Sons Ltd.
- Lin JY, Fisher DE. 2007. Melanocyte biology and skin pigmentation. Nature. 445(7130):843–850.
- McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GR, Thormann A, Flicek P, Cunningham F. 2016. The Ensembl variant effect predictor. Genome Biol. 17(1):122.
- Montoliu L, Oetting WS, Bennett DC. 2009. Color Genes. European Society for Pigment Cell Research. [accessed 2020 August 17]. Available from: http://www.espcr.org/micemut.
- Oetting WS, King RA. 1999. Molecular basis of albinism: mutations and polymorphisms of pigmentation genes associated with albinism. Hum Mutat. 13(2):99–115.
- Rees JL. 2011. The genetics of human pigmentary disorders. J Invest Dermatol. 131(E1):E12–E13.
- Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory.
- Sanabria D, Groot H, Guzmán J, Lattig MC. 2012. [An overview of oculocutaneous albinism: TYR gene mutations in five Colombian individuals]. Biomedica. 32(2):269–276.
- Schmutz SM, Berryere TG, Ciobanu DC, Mileham AJ, Schmidt BH, Fredholm M. 2004. A form of albinism in cattle is caused by a tyrosinase frameshift mutation. Mamm Genome. 15(1):62–67.
- Searle AG. 1968. Comparative genetics of coat colour in mammals. London (UK): Logos Press Limited.
- Searle AG. 1990. Comparative genetics of albinism. Ophthalmic Paediatr Genet. 11(3):159–164.
- Song Y, Zhang Y, Chen M, Deng J, Sui T, Lai L, Li Z. 2018. Functional validation of the albinism-associated tyrosinase T373K SNP by CRISPR/Cas9-mediated homology-directed repair (HDR) in rabbits. EBioMedicine. 36:517–525.
- Stephens M, Smith N, Donnelly P. 2001. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 68(4):978–989.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 30(12):2725–2729.
- Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG. 2012. Primer3-new capabilities and interfaces. Nucleic Acids Res. 40(15):e115
- Utzeri VJ, Bertolini F, Ribani A, Schiavo G, Dall'Olio S, Fontanesi L. 2016. The albinism of the feral Asinara white donkeys (Equus asinus) is determined by a missense mutation in a highly conserved position of the tyrosinase (TYR) gene deduced protein. Anim Genet. 47(1):120–124.
- Utzeri VJ, Ribani A, Fontanesi L. 2014. A premature stop codon in the TYRP1 gene is associated with brown coat colour in the European rabbit (Oryctolagus cuniculus). Anim Genet. 45(4):600–603.
