Abstract
The aim of the present study was to evaluate the effect of different dietary zinc sources (ZnSO4, Zinc-amino acid and Zn5(OH)2Cl2H2O), on hormone levels, testicular histology and sperm quality in broiler breeder roosters. Twenty, 54-week-old broiler breeder roosters (Ross 308) were reared in individual cages. Dietary treatments were T1: a basal diet without any addition of zinc (0 Zn) (Control), T2: control diet supplemented with 110 mg Zn (sulphate)/kg, T3: control diet supplemented with 110 mg Zn (organic)/kg and T4: control diet supplemented with 110 mg Zn (hydroxide)/kg. The traits measured included blood serum IGF- and testosterone, motility, concentration, viability, and membrane integrity of sperm and testicular histology. The results indicated that, significant differences between the experimental treatments in terms of serum levels of IGF and testosterone in the 32nd and 45th weeks (p < .05). The highest levels of IGF and testosterone were observed in the organic zinc treatment (T3). The results of testicular histology, fertility and sperm quality showed that there was a significant difference between experimental treatments in terms of diameter of seminiferous tubules, spermatogonia and the motility, integrity, morphology, viability and concentration of sperm (p < .05). The highest levels of these traits were observed in the organic zinc treatment (T3) and the lowest in the control treatment. In conclusion, organic zinc compared to other forms of zinc supplement can be used as an efficient feed additive for improving the fertility status of the flock of broiler breeder roosters.
Mineral resources are used in various ways in poultry feed.
Zinc is one of the most important mineral sources in poultry nutrition.
Organic zinc has been shown to perform better than other sources of zinc.
HIGHLIGHTS
Introduction
Zinc potentiates the activity of some hormones including glucagon, insulin, sex and growth hormones. In addition, it plays a substantial role in the immune system, and in the transport and use of vitamin A (Chand et al. Citation2014). Inorganic minerals, such as sulphates and oxides, are usually supplemented in poultry rations above the recommended NRC requirement to improve feed utilisation and maximise production performances (Leeson and Caston Citation2008). There are two inorganic feed-grade Zn sources in a commercial sense, which are utilised by the poultry feed industries (Leeson and Summers Citation1997): Zn oxide (ZnO: 72% Zn) and Zn sulphate monohydrate (ZnSO4–H2O: 36% Zn). Most (80–90%) of the supplemental Zn used in poultry diets comes from ZnO source, which is less bioavailable than Zn sulphate for poultry (Sandoval et al. Citation1997). However, sulphate (acid salt) is more water-soluble, allowing reactive metallic ions to promote free-radical formation. This can support chemical reactions responsible for the breakdown of vitamins and eventually the destruction of fats and essential oils, down rating the nutritive value of the diets. Oxides are less reactive but are again less bioavailable (Batal et al. Citation2001). Organic Zn sources have become a component in more recent diets used in the feed industry (Mohammadi et al. Citation2015). The reproductive performance in most animal species was significantly influenced by nutrition (Kidd Citation2004). Hence, using an adequate level of nutrients in the diet would improve the reproductive function in broiler breeders (Zhandi et al. Citation2016). In poultry, diets trace minerals are essential as they play important roles in male and female breeders’ reproduction performance (Ruan et al. Citation2012). Zinc is one of the most important essential trace minerals for the reproduction, biochemical processes, and also is a cofactor for many metalloenzymes. Zinc is an essential mineral for all farm animals and it is the mineral among others that has a higher biological activity. This element is important for fertility, infertility and in various enzyme structures (Naz et al. Citation2016).
Li et al. (Citation2019) had also reported higher fertility in Chinese yellow feathered chicken supplemented with zinc (48–120 mg/kg). Cobb 500 broiler breeder hens supplemented with zinc oxide (60–120 mg/kg) had higher fertility at the later phase of the laying period (Sharideh et al. Citation2016). The result of a study showed that the addition of ZnO to the semen extender had some beneficial effects on freezability of rooster spermatozoa, and ameliorated deleterious-associated effects due to cryopreservation on post-thaw motility, viability and apoptosis status (Zhandi et al. Citation2020). More recently, a study by Khoobbakht et al. (Citation2018) suggested that dietary supplementation with zinc-methionine can improve reproductive parameters of male Japanese quail. The majority of studies in this respect confirmed that organic Zn sources like ZnMet or Zn-propionate were better than inorganic sources such as ZnO or ZnSO4 because they are more bioavailable than inorganic sources (Abd El-Hack et al. Citation2017).
The key role of zinc is associated with its presence as a vital part of enzymes involved in producing sex hormones . Zinc supplementation in broiler roosters’ diets increases semen volume, improves semen quality, significantly increases the concentrations of oestrogen and progesterone, improves sperm motility and reduces the percentage of abnormal or dead sperms and chromosomal anomalies. Adding zinc to the diet of broiler breeder roosters can therefore improve the fertility of roosters and the entire flock (Amen and Al-Daraji Citation2011a, Citation2011b). However, few studies have reported no effect of dietary zinc supplementation on the fertility of broiler breeder roosters (Stahl et al. Citation1986; Alagarsamy and Mahapatra Citation2020). Therefore, the following study was designed to investigate the effect of different sources of zinc on the level of hormones such as insulin-like growth factor 1 (IGF-1) and testosterone, testicular histology and sperm quality in a flock of broiler breeder roosters.
Materials and methods
Birds management and experimental groups
The experiment was approved by the Animal Care Committee and was conducted in a broiler breeder farm in Savadkoh, Mazandaran province, Iran. The experimental trial lasted 45 weeks after the bird’s arrival.
Chemicals utilised in the current trial were provided from Merck (Darmstadt, Germany) and Sigma-Aldrich Company (St. Louis, MO, USA).
Twenty, 54-week-old broiler breeder roosters (Ross 308) were reared in individual cages under 14 L:10D photoperiods and at a temperature of 22–24 °C. Birds were fed a diet containing 2754.57 kcal ME/kg, 12% crude protein, 0.9% calcium and 0.45% available phosphorus (). After 2 weeks of habituation (to 60 weeks of age) to the experimental conditions and semen collection by abdominal massage (Burrows and Quinn Citation1937), semen samples were weekly collected for five successive weeks (during weeks 61 to 65 of age). Semen samples reaching the desired primary semen quality, that is, normal appearance, 0.3–0.6 mL volume, semen concentration of ≥3 × 109 spermatozoa/mL and 80% motility, were mixed to minimise individual rooster effects. After that, it was split into three similar amounts and diluted with a semen extender containing ZnO at either 0, 1 or 2 µg/mL concentration. Treatment groups were as following: T1: Birds fed the basal diet without any addition of zinc (0 Zn) (Control), T2: Birds fed diet supplemented with 110 mg Zn (sulphate)/kg of GIVAN SHIMI, T3: Birds fed a diet supplemented with 110 mg Zn (organic)/kg of ZINPRO and T4: Birds fed a diet supplemented with 110 mg Zn (hydroxide)/kg of IntelliBond.
Measured traits
Insulin-like growth factor-1 (IGF-1) and testosterone
Ten birds were randomly selected from each group. Blood (4 mL each) was collected from the wing vein and centrifuged at 3000 × g and 4 °C for 10 min to obtain serum, which was stored at −80 °C for further analysis. The serum concentration of IGF-1 were determined using an enzyme-linked immunosorbent assay kit following the manufacturer’s protocols (ELISA; Uscn Life Science Inc. Wuhan, China) with minimum detectable concentrations of 7.4 pg mL−1.
Testosterone was sampled at two stages during the 32nd and 45th weeks by taking 2 mL of blood from the wing vein of the roosters. After preparing the kits according to the manufacturer’s instructions, the samples were analysed by pouring 25 µL of the sample serum along with 100 µL of a conjugated solution into a special progesterone kit and performing incubation at 37 °C for 1 h. Afterwards, the kit was emptied and rinsed three times with 300 µL of the special solution. After adding 100 µL of a substrate solution, the kit was placed in a dark room at 22–28 °C for 15 min. One hundred microlitre of a stop solution was ultimately added to the kit, and optical density was read at 450 nm using ELISA. The commercial kit used for testosterone was DIMEDIC Diagnostic GmbH, Kiel, Germany; EIA1559.
Fertilisation
Sperm motility
To measure sperm motility, a drop of the thawed sample was put on a Makler chamber (37 °C) and then conveyed under a phase-contrast microscope (Labomed, Lx 400, USA) at a magnification of 100×. In this study, total motility (TM, %), progressive motility (PM, %), straight linear velocity (VSL, μm/s), curvilinear velocity (VCL, μm/s), average path velocity (VAP, μm/s), linearity (LIN, %), sperm track straightness (STR, %), the amplitude of lateral head displacement (ALH, μm), and wobble coefficient (WOB %: VAP/VCL) were measured (Zhandi et al. Citation2020).
Sperm concentration
Semen samples were initially evaluated for sperm concentration. The samples were diluted with cryoprotectant free diluent such that the sperm concentration arrived at 4 million/μL. The samples were equilibrated at 5 °C for 30 min and were diluted in 1:1 proportion with a diluent containing 8% dimethyl sulfoxide (DMSO) so that the final concentration of DMSO was 4% and the final sperm concentration was 2 million/μL in each treatment. The semen mixed with DMSO was immediately loaded into 0.5 mL French straws and sealed with polyvinyl alcohol powder. The filled straws were placed 4.5 cm above the liquid nitrogen (LN2) on a Styrofoam raft floating on LN2 in a thermocol box. The straws were exposed to nitrogen vapours for 30 min, plunged into LN2 and stored at −196 °C until further use. Semen straws were stored for a minimum of 7 days before evaluation. Cryopreserved semen after thawing at 5 °C for 100 s in ice water was evaluated on nine different occasions for progressive sperm motility, live and abnormal sperm and intact sperm acrosome (Alagarsamy and Mahapatra Citation2020).
Sperm viability
A 20 µL of sperm suspension (1:10) was mixed with an equal volume of 0.05% eosin-Y on a slide. After 20–30 s, 20 µL nigrosin was added. Slides were examined using a light microscope with 400× magnification following 2 min incubation at room temperature. Dead sperms appeared to be pink and live sperms were not stained. In each sample, 200 sperms were counted and their viability (%) were recorded (Zahra et al. Citation2013).
Sperm membrane integrity (hypo-osmotic swelling test)
The hypoosmotic swelling (HOS) test evaluates the functional integrity of the sperm’s plasma membrane and also serves as a useful indicator of the fertility potential of sperm. The HOS test predicts membrane integrity by determining the ability of the sperm membrane to maintain equilibrium between the sperm cell and its environment. Influx of the fluid due to hypo-osmotic stress causes the sperm tail to coil and balloon or ‘swell’. A higher percentage of swollen sperm indicates the presence of sperm having a functional and intact plasma membrane (Ramu and Jeyendran Citation2013). The sperm membrane integrity was determined through the hypo-osmotic swelling test as described for breeder roosters spermatozoa (Santiago-Moreno et al. Citation2009). In a glass tube, 25 L of diluted semen was taken and 500 L of hypo-osmotic solution (100 mOsm/kg; 1 g of sodium citrate in 100 mL distilled water) was added and incubated for 30 min at 37 °C. The samples fixed in 25 L of 2% glutaraldehyde were examined at 400× under a phase-contrast microscope by counting 200 sperm. The percentage of spermatozoa having coiled mid-pieces and tails were calculated (Ramu and Jeyendran Citation2013).
Testicular histology
Roosters were humanely slaughtered at the end of the experiment and both testicles were carefully removed, weighed and the testis index calculated (as testis weight/bodyweight; Sarabia Fragoso et al. (Citation2013). The right testicle from each rooster was removed and was immersed in 10% buffered formalin (pH 7.4) and embedded in paraffin. Sections (4 mm) were cut and subsequently stained using the haematoxylin–eosin technique (Sarabia Fragoso et al. Citation2013). Morphometric data were generated from 20 photomicrographs randomly selected from four cross-sections of each testis at a magnification of 10 using a light microscope (Carl Zeiss Microscopy GmbH) equipped with Dino-Eye Eyepiece Camera (AnMo Electronics) and analysed with ImageJ software (National Institutes of Health). The diameter of the seminiferous tubules, the thickness of the seminiferous epithelium and the number of spermatogonia (identified by their cytoplasmic and nuclear morphology) were determined in 20 randomly selected cross-sections of seminiferous tubules from each bird and averaged (Islam et al. Citation2010). The number of Leydig cells and blood vessels was also determined in each photomicrograph (0.37 mm) and averaged.
Statistical analysis
Data were analysed statistically using the General Linear Models (GLM) procedure of SAS 9.4 (SAS Citation1999). Significant differences between treatment means are separated using Duncan’s multiple range test with 5% probability (Duncan Citation1955).
Results
Serum levels of sex hormones
The results of Table show significant differences between the experimental treatments in terms of serum levels of IGF and testosterone in the flock of broiler breeder roosters in the 32 and 45 weeks (p < .05). The highest levels of IGF and testosterone were observed in the organic zinc treatment and the lowest in the control treatment. No significant differences were observed between the experimental treatments in terms of serum levels of oestrogen and progesterone in the broiler breeder roosters in weeks 32 and 45.
Table 2. The effect of different sources of zinc on serum levels of testosterone in the birds.
Testicular histology, fertility and sperm quality
The results of Table shows significant differences between the treatments in terms of diameter of seminiferous tubules, spermatogonia (Figure ) and the sperm motility, integrity, morphology, viability and concentration of sperm (p < .05). The highest levels of these traits were observed in the organic zinc treatment and the lowest in the control treatment. No significant differences were observed between the treatments in terms of epithelial thickness.
Figure 1. Cross sections in birds from the 4 experimental groups in the roosters fed with different sources of zinc (A) no zinc supplements, (B) zinc sulphate supplement, (C) organic zinc, (D) zinc hydroxide. 1. Seminiferous tubule diameter, 2. Spermatogonia, 3. Thickness of the epithelium of seminiferous tubules; magnification: ×100, scale bar: 50 µm.
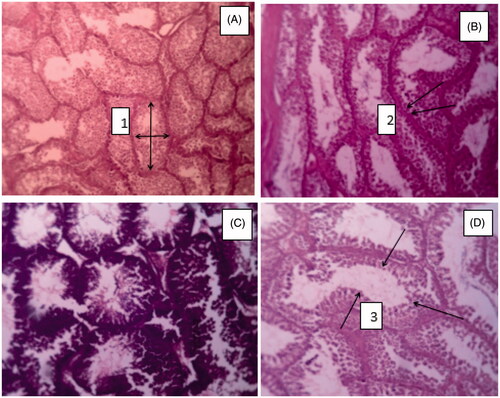
Table 3. Effects of different sources of zinc on testicular histology, fertility and sperm quality in the flock of broiler breeder roosters.
Discussion
The level of serum testosterone and IGF
Investigating sex hormones’ levels the highest levels of IGF and testosterone were higher in organic zinc supplement birds, which showed that this organic source improves sex hormone levels in roosters. The results of a study showed that significant increases in concentrations of oestrogen and progesterone by adding different levels of zinc to the diet and reported significant improvements in sex hormones and reproductive function using the zinc-containing diet (Hazim and Mahmood Citation2011). In the literature, it is reported that organic trace minerals premix fed roosters presented higher serum testosterone levels at 45 weeks of age (Shan et al. Citation2017).
In the present study, dietary organic zinc treatment increased serum testosterone levels. In order to investigate the potential mechanisms underlying the organic zinc-induced testosterone increase, the mRNA expression of enzymes related to the testosterone biosynthesis was measured. The HSD3B2, CYP17A1, hydroxysteroid 17-beta dehydrogenase 4 (HSD17B4) genes are known to encode enzymes involved in the biosynthesis of testosterone (Rabbani et al. Citation2012).
In a recent study, Daragò et al. (Citation2020) reported that zinc alone leads to an increase in serum testosterone concentrations in rats(Daragó et al. Citation2020). Badran et al. (Citation2018) indicated that serum testosterone level was significantly higher in the group Zn group. Brown and Pentland (Citation2007) reported that Zn is the most critical trace mineral for the synthesis of testicosteroid, spermatogenesis, successful fertilisation, testosterone secretion, and male sexual health. The results of some studies report that zinc plays an important role in the synthesis of steroid hormones and prostaglandins (Guo et al. Citation2002; Brown and Pentland Citation2007).
Our results showed that organic zinc (Zinc-amino acid) to be more effective than other sources in improving reproductive indices in this rooster flock (Table ). Alagarsamy and Mahapatra (Citation2020) reported that organic zinc supplementation did not improve fertility in white Leghorn rooster. The results of a study showed that zinc oxide (60–120 mg/kg) had higher fertility at the later phase of the laying period in Cobb 500 broiler breeder hens (Sharideh et al. Citation2016).
The results of Zhandi et al. (Citation2020) showed that the addition of ZnO affected total motility, progressive motility, and average path velocity with the highest values were noted in the ZnO-1 group. Levels of ZnO affected percentages of live and dead spermatozoa, where the highest percentage of living or dead spermatozoa was for the ZnO-1 group. Sperm motility has a direct correlation with fertilising ability. Among different sperm quality indices, motility (especially progressive motility) has the greatest impact on prolonging the fertilisation period in avian species (Froman Citation2003).
The results of several studies showed that the addition of ZnO had some beneficial effects on freezability of rooster spermatozoa (Zhandi et al. Citation2020) and improve reproductive parameters (Khoobbakht et al. Citation2018). Ali et al. (Citation2007) and Fernandez (Citation2008) reported that organic zinc improves sperm motility, which is consistent with the present results. Moreover, improved density and relative weight of seminiferous tubules observed in the roosters supplemented with organic zinc was potentially caused by the thickening of germ cells. Increasing sperm motility constitutes the main role of zinc in fertility (Bjorndahl and Kvist Citation1982). Zinc also affects sperm motility by providing the required energy through activating ATPase and thereby releasing energy from phospholipids (Swarup and Sekhon Citation1976).
Talebi et al. (Citation2013) found zinc nanoparticles to increase sperm count and motility. Manuja et al. (Citation2012) found zinc supplements to significantly increase sperm penetration. Zinc contributes to cell proliferation and differentiation, especially in the metabolism of nucleic acid. Kumar et al. (Citation2006) found significant increases in the qualitative and quantitative characteristics of semen such as semen volume and density, the motility and percentage of viable sperms as well as testosterone concentration by adding zinc to the diet.
Zinc deficiency in poultry reduces semen quality, that is, an approximate 10% reduction in sperm motility, and leads to frail chickens and their mortality (Huang et al. Citation2019). A study on Cobb 500 roosters found zinc supplementation to significantly increase spermatocrit, semen volume, mass motility and individual motility and reduce the percentage of dead and abnormal sperms and acrosomal anomalies (Amen and Al-Daraji Citation2011a). Sahin and Tasdemir (Citation2017) reported that adding 60 mg/kg of zinc supplements significantly improved hatchability, fertility, mortality and conversion rate in breeder hens. The result of a study showed that the addition of zinc supplements (100 mg/kg) led to improvements in semen quality, such as live spermatozoa in broiler breeder males (Gallo et al. Citation2003). Shanmugam et al. (Citation2015) found organic zinc supplementation improved semen quality in broiler breeder roosters.
Conclusions
The study showed that the addition of organic zinc supplementation in the diet, increased blood serum IGF-1 and testosterone. In addition, zinc supplementation (Zinc-amino acid) improved testicular histology, fertility and sperm quality in the flock of broiler breeder roosters. Organic zinc supplementation compared to other forms of zinc, improved some of the reproductive indices studied in the present study and is recommended to increase the sperm quality of roosters.
Ethical approval
All experimental procedures were performed according to the guidelines for the ethical treatment of experimental animals and approved by the Department of Animal Science, Islamic Azad University (Qaemshahr Branch, Qaemshahr, Iran), Animal Care and Use Local Ethics Committee.
Acknowledgements
The authors appreciate the support of Islamic Azad University, Qaemshahr Branch.
Disclosure statement
The authors declare that there is no conflict of interest associated with the paper. The authors alone are responsible for the content and writing of this article.
Table 1. Feed ingredients and chemical composition of basal diets.
References
- Abd El-Hack ME, Alagawany M, Arif M, Chaudhry MT, Emam M, Patra A. 2017. Organic or inorganic zinc in poultry nutrition: a review. World’s Poult Sci J. 73(4):904–915.
- Alagarsamy K, Mahapatra R. 2020. Effect of organic zinc supplementation in hens on fertility from cryopreserved semen. bioRxiv
- Ali H, Ahmed M, Baig M, Ali M. 2007. Relationship of zinc concentrations in blood and seminal plasma with various semen parameters in infertile subjects. Pak J Med Sci. 1l:111.
- Amen M, Al-Daraji HJ. 2011a. Effect of dietary zinc on semen quality of Cobb 500 broiler breeder males. Poult Sci. 10:447–482.
- Amen M, Al-Daraji HJ. 2011b. Effect of dietary zinc supplementation on some seminal plasma characteristics of broiler breeders males. Int J of Poultry Science. 10(10):814–818.
- Badran AM, Jatab MH, Sabic EM. 2018. Physiological response of growing Gimmizah chicks to zinc and/or creatinemonohydrate supplementation in drinking water. Egypt J Poult Sci. 2:483–496.
- Batal AB, Parr TM, Baker DH. 2001. Zinc bioavailability in tetrabasic zinc chloride and the dietary zinc requirement of young chicks fed a soy concentrate diet. Poult Sci. 80(1):87–90.
- Bjorndahl L, Kvist U. 1982. Importance of zinc for human sperm head tail connection. Acta Physiol Scand. 116(1):51–55.
- Brown L, Pentland S. 2007. Health infertility organization: male infertility-improving sperm quality. Vancouver (Canada): Acubalance Wellness Centre Ltd.
- Burrows WH, Quinn JP. 1937. The collection of spermatozoa from the domestic fowl and turkey. Poult Sci. 16(1):19–24.
- Chand N, Naz S, Khan A, Khan S, Khan RU. 2014. Performance traits and immune response of broiler chicks treated with zinc and ascorbic acid supplementation during cyclic heat stress. Int J Biometeorol. 58(10):2153–2157.
- Daragó A, Klimczak M, Stragierowicz J, Stasikowska-Kanicka O, Kilanowicz A. 2020. The effect of zinc, selenium, and their combined supplementation on androgen receptor protein expression in the prostate lobes and serum steroid hormone concentrations of Wistar rats. Nutrients. 12(1):153.
- Duncan DB. 1955. The new multiple range and F test. Biometrics. 11(1):1–42.
- Fernandez LC, Perez-Llano B, Garcia-Casado P, Sala R, Gosalbez A, Arroyo F, Fernandez JL, Gosalvez J. 2008. Sperm DNA fragmentation in a random sample of the Spanish boar livestock. Anim Reprod Sci. 103:87–98.
- Froman D. 2003. Deduction of a model for sperm storage in the oviduct of the domestic fowl (Gallus domesticus). Biol Reprod. 69(1):248–253.
- Gallo R, Veronico M, Nacucchi O, Tafaro E, Barile P, Nicastro F, Zezza L. 2003. The effects of selenium, zinc and vitamin E supplementation on performance of broiler breeder males. Ital J AnimSci. 2:471–473.
- Guo YM, Yang R, Yuan J, Ward TL, Fakler TM. 2002. Effect of Available Zn and ZnSO4 on laying hen performance and egg quality. Poult Sci. 81:40.
- Hazim J, Mahmood HM. 2011. Effect of dietary zinc on certain blood traits of broiler breeder chickens. Int J Poult Sci. 10:807–813.
- Huang L, Li X, Wang W, Yang L, Zhu Y. 2019. The role of zinc in poultry breeder and hen nutrition: an update. Biol Trace Elem Res. 192(2):308–311.
- Islam MN, Zhu ZB, Aoyama M, Sugita S. 2010. Histological and morphometric analyses of seasonal testicular variations in the jungle crow (Corvus macrorhynchos). Anat Sci Int. 85(3):121–129.
- Khoobbakht Z, Mohammadi M, Mehr MRA, Mohammadghasemi F, Sohani MM. 2018. Comparative effects of zinc oxide, zinc oxide nanoparticle and zinc-methionine on hatchability and reproductive variables in male Japanese quail. Anim Reprod Sci. 192:84–90.
- Kidd M. 2004. Nutritional modulation of immune function in broilers. Poult Sci. 83(4):650–657.
- Kumar N, Verma RP, Singh LP, Varshney VP, Dass RS. 2006. Effect of different levels and sources of zinc supplementation on quantitative and qualitative semen attributes and serum testosterone level in crossbred cattle (Bos indicus × Bos taurus) bulls. Reprod Nutr Dev. 46(6):663–675.
- Leeson S, Caston L. 2008. Using minimal supplements of trace minerals as a method of reducing trace mineral content of poultry manure. Anim Feed Sci Technol. 142(3–4):339–347.
- Leeson S, Summers JD. 1997. Commercial poultry nutrition. 2nd ed. Guelph (Canada): University Books.
- Li L, Abouelezz KFM, Gou Z, Lin X, Wang Y, Fan Q, Cheng Z, Ding F, Jiang S, Jiang Z. 2019. Optimization of dietary zinc requirement for broiler breeder hens of Chinese yellow-feathered chicken. Animals. 9(7):472.
- Manuja A, Kumar B, Sing RK. 2012. Nanothechnology developments: opportunities for animal health and production. Nanotechnol Dev. 2(1):4–12.
- Mohammadi V, Ghazanfari S, Mohammadi-Sangcheshmeh A, Nazaran MH. 2015. Comparative effects of zinc-nano complexes, zinc-sulphate and zinc-methionine on performance in broiler chickens. Br Poult Sci. 56(4):486–493.
- Naz S, Idris M, Khalique MA, Zia-Ur-RahmanAlhidary IA, Abdelrahman MM, Ahmad S. 2016. The activity and use of zinc in poultry diets. World’s Poult Sci J. 1:159–167.
- Rabbani B, Mahdieh N, Ashtiani MTH, Setoodeh A, Rabbani A. 2012. In silico structural, functional and pathogenicity evaluation of a novel mutation: an overview of HSD3B2 gene mutations. Gene. 503(2):215–221.
- Ramu S, Jeyendran RS. 2013. The hypo-osmotic swelling test for evaluation of sperm membrane integrity. In Spermatogenesis. Totowa (NJ): Humana Press; p. 21–25.
- Ruan H, Zhang Z, Wu Q, Yao H, Li J, Li S, Xu S. 2012. Selenium regulates gene expression of selenoprotein W in chicken skeletal muscle system. Biol Trace Elem Res. 145(1):59–65.
- Sahin T, Tasdemir AN. 2017. The effects of organic and inorganic zinc supplemented in breeder hens’ rations on hatching traits and chick quality (II). Vet Hekim Der Derg. 1:3–12.
- Sandoval M, Henry PR, Ammerman CB, Miles RD, Littell RC. 1997. Relative bioavailability of supplemental inorganic zinc sources for chicks. J Anim Sci. 75(12):3195–3205.
- Santiago-Moreno J, Castaño C, Coloma MA, Gómez-Brunet A, Toledano-Díaz A, López-Sebastián A, Campo JL. 2009. Use of the hypo-osmotic swelling test and aniline blue staining to improve the evaluation of seasonal sperm variation in native Spanish free-range poultry. Poultr Sci. 88(12):2661–2669.
- SAS. 1999. SAS Statistics user's guide. Statistical analytical system (5th revised edn). SAS Institute Inc, Carry.
- Sarabia Fragoso J, Pizarro Díaz M, Abad Moreno JC, Casanovas Infesta P, Rodriguez-Bertos A, Barger K. 2013. Relationships between fertility and some parameters in male broiler breeders (body and testicular weight, histology and immunohistochemistry of testes, spermatogenesis and hormonal levels. Reprod Domest Anim. 48(2):345–352.
- Shan T, Dai P, Zhu P, Chen L, Wu W, Li Y, Li C. 2017. Effect of an organic trace mineral premix on the semen quality, testicular morphology and gene expression related to testosterone synthesis of male broiler breeders. Rev Bras Cienc Avic. 19(3):481–488.
- Shanmugam M, Prakash B, Reddy EPK, Panda AK. 2015. Dietary organic zinc and selenium supplementation improves semen quality and fertility in layer breeders. Indian J AnimSci. 85:202–204.
- Sharideh H, Zhandi M, Zaghari M, Akhlaghi A. 2016. Dietary zinc oxide and 6-phytase effects on fertility rate in old broiler breeder hens. J Agr Sci Tech. 18:327–336.
- Stahl JL, Cook ME, Sunde ML. 1986. Zinc supplementation: its effect on egg production, feed conversion, fertility, and hatchability. Poult Sci. 65(11):2104–2109.
- Swarup D, Sekhon H. 1976. Correlation of vitamin A and zinc concentration of seminal plasma to fertility of bovine semen [Cattle]. Nutr Rep Int. 13:37–42.
- Talebi AR, Khorsand L, Moridian M. 2013. The effect of zinc oxide nanoparticles on mouse spermatogenesis. J Assist Reprod Genet. 30(9):1203–1209.
- Zahra A, Gholamreza N, Farokhi F, Jalali AS. 2013. Attenuation of cyclosporine-induced sperm impairment and embryotoxicity by Crataegus monogyna fruit aqueous extract. Cell J. 3:198.
- Zhandi M, Sharideh H, Zaghari M, Akhlaghi A. 2016. Dietary zinc oxide and 6-phytase effects on fertility rate in old broiler breeder hens. J Agric Sci Technol. 2:327–336.
- Zhandi M, Talebnia-Chalanbar A, Towhidi A, Sharafi M, Yousefi AR, Hussaini SMH. 2020. The effect of zinc oxide on rooster semen cryopreservation. Br Poult Sci. 61(2):188–194.
