Abstract
In this study, we compared three popular textural tests: the compression, Meullenet–Owens razor blade (MORS), and Allo–Kramer (AK) tests, which are used to detect the wooden breast (WB) and spaghetti meat (SM) myopathies. A total of 209 fillets (71 WB, 71 SM, 67 normal) were selected from three different flocks at a large commercial plant. Thawed fillets were subjected to 20% compression tests before and after cooking, and cooked samples were subjected to the MORS and AK tests. The compression test on raw samples showed that normal and SM fillets had lower force (5.61 and 4.69 vs. 9.52 N), work (25 and 22 vs. 45 N mm), and Young’s modulus (2.71 and 2.11 vs. 4.29 N/s, p < .001) values than those of WB. The results of the compression test were confirmed by the cooked fillet results. The MORS test showed that SM had lower shear force (12.8 vs. 14.7 N) and work (249 vs. 288 N mm) values than those of the normal fillets, while WB showed intermediate values. The AK test results showed that SM had lower shear force (10.5 vs. 14.5 N) and Young’s modulus (31.0 vs. 46.0 N/s; p ≤ .01) values than those of WB fillets, whereas normal fillets had intermediate values. The compression test can be used to identify WB in both raw and cooked meat. The MORS test could distinguish cooked SM fillets from normal fillets, whereas the AK test differentiated SM from WB.
This study compared the compression, Meullenet–Owens razor blade, and Allo–Kramer tests for detecting wooden breast (WB) and spaghetti meat (SM).
The compression test identified WB in both raw and cooked fillets.
Meullenet–Owens razor blade test distinguished SM from normal fillets.
Allo–Kramer test accurately distinguished SM from WB fillets.
HIGHLIGHTS
Introduction
Poultry markets have changed substantially over the past 50 years. Increased demand has driven producers to improve farming techniques and produce more meat over a shorter period of time. Today, broilers are marketed in about half the time and at about twice the body weight as compared to 50 years ago (Barbut Citation2015). Boneless and skinless broiler breast meat is one of the most consumed products worldwide because it is viewed as a low-fat product with a bland flavour profile and is reasonably priced (Morey and Owens Citation2017). In addition, consumers prefer broiler meat as a tender product. However, there are factors that can negatively impact the tenderness of broiler fillets (Morey and Owens Citation2017).
Overall, the poultry industry is facing increased occurrences of meat abnormalities due to both pre-slaughter and slaughtering factors (Kuttappan et al. Citation2016; Petracci et al. Citation2019). Some of these defects have also been observed in other species (e.g. pale, soft, and exudative meat in pork) and are often described in the literature. Other abnormalities are specific to poultry, such as white striping (WS), wooden breast (WB), and spaghetti meat (SM), all of which affect the pectoralis major muscle of broiler breasts (Petracci et al. Citation2019). Among these myopathies, WS is usually most prevalent in both males and females, whereas WB is more prevalent in males (Gratta et al. Citation2019) and SM in females (Pascual et al. Citation2020). In commercial processing plants, these myopathies are typically identified in raw breast fillets through subjective tactile and visual assessments.
The myopathies affect different quality attributes (texture, juiciness, tenderness, colour, and flavour). Therefore, they can significantly affect consumer judgement (Kuttappan et al. Citation2012; Tasoniero et al. Citation2016) and compromise the technological properties of meat (pH, water holding and binding capacity, texture) (Mudalal et al. Citation2015; Trocino et al. Citation2015; Soglia et al. Citation2016). The significant rise in myopathies over the last 5 years has become a challenge for the global poultry industry (Aguirre et al. Citation2018; Petracci et al. Citation2019). The WS and WB fillets have increased intramuscular fat content, decreased protein levels, and decreased soluble salt content as well as some dysfunctions in muscle tissues, such as degeneration of the myofibrillar structure (Mudalal et al. Citation2015; Radaelli et al. Citation2017; Soglia et al. Citation2017). Furthermore, fillets with WB have a lower water holding capacity and, therefore, higher cooking losses than normal meat (Soglia et al. Citation2016). However, the SM effects on meat quality are not fully understood because the condition is much more recent (Baldi et al. Citation2018).
The current methods for diagnosing fillets affected by myopathies are visual and palpitation methods, and near-infrared reflectance spectroscopy, which is used to check raw broiler breast fillets. In both commercial and research situations, subjective scoring is still the predominant method used to categorise chicken fillets under WB conditions (Pang et al. Citation2020). Nevertheless, objective criteria (e.g. instrumental texture measurements) that assess the presence and severity of the different myopathies are required. Literature data on the shear force of cooked WB meat are not always consistent (Chatterjee et al. Citation2016; Tijare et al. Citation2016; Cai et al. Citation2018; Dalgaard et al. Citation2018; Maxwell et al. Citation2018). Overall, with compression-based texture methods, as opposed to shear-based methods, the results for WB fillets are rather consistent, particularly for raw samples (Soglia et al. Citation2016, Citation2017; Dalgaard et al. Citation2018; Sun et al. Citation2018). Nevertheless, a more thorough understanding of the textural characteristics of WB and SM in both raw and cooked meat is needed. Furthermore, it is important to evaluate which method is the most reliable one for detecting affected fillets. Therefore, we evaluated and compared normal, WB, and SM fillets after testing them using the compression of raw and cooked meat method and shear with a single blade (Meullenet–Owens razor, MORS) method and shear with multiple blades (Allo–Kramer, AK) method on cooked meat. The second aim was to compare the methods in order to determine the best method for all or a certain myopathy.
Materials and methods
Sampling of broiler breast fillets
Breast fillets were collected from the deboning line of a commercial processing plant (Maple Leaf Poultry, Brampton, ON, Canada) 3 h post mortem. A total of 209 fillets (67 normal, 71 WB, 71 SM) was selected from three flocks (79, 97, and 33 in each flock) on three different days. They were scored as normal, severe WB, and severe SM based on subjective hardness by palpation for WB (fillets that were extremely hard and rigid throughout from cranial region to caudal tip) and by examining fibre separation in the cranial and caudal parts of the fillets for SM (Tijare et al. Citation2016; Baldi et al. Citation2018). Fillets that were selected did not present any crossover between WB and SM. The fillets were placed in plastic bags and transported on ice to the Food Science Department, University of Guelph, Canada, within 60 min. On arrival at the processing facility, 182 fillets out of the 209 samples (58 normal, 62 WB, 62 SM) were used to measure the pH at the cranial end of the fillets (Hanna Instruments 99163N, Woonsocket, RI, USA). The fillets were individually weighed, and the ventral side L*, a* and b*colour coordinates (Nix Pro Colour Sensor; Nix Ltd., Ontario, Canada) were measured. The fillets were then individually packed and stored in a freezer at −18 °C for 4 days for subsequent texture analysis.
Chemical analysis
A total of 27 fillets (9 normal, 9 WB, and 9 SM; 9 from each flock) out of the 209 collected at the processing facility was homogenised and frozen at −20 °C. Then, the moisture and fat contents were analysed (Meat Trac Fat and Moisture Analyser; CMC, Matthews, NC, USA; AOAC official method 2008.06), and the protein content was determined (Sprint Rapid Protein Analyser; CMC) (AOAC official method 967.12, 930.33).
Sample preparation and texture analysis
The fillets were defrosted overnight at 4 °C, and the thawing losses were determined. Raw samples were subjected to a compression test (CT). The raw fillets were compressed to 20% of their original height three times at different locations (cranial, medium, and caudal parts) using a 10-mm round flat probe mounted on the load cell at a test speed of 3 mm/s. The compression force (N), work (N m), and Young’s modulus (N/s) were obtained using the macro program software (Exponent Connect version 6.1, 7.0; Texture Technologies Corp.). The ‘maximum compression’ or ‘shear force’ is the highest point in the curve and is related to the hardness of the sample; the ‘work to shear’ is the area under the curve and is calculated as the force per distance, this parameter is the relationship between the hardness and the elasticity of the sample; and the ‘Young’s modulus’ is the linear part of the slope of the curve (Barbut Citation2015).
After compression of the raw samples, the fillets were individually vacuum-packed in plastic bags and cooked in a water bath set at 75 °C until an internal temperature of 72 °C was reached. After a 40-min cooling period, the fillets were weighed and the cooking losses were calculated.
The cooked samples were subjected to CT (with the same procedure described above for raw samples), MORS, and AK tests.
The samples were subjected to the MORS test using a 0.5-mm thick, 8.9-mm wide, and 30-mm tall blade to shear the fillets perpendicular to the muscle fibre direction (Cavitt et al. Citation2005). There were four shears per fillet in different areas (2-cm apart) at a depth of 20 mm (Bowker and Zhuang Citation2019). The shear force (N), number of peaks, work (N mm), and Young’s modulus (N/s) were obtained.
In the AK test, a small parallelepiped meat portion (3 cm × 2 cm × 1 cm) was sheared using a 5-bladed probe (distance between the blades: 5 mm; blade thickness: 2 mm; cutting speed: 5 mm/s) as described by Mudalal et al. (Citation2015). Then, the force (N), work (N mm), and Young’s modulus (N/s) values were obtained.
A texture analyser with a 50-kg load cell was used for all the texture analyses (Model TA-XT-PlusC; Texture Technologies Corp., Hamilton, MA, USA).
Statistical analysis
Individual data for fillet weight, chemical composition, rheological traits, and texture measurements (CT, MORS, and AK tests) were subjected to analysis of variance (ANOVA) with meat type (normal, WB, SM), flock, and their interactions as the main effects and the replications (per fillet) as the random effect. The analyses were carried out using the PROC MIXED component in SAS (SAS Institute Citation2013). The significance level was set at p ≤ .05. Data related to interactions between meat type and flock are provided as Supplementary Tables S1 and S2.
Results and discussion
In accordance with previous studies (Trocino et al. Citation2015; Bowker and Zhuang Citation2019; Gratta et al. Citation2019; Tasoniero et al. Citation2020), the fillets affected by SM and WB myopathies were heavier (p ≤ .01) than the normal fillets (Table ). Normal fillets had a lower fat content than WB fillets (0.91 vs. 1.54%; p ≤ .05), whereas SM fillets had an intermediate value (1.25%). The myodegraded fillets also had lower protein contents than normal fillets (22.7 and 22.3% in SM and WB, respectively, vs. 23.9% in normal fillets; p ≤ .01). Myodegeneration causes the replacement of muscle fibres with adipose and connective tissues, resulting in higher fat contents (Sihvo et al. Citation2014; Radaelli et al. Citation2017; Baldi et al. Citation2018; Gratta et al. Citation2019). The decreased protein content is associated with the large changes in muscle architecture that occur in pectoralis major tissue affected by muscular abnormalities (Soglia et al. Citation2016; Cai et al. Citation2018; Baldi et al. Citation2019).
Table 1. Chemical composition and quality traits (LS means) of normal, spaghetti meat, and wooden breasts of broiler chickens from three flocks that had been sampled at slaughtering.
Similar to other studies (Trocino et al. Citation2015; Baldi et al. Citation2018), the ultimate meat pH was not significantly different among the three types of meat. Previous studies (Petracci et al. Citation2019) found that the ultimate pH of WB, WS, and SM breasts is often some decimal points (0.2–0.4) higher than that of normal breasts. The colour results for the SM and WB fillets showed that they had significantly higher lightness (L*) values than that of the normal ones (p ≤ .001), while yellowness (b*) was higher in SM fillets (p ≤ .001). The results reported by previous studies are variable for these characteristics. Some reported no differences in lightness (Petracci et al. Citation2013; Trocino et al. Citation2015; Tasoniero et al. Citation2016) or yellowness and redness values due to myopathies (Sanchez Brambila et al. Citation2016; Zambonelli et al. Citation2016). Others observed a higher redness (Gratta et al. Citation2019) and yellowness index (Mudalal et al. Citation2015) in affected fillets than in normal fillets, which have been associated with increased fibrotic responses and reduced haem pigment levels (Petracci et al. Citation2017).
In this study, SM fillets had greater (p ≤ .01) dripping losses on average than normal fillets, with WB showing intermediate values (Table ). The fillets affected by SM and WB showed higher cooking losses than normal ones, which confirms the results reported by Soglia et al. (Citation2016) and Baldi et al. (Citation2018). This is assumed to be due to the degeneration of muscle proteins and the replacement of myofibrillar proteins with connective tissues (Mudalal et al. Citation2015; Soglia et al. Citation2016; Tijare et al. Citation2016).
In this trial, the compression force and work values obtained for raw WB fillets were higher than those obtained for normal fillets (p ≤ .001) (Table ), which is consistent with Sun et al. (Citation2018) and Baldi et al. (Citation2019) and suggests that CT could accurately detect WB fillets and distinguish them from normal fillets. Conversely, CT did not distinguish between SM fillets and normal raw or cooked fillets (Table ; p ≤ .001; Figure ), which indicated that SM and normal fillets had similar hardness and elasticity in the CT. Baldi et al. (Citation2019) reported that raw SM fillets had a compression force value between those of WB and normal fillets. They also showed that after cooking, SM meat became softer in the CT as compared to normal and WB fillets. This result was attributed to a lower collagen content in the SM samples than in normal and WB muscles, which were gelatinised during cooking. However, in this study, the SM and normal fillet compression forces were always lower than those for WB for both raw (Figure ) and cooked fillets (Figure ). This means that cooking did not change the toughness of SM fillets as compared to the normal ones. Indeed, in this trial, the compression force was higher for cooked meat than for raw meat (Figure ), which could be due to moisture losses during cooking and changes in the protein matrix of the muscle fibres.
Figure 1. Compression curves for raw fillets (a) and cooked fillets (b). Shear curves for the MORS test (c) and the Allo–Kramer test (d) on cooked fillets. WB: wooden breast; SM: spaghetti meat.
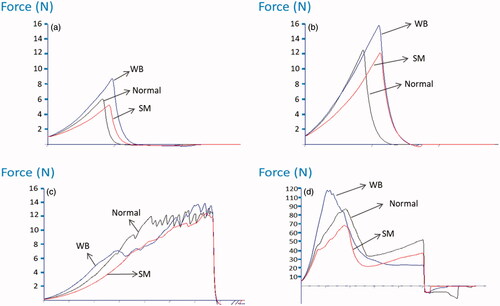
Table 2. Results of the compression, MORS, and Allo–Kramer tests on normal, spaghetti meat, and wooden breasts of broiler chickens from three flocks that had been sampled at slaughtering.
Shear force tests are frequently used on cooked meat samples to determine tenderness, and increased shear force values are associated with increased hardness. In this study, the MORS test on cooked samples did not reveal any differences in shear force, work, peak counts, and Young’s modulus between normal and WB fillets (Table ), which is consistent with the results reported by Pang et al. (Citation2020) on raw samples and those of Bowker and Zhuang (Citation2019) on cooked samples. Likely, the higher shearing than compression action exerted by the MORS test could explain the absence of differences when comparing normal with WB meat. In contrast, Chatterjee et al. (Citation2016) recorded greater shear force and energy values using the MORS test for cooked WB fillets than that of the normal ones. According to the literature, the number of peaks along with the shear curve increases as the severity of the woody breast condition increases (Sun et al. Citation2018). In this trial, the MORS test accurately separated normal fillets from SM fillets. In fact, shear force, number of peaks, and work were significantly lower in SM meat than in normal fillets (Table ; p ≤ .01; Figure ).
In this trial, the SM fillets had significantly different AK shear force, work, and Young’s modulus values from those of normal and WB fillets (Table ; p ≤ .01; Figure ). However, Soglia et al. (Citation2017) and Gratta et al. (Citation2019) did not find a difference in shear force between WB and normal fillets with the same AK test. In this study, the results showed that WB was more resistant to cutting than SM and needed more force to shear the samples because more work was needed, and there was a larger area under the force deformation curve. In this case, the higher compression than shearing action exerted by the AK test could explain the differences found between WB and SM meat.
Baldi et al. (Citation2019) found that the higher collagen content in WB fillets was responsible for the distinctive hardness of WB fillets, which could be the reason for the lower number of peaks observed in the MORS test than that of the normal fillets. Overall, peaks appear when a group of muscle fibres/connective tissue layers has been cut. If the resistance to cutting is very strong, the blade pushes and destroys the muscle structure instead of cutting one layer at a time, and it takes more force to start cutting the fibres. Therefore, few peaks were observed at the beginning of the curve in the WB fillets. The lowest number of peaks (Figure ) appeared in the MORS test, and the lowest AK force (Figure ) was needed to cut the SM samples, which could be explained by the lower collagen level or cross-linking degree in the SM samples where fibre bundles had separated within the muscle tissue itself. However, when the shearing methods were compared, the MORS shear provided apparent advantages over the AK method because no sample preparation or weighing was required to execute the test nor was it excessively destructive because only a small incision was made into the sample (Cavitt et al. Citation2005).
Conclusions
The results of this study revealed that texture tests have different sensitivities with respect to meat type and the state of the meat (raw vs. cooked). The CT, which measures hardness and elasticity, could identify severe WB in both raw and cooked meat. This test, when applied to raw meat, can guarantee robust and consistent results and avoids the variability linked to the cooking process. Furthermore, the MORS test could distinguish between severe SM and normal fillets when the samples had been cooked, whereas the AK test was better at distinguishing between cooked severe SM and WB fillets. Further investigation should compare the sensitivities of different methods in the case of fillets affected by mild myopathies, which occur at the highest rate in the field.
Ethical approval
This study was conducted in post-mortem and the animals were reared within the regular farm management practices that did not require any stressful procedures. All procedures and animal care were in compliance with national regulations on the protection of animals during transport at the time of the killing. No ethical approval was therefore requested.
Supplemental Material
Download MS Word (20.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Aguirre ME, Owens CM, Miller RK, Alvarado CZ. 2018. Descriptive sensory and instrumental texture profile analysis of woody breast in marinated chicken. Poult Sci. 97(4):1456–1461.
- Baldi G, Soglia F, Laghi L, Tappi S, Rocculi P, Tavaniello S, Prioriello D, Mucci R, Maiorano G, Petracci M. 2019. Comparison of quality traits among breast meat affected by current muscle abnormalities. Food Res Int. 115:369–376.
- Baldi G, Soglia F, Mazzoni M, Sirri F, Canonico L, Babini E, Laghi L, Cavani C, Petracci M. 2018. Implications of white striping and spaghetti meat abnormalities on meat quality and histological features in broilers. Animal. 12(1):164–173.
- Barbut S. 2015. The science of poultry and meat processing [accessed 2021 February 23] [ISBN 978-0-88955-626-3] Guelph (Canada). www.poultryandmeatprocessing.com
- Bowker B, Zhuang H. 2019. Detection of razor shear force differences in broiler breast meat due to the woody breast condition depends on measurement technique and meat state 1. Poult Sci. 98(11):6170–6176.
- Cai K, Shao W, Chen X, Campbell YL, Nair MN, Suman SP, Beach CM, Guyton MC, Schilling MW. 2018. Meat quality traits and proteome profile of woody broiler breast (pectoralis major) meat. Poult Sci. 97(1):337–346.
- Cavitt LC, Meullenet JF, Gandhapuneni RK, Youm GW, Owens CM. 2005. Rigor development and meat quality of large and small broilers and the use of Allo-Kramer shear, needle puncture, and razor blade shear to measure texture. Poult Sci. 84(1):113–118.
- Chatterjee D, Zhuang H, Bowker BC, Rincon AM, Sanchez-Brambila G. 2016. Instrumental texture characteristics of broiler pectoralis major with the wooden breast condition. Poult Sci. 95(10):2449–2454.
- Dalgaard LB, Rasmussen MK, Bertram HC, Jensen JA, Møller HS, Aaslyng MD, Hejbøl EK, Pedersen JR, Elsser‐Gravesen D, Young JF. 2018. Classification of wooden breast myopathy in chicken pectoralis major by a standardised method and association with conventional quality assessments. Int J Food Sci Technol. 53(7):1744–1752.
- Gratta F, Fasolato L, Birolo M, Zomeño C, Novelli E, Petracci M, Pascual A, Xiccato G, Trocino A. 2019. Effect of breast myopathies on quality and microbial shelf life of broiler meat. Poult Sci. 98(6):2641–2651.
- Kuttappan VA, Hargis BM, Owens CM. 2016. White striping and woody breast myopathies in the modern poultry industry: a review. Poult Sci. 95(11):2724–2733.
- Kuttappan VA, Lee YS, Erf GF, Meullenet JF, McKee SR, Owens CM. 2012. Consumer acceptance of visual appearance of broiler breast meat with varying degrees of white striping. Poult Sci. 91(5):1240–1247.
- Maxwell AD, Bowker BC, Zhuang H, Chatterjee D, Adhikari K. 2018. Descriptive sensory analysis of marinated and non-marinated wooden breast fillet portions. Poult Sci. 97(8):2971–2978.
- Morey A, Owens CM. 2017. Methods for measuring meat texture. In: Petracci M, Berri C, editors. Poultry quality evaluation. Sawston (UK): Woodhead Publishing; p. 115–132.
- Mudalal S, Lorenzi M, Soglia F, Cavani C, Petracci M. 2015. Implications of white striping and wooden breast abnormalities on quality traits of raw and marinated chicken meat. Animal. 9(4):728–734.
- Pang B, Bowker B, Yang Y, Zhang J, Zhuang H. 2020. Relationships between instrumental texture measurements and subjective woody breast condition scores in raw broiler breast fillets. Poult Sci. 99(6):3292–3298.
- Pascual A, Trocino A, Birolo M, Cardazzo B, Bordignon F, Ballarin C, Carraro L, Xiccato G. 2020. Dietary supplementation with sodium butyrate: growth, gut response at different ages, and meat quality of female and male broiler chickens. Ital J Anim Sci. 19:1135–1146.
- Petracci M, Mudalal S, Bonfiglio A, Cavani C. 2013. Occurrence of white striping under commercial conditions and its impact on breast meat quality in broiler chickens. Poult Sci. 92(6):1670–1675.
- Petracci M, Soglia F, Berri C. 2017. Muscle metabolism and meat quality abnormalities. In: Petracci M, Berri C, editors. Poultry quality evaluation. Sawston (UK): Woodhead Publishing; p. 51–75.
- Petracci M, Soglia F, Madruga M, Carvalho L, Ida E, Estévez M. 2019. Wooden-breast, white striping, and spaghetti meat: causes, consequences and consumer perception of emerging broiler meat abnormalities. Compr Rev Food Sci Food Saf. 18(2):565–583.
- Radaelli G, Piccirillo A, Birolo M, Bertotto D, Gratta F, Ballarin C, Vascellari M, Xiccato G, Trocino A. 2017. Effect of age on the occurrence of muscle fiber degeneration associated with myopathies in broiler chickens submitted to feed restriction. Poult Sci. 96(2):309–319.
- Sanchez Brambila G, Bowker BC, Zhuang H. 2016. Comparison of sensory texture attributes of broiler breast fillets with different degrees of white striping. Poult Sci. 95(10):2472–2476.
- SAS Institute. 2013. The SAS/STAT 9.4 user’s guide. Cary (NC): SAS Inst. Inc.
- Sihvo HK, Immonen K, Puolanne E. 2014. Myodegeneration with fibrosis and regeneration in the pectoralis major muscle of broilers. Vet Pathol. 51(3):619–623.
- Soglia F, Gao J, Mazzoni M, Puolanne E, Cavani C, Petracci M, Ertbjerg P. 2017. Superficial and deep changes of histology, texture and particle size distribution in broiler wooden breast muscle during refrigerated storage. Poult Sci. 96(9):3465–3472.
- Soglia F, Mudalal S, Babini E, Di Nunzio M, Mazzoni M, Sirri F, Cavani C, Petracci M. 2016. Histology, composition, and quality traits of chicken pectoralis major muscle affected by wooden breast abnormality. Poult Sci. 95(3):651–659.
- Sun X, Koltes DA, Coon CN, Chen K, Owens CM. 2018. Instrumental compression force and meat attribute changes in woody broiler breast fillets during short-term storage. Poult Sci. 97(7):2600–2606.
- Tasoniero G, Cullere M, Cecchinato M, Puolanne E, Dalle Zotte A. 2016. Technological quality, mineral profile, and sensory attributes of broiler chicken breasts affected by White Striping and Wooden Breast myopathies. Poult Sci. 95(11):2707–2714.
- Tasoniero G, Zhuang H, Gamble GR, Bowker BC. 2020. Effect of spaghetti meat abnormality on broiler chicken breast meat composition and technological quality 1. Poult Sci. 99(3):1724–1733.
- Tijare VV, Yang FL, Kuttappan VA, Alvarado CZ, Coon CN, Owens CM. 2016. Meat quality of broiler breast fillets with white striping and woody breast muscle myopathies. Poult Sci. 95(9):2167–2173.
- Trocino A, Piccirillo A, Birolo M, Radaelli G, Bertotto D, Filiou E, Petracci M, Xiccato G. 2015. Effect of genotype, gender and feed restriction on growth, meat quality and the occurrence of white striping and wooden breast in broiler chickens. Poult Sci. 94(12):2996–3004.
- Zambonelli P, Zappaterra M, Soglia F, Petracci M, Sirri F, Cavani C, Davoli R. 2016. Detection of differentially expressed genes in broiler pectoralis major muscle affected by white striping – wooden breast myopathies. Poult Sci. 95(12):2771–2785.
