 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study was aimed to investigate the effects of dietary fat sources and green tea extract (GTE) on gene expression associated with lipid metabolism and inflammation in broiler chicken. A total of 300 female Ross 308 broiler chicks were allocated to six dietary treatments in a completely randomised design with a factorial arrangement of two levels of GTE (0 and 500 mg/kg diet) × three levels of fat inclusion [without fat (control group), soybean oil (SO) and tallow (Ta)]. Liver fat (LF), liver methallothionin (LMT) content, and lipoprotein lipase (LPL), adipose triglyceride lipase (ATGL), tumour necrosis factor α (TNF-α), and interleukin (IL-6)] genes expression were investigated. Relative expression and statistical analyses of genes expression were assessed using REST software. The effect of added dietary fats was significant and improved performance parameters compared to the control group (p < .05). The highest abdominal fat and blood triglyceride, and the lowest carcase yield, were achieved in chicks fed SO-supplemented diet (p < .05). Chicks fed a diet supplemented with SO showed an overexpression and a decrease of LPL and ATGL compared to the Ta-supplemented and control groups, respectively (p < .05). GTE supplementation was effective on LPL and ATGL expression and ameliorated the effect of SO on abdominal fat percentage. TNF-α expression, LMT content, and relative weight of lymphoid organs were affected neither by GTE nor by the fat sources. There was no detection of IL-6 gene expression in adipose tissue. The results of this study demonstrated that diets rich in SO were capable increase fat deposition by an increase and decrease in LPL and ATGL gene expression, respectively. However inclusion of GTE in broiler diets alleviated the LPL-increasing and ATGL-reducing effects of SO and ameliorated the effect of SO on abdominal fat mass.
Diets supplemented with SO increased abdominal fat mass by changes in LPL and ATGL gene expression.
Obesity was not effective in the expression of inflammatory genes (TNF-α and IL-6), indicating a lack of connection between inflammation and obesity in broiler chicken.
GTE (500 mg/kg diet) addition to the diets supplemented with SO ameliorated the effect of SO on LPL and ATGL gene expression and abdominal fat mass.
Highlights
Introduction
During the last several decades, it has been seen a great improvement in body weight gain and feed conversion ratio of broilers (Huang et al. Citation2013). However, this breeding strategy only targets body weight gain and feed conversation efficiency without considering some inevitable problems such as metabolic disorders, reduction in meat quality and increase in waste by fat accumulation (Huang et al. Citation2013; Bahareldin et al. Citation2018). Adipose tissue, especially abdominal fat, is an essential endocrine organ producing hormones and other substances (Marisa et al. Citation2013) and needs to be controlled to reduce negative effects on productivity, acceptability, feed efficiency, and health of consumers. Therefore, regulating fat deposition plays an important role in broiler chicken (Claire D'Andre et al. Citation2013).
Lipoprotein lipase (LPL) plays a crucial role in fat accumulation in adipose tissue of chickens in a rate-limiting step (Loongyai et al. Citation2018). LPL hydrolyses triglycerides of chylomicrons and very-low-density lipoproteins (VLDL) to produce glycerol and free fatty acids which that are capable of esterifying to form triglycerides in adipocytes. Adipose triglyceride lipase (ATGL) is one of the key lipid-degrading enzymes and catalysis the initial step in triglycerides hydrolysis (Huang et al. Citation2013). It has been demonstrated that triglyceride storage is mainly influenced by the expression of ATGL in the basal state (Miyoshi et al. Citation2008).
There is general agreement that the circulating of some pro-inflammatory cytokines are elevated in obesity. Some molecular studies show that adipocytes can synthesise and secrete two important pro-inflammatory cytokines, including tumour necrosis factor α (TNF-α) and interleukin-6 (IL-6). (Coppack Citation2001). Feeding animals with high-fat diets increased the TNF-α content of their adipose tissue (Bastard et al. Citation2000; Coppack Citation2001). Korver et al. (Citation1998) reported that the pro-inflammatory factors decreased feed intake, body weight gain, and consequently increased feed conversion ratio in broiler chicks. As part of the host inflammatory response, the liver synthesises a variety group of acute-phase proteins such as metallothionein in reaction to both pathogenic and non-pathogenic inflammatory stimuli. It was suggested that the severity of the inflammatory response to a specified imposed stimulus can be influenced by the type of dietary fat sources (Korver et al. Citation1998). Reda et al. (Citation2020) reported that different source of the oil can affect productive, reproductive, and health aspects of Japanese quail. While most of the studies on diet-induced obesity reported that the use of diets supplemented with animal fat led to obesity, a few studies have recently investigated the effects of vegetable oil on obesity, insulin resistance, diabetes, and liver fatty acid composition in broilers (Costa et al. Citation2011; Deol et al. Citation2015).
Green tea (Camellia sinensis) is a medicinal herb with high levels of polyphenol compounds such as epigallocatechin gallate, epigallocatechin, epicatechin, and epicatechin gallate (Huang et al. Citation2013). Among the beneficial effects of polyphenols, their anti-adipogenic effect has been frequently investigated in animal models such as rodent obese (Huang et al. Citation2013). That has been also demonstrated the addition of green tea leaves increases the level of interleukin 2 and interferon gamma in the serum of chickens and enhances production of anti‐inflammatory cytokines in pigs (Alagawany et al. Citation2020). Here, we hypothesised that genes associated with lipid metabolism and inflammatory responses are differentially expressed in fat tissue between broilers fed soybean oil and tallow with and without green tea extract and there is a relationship between their expression levels and abdominal fat content in broiler chicken. Therefore, the present study was aimed to investigate the effects of different sources of dietary fat and green tea extract (GTE) on gene expression associated with lipid metabolism and inflammatory responses in broiler chicken.
Materials and methods
Birds and experimental design
This study was carried out at the Poultry Research Station and the nutrition and biotechnology laboratories of the Department of Animal Science at Guilan University (Rasht, Iran) in 2018–2019. All applicable international guidelines for the care of animals, experimental procedures and sample collection methods were followed when carrying out the present research.
A total of 300 one-day-old female Ross 308 broiler chicks in a completely randomised design with a 2 × 3 factorial arrangement were allocated to six dietary treatments. Each treatment replicated five times with 10 birds per replicate. The dietary treatments were: (T1) without dietary GTE and fat supplementation, (T2) without GTE + soybean oil (SO), (T3) without GTE + tallow (Ta), (T4) 500 mg GTE/kg diet without dietary fat, (T5) 500 mg GTE/kg diet + SO, (T6) 500 mg GTE/kg diet + Ta. Because of different levels of tallow or soybean oil, the diets were not isocaloric in the present experiment. Table shows the ingredients and chemical composition of the experimental diets. Chicks were raised for 49 days with free access to feed and water through the entire experimental period. The Green tea used in this study was purchased from Lahijan Tea Research Centre (Gulian, Iran). Purified GTE was obtained by the maceration method. Briefly, 300 mg powdered green tea was extracted overnight with 1 L of ethanol 75% by maceration. The extract was filtered with Whitman paper and then was concentrated as powder by rotary evaporator. The polyphenolic compositions of GTE were determined by high-performance liquid chromatography (HPLC, KNAUER AZURA, Germany, UV-VIS detector, LiChrosorb RP-18 column), which contained 9.17% Epigallocatechin gallate, 4.16% Epicatechin gallate, 3.05% Epigallocatechin, 2.30% Gallocatechin gallate, 1.62% Gallocatechin, 1.45% Epicatechin, and 0.92% Catechin.
Table 1. Ingredients and chemical composition of the dietsCitation1.
Sampling and storage
On day 49, 10 birds per treatment were selected and slaughtered after bleeding. The bloods, livers, and abdominal fats of slaughtered chicks were immediately frozen at −80 °C until further analysis and were used for fasting concentration of blood triglyceride, LF (liver fat), LMT (liver methalothionin), and genes (LPL, ATGL, TNF-α and IL-6) expression determinations, respectively.
LMT and LF assays
The 1 gr liver tissue was homogenised with 3 mL of the homogenisation buffer (0.5 M sucrose, 20 mM Tris-HCl buffer (Ph = 8.6), containing 0.01% mercaptoethanol) by a tissue homogeniser (IKA, T25, Ultra Turrax). The homogenates were centrifuged at 30,000 g for 20 min to obtain a supernatant containing metallothionein. Then, 80 µl of chloroform and 1.05 mL of cold absolute ethanol were added per 1 mL of the supernatant. The samples were centrifuged at 6000 g for 10 min (at 0–4 °C). 3 mL of cold ethanol was added to the supernatant and was stored at −20 °C for 1 hour and then centrifuged at 6000 g for 10 min. The resulting pellets were washed with ethanol: chloroform: homogenisation buffer (87:1:12) and centrifuged again at 6000 g for 10 min and then was dried. The dried pellet was resuspended in 300 µl of 5 mM Tris-HCl, 1 mM EDTA (pH = 7). The resuspended metallothionein fraction was added to 4.2 mL of 0.43 mM nitrobenzoic acid in 0.2 M phosphate buffer, pH = 8. It left for 30 min at room temperature. Then, the concentration of reduced sulfhydryl was evaluated by reading the absorbance at 412 nm in a spectrophotometer. A standard absorbance curve with glutathione measured at 412 nm was used as a standard reference for correct quantification of MT in the samples of liver tissue (Linde and Vazquez Citation2006).
The AOAC (Citation2005) method was used to determine the crude fat of the liver. Exactly two grams of dried sample was weighted into glass Soxhlet thimbles and mixed with 120 mL of n-hexane. The fat was extracted by continuously refluxing solvent through the sample for eight hours. The solvent was finally evaporated, and fat content was calculated as a percentage of the dried sample weight.
Gene expression
Total RNA extraction
RNX-Plus kit (Sinaclon, Iran) was used for total RNA isolating from the chicken abdominal fat tissue by using the solution procedure according to the manufacturer’s instructions. Integrity and quantity assessments of isolated RNA for each sample were made both by 1% agarose gel electrophoresis and NanoDrop spectrophotometer. The values of A260/A280 were 1.94 ± 0.042. RNA samples were treated with DNase (Sinaclon, Iran) to remove any DNA contamination, and RNA samples were stored at −80 °C until cDNA synthesis.
cDNA synthesis
First-strand cDNA was synthesised by Thermo scientific kit, and the following reagents were added into a sterile, nuclease-free tube on ice: 1 µg mRNA, 1 µL oligo (dT)18 primer, and the nuclease free water and the mixture was incubated at 65 °C for 5 min. Then, 4 µL of 5x reaction buffer, 1 µL of RiboLock RNase Inhibitor (20 U/μL), 2 µL deoxynucleoside triphosphate mix (10 mM), and 1 µL of RevertAid M-MuLV Reverse Transcriptase (200 U/Μl) were added to the above mixture and was incubated (42 °C, 60 min). At the final stage, the mixture was heated (70 °C, 5 min) and stored at −20 °C.
Quantitative real-time PCR (qRT-PCR)
The gene expression was quantified by SYBR Blue HS-qPCR mix real-time PCR. The q-RT-PCR was done in 12.5 µL containing 6.25 µL of master mix containing SYBR Blue HS-qPCRMix (Sinaclon, Iran), 4.25 µL nuclease-free water, 0.5 µL of forward-reverse primers, and 1 µL cDNA. Thermal cycling parameters were as follows: one cycle of preliminary denaturation at 95 °C for 7 min, 40 cycles of amplification was followed by a 2-steps program (for LPL: 95 °C for 15 seconds and 60 °C for 40 seconds, for ATGL: 95 °C 15 seconds and 61 °C for 40 seconds, and for TNF-α and IL-6: 95 °C for 15 seconds and 63 °C for 40 seconds). Chicken LPL, ATGL, TNF-α, IL-6, and β-actin primers sequences were reported by Huang et al. (Citation2013), Ramiah et al. (Citation2014), Bhanja et al. (Citation2014), Wang et al. (Citation2012), and Khoobbakht et al. (Citation2020) respectively (Table ). Runs were performed in triplicate. Relative expression and statistical analyses of gene expression were assessed using the REST software (Pfaffl et al. Citation2002). The data were normalised for β-actin as an internal control gene.
Table 2. Oligonucleotide primer sequence for RT-PCR amplification.
Statistical analysis
The statistical analyses of growth performance traits, carcase characteristics, blood triglyceride, and LF and LMT content were performed using the GLM procedure of SAS (SAS Institute Citation2000), and comparisons of means were analysed by Tukey’s tests at p < .05.
The following statistical model was used:
where Yijk is the observerd value, Ai is the effect of factor A (GTE), Bj is the effect of factor B (dietary fat), ABij is the interaction effect of factors, and eijk is the experimental error.
Results
The effects of GTE and dietary fat sources on growth performance (feed intake (FI), body weight gain (BWG), feed conversion ratio (FCR), and energy intake (EI)) are shown in Table . Despite the non-significant effect of dietary GTE supplementation on FI, BWG, FCR, and EI (Table ), the effect of added dietary fats was significant and improved BWG and FCR compared to the control group (diets without supplementary fat) (p < .05). There were no significant differences for BWG, FCR and EI between SO and Ta-supplemented groups (p > .05). There were no interaction effects for GTE and dietary fat on FI, BWG, FCR and EI.
Table 3. The effects of dietary green tea extract (GTE) and fat sources on growth performance of female broiler chicks at 49 days of age.
The main and interaction effects of GTE and dietary fat sources on carcase yield, abdominal fat percentage, blood triglyceride concentration, and liver fat content at 49 days are presented in Table . SO-supplemented diet significantly increased abdominal fat percentage, blood triglyceride concentration, and liver fat content but decreased carcase yield compared to the control group (p < .05). No significant differences were observed for blood triglyceride concentration, abdominal fat percentage, carcase yield, and liver fat content between SO and Ta-supplemented groups. GTE was not effective on carcase yield, abdominal fat percentage, blood triglyceride concentration, and liver fat content.
Table 4. The effects of dietary green tea extract (GTE) and fat sources on carcase yield (CY), abdominal fat (AF), blood triglyceride concentration (Blood TG), liver fat (LF), and liver MT (LMT) content in female broiler chicks at 49 days of age.
The LPL expression was significantly (p < .05) higher in the SO-supplemented chicks compared to the control group (Figure ). There was also a significant difference in LPL expression between SO and Ta-supplemented groups (p < .05). There were no significant differences in LPL expression by chicks fed with Ta-supplemented diets compared with control and those fed SO/Ta-supplemented and GTE groups (p>.05). GTE supplementing was effective on LPL expression in a diet rich in SO. The Co-supplementation of SO and GTE significantly decreased LPL gene expression compared to SO (p < .05). Whereas a slight and no significant decrease of gene expression of LPL was observed in Ta and GTE-supplemented group compared to Ta-supplemented. A fat-free diet plus GTE administration also led to a slight decrease in LPL gene expression in comparison to a fat-free diet alone.
Figure 1. QRT-PCR analysis of LPL (lipoprotein lipase) gene expression in abdominal fat tissue of female broiler chicks in response to green tee extract (GTE) and fat supplementation. GTE0: without green tea extract; GTE500: 500 mg green tea extract/kg diet; SO: soybean oil; Ta: tallow; SO0/Ta0: without soybean oil/without tallow.
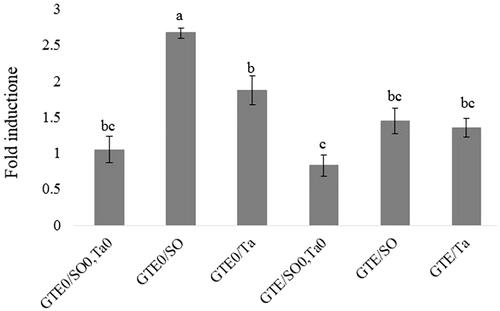
The changes to the ATGL gene expression in the abdominal fat tissue are shown in Figure . In the abdominal adipose tissue, the ATGL gene expression was significantly increased in broiler fed with or without fat in comparison to those fed SO. There was also a significant difference in ATGL gene expression between two sources of fat when used without GTE (p < .05). ATGL gene expression was significantly decreased by threefold in response to dietary SO compared to Ta-supplemented groups. A similar result was found between SO-supplemented and control groups. We observed a significant down-regulated in ATGL gene expression of broilers fed SO in comparison to the control group. No alteration of the gene expression of ATGL was found regarding the GTE treatment except in SO-supplemented diets. GTE significantly increased ATGL gene expression in broiler fed a diet rich in SO and GTE compared to a SO-supplemented diet.
Figure 2. QRT-PCR analysis of ATGL (adipose triglyceride lipase) gene expression in abdominal fat tissue of female broiler chicks in response to green tee extract (GTE) and fat supplementation. GTE0: without green tea extract; GTE500: 500 mg green tea extract/kg diet; SO: soybean oil; Ta: tallow; SO0/Ta0: without soybean oil/without tallow.
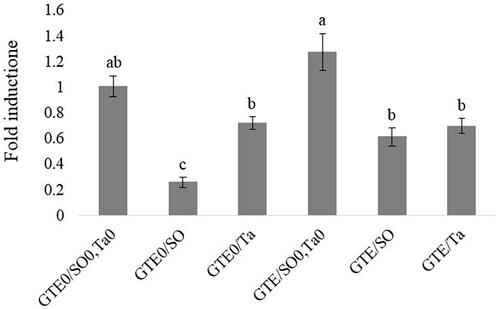
The changes to TNF-α in the abdominal adipose tissue of female broilers fed the different dietary treatments are shown in Figure . There were statistically no differences in TNF-α gene expression between the control and fat-supplemented groups. Besides, no alteration of the gene expression of TNF-α was found regarding the GTE treatment.
Figure 3. QRT-PCR analysis TNF-α (tumour necrosis factor α) in abdominal fat tissue of female broiler chicks in response to green tee extract (GTE) and fat supplementation. GTE0: without green tea extract; GTE500: 500 mg green tea extract/kg diet; SO: Soybean oil; Ta: tallow; SO0/Ta0: without soybean oil/without tallow.
Data from LMT content and the relative weight of lymphoid organs are shown in Table . Dietary supplementation of GTE and different sources of dietary fat did not affect LMT content and the relative weight of lymphoid organs in female chickens. LMT (p = .16) tended to be greater in SO-supplemented chicks compared to Ta-supplemented and free fat diets.
Table 5. The effects of dietary green tea extract (GTE) and fat sources LMT and some relative immune organs weight (Bursa of Fabricius (BF), thymus and spleen) in female broiler chicks at 49 days of age.
Discussion
The findings of the current study indicated that broilers fed with diets containing SO and Ta showed significantly higher BWG, FCR, and EI compared with those fed diets without fat. There were no significant differences between SO and Ta-supplemented groups for the growth performance traits, which are in line with the findings of previous studies (Sanz et al. Citation2000; Azman et al. Citation2004).
There are many reports that dietary manipulation can be effective on the growth pattern of fat depots in chickens, especially dietary fat source (Ramiah et al. Citation2014). While most experimental diet-induced obesity studies were based on diets high in saturated fat, only a few studies attempted to examine the effects of unsaturated fat sources such as SO (Costa et al. Citation2011; Deol et al. Citation2015, Citation2017). Deol et al. (Citation2017) indicated that oxylipin metabolites of linoleic acid (LA) correlated positively with obesity. LA is an omega-6 fatty acid that makes up about 55% of SO. Unsaturated fatty acids (USFA) also, in comparison with saturated ones, produced lower faecal energy losses, and therefore better metabolism energy supply. Higher metabolism energy supply from USFA could be expected to cause higher fat deposition (Crespo and Esteve-Garcia Citation2001). The result of our study is in agreement with these reports, while chickens fed with SO had the highest abdominal fat mass and the lowest carcase yield compared to the control group. Based on the results of the current study, SO also significantly increased liver fat and serum triglyceride compared to the control group.
Based on the results in Figure , the LPL and ATGL were highly expressed in chicken adipose tissue and clearly affected by dietary fat. The expression level for lipid anabolism gene, LPL, significantly increased in chicken fed diet supplemented with SO compared to the control group. The present study showed that an increase in LPL activity produced more abdominal accumulation of lipids. We also found a significant difference in the serum triglyceride and a higher fat pad accumulation in broilers fed SO than the control group. These results suggest that LPL gene expression, as a lipogenic enzyme, regulated fat accumulation deposition in adipose tissues during adipogenesis in a rate-limiting step (Claire D'Andre et al. Citation2013; Loongyai et al. Citation2018).
The results from the present study also showed that diets rich in SO suppressed the expression of the lipolytic gene ATGL compared to the control group. ATGL is a key lipid-degrading and an adipose-specific enzyme (Serr et al. Citation2009) that acts as an energy supply for other tissues. A reduction in the lipolytic activity of adipocytes was reported for dietary fat inclusion due to the inhibitory action exerted by insulin on intracellular lipases (Camargo et al. Citation2014).
In contrast with mammals, there is relatively little information on immunity gene expression in chicken adipose tissue. TNF-α and IL-6 are the most prominent adipokines in mammals implicated in the regulation of inflammation. There is general agreement among researcher that TNF-α and IL-6 in mammal’s serum are elevated in obesity (Lumeng et al. Citation2007; Chavey et al. Citation2009). Although we detected TNF-α gene expression in broiler chicken adipose tissue, we found no correlation between abdominal fat mass and expression TNF-a level. Bornelov et al. indicated that TNF-α mRNA was poorly expressed in the visceral fat of female broilers and layer chickens and was not affected by feed deprivation (Bornelov et al. Citation2018). The ILs also regulates the host immune response to infection (Wang et al. Citation2012; Al-Zghoul et al. Citation2019). However, there is virtually nothing known about the expression of IL or any immune system-related gene in chicken abdominal fat tissue. Hausman et al. reported that IL-6 gene expression was not detected in chicken adipose tissue, which is in line with the result of the current study (Hausman et al. Citation2012).
The concentration of LMT and the relative weight of lymphoid organs are often used to evaluate the immune status and inflammatory response in animals ( Inoue et al. Citation2009). While there are conflicting reports about the role of LMT in inflammatory reactions (Inoue et al. Citation2009), it has been known as an acute-phase protein produced in response to a wide range of pro-inflammatory cytokines including IL-6, and TNF-α in vivo (Korver et al. Citation1998). However, in the current study, there were no significant differences between treatment groups in the LMT level or the relative weight of lymphoid organs.
It has been reported that abdominal fat mass in broiler chicken can be decreased by supplementing specific anti-lipogenic natural compounds such as green tea (Chen et al. Citation2009; Lee et al. Citation2009). In the current study, 500 mg GTE/kg diet was significantly decreased LPL and increased ATGL genes expression and tended to decrease abdominal fat deposition in broiler fed diet rich in SO (p = .1). But it did not alter the blood triglyceride concentration and carcase yield. These results of the present study are in agreement with Shomali et al. (Citation2012) who reported that adding green tea powder to the dietary of broiler chickens did not affect the serum concentration of triglycerides. However, Huang et al. (Citation2013) demonstrated that abdominal fat mass, blood triglyceride, and cholesterol were significantly decreased in the green tea polyphenols-supplemented groups and their data revealed that green tea administration decreased and increased the expression of some key genes associated to lipogenesis, and fatty acid β-oxidation in the liver respectively. Vitali et al. (Citation2018) also reported that the use of linolenic acid and polyphenols in the swine diet, stimulates the expression of genes involved in lipogenesis. Although in the present study GTE was effective on anabolism and catabolism related genes, no significant alteration of the blood triglyceride concentration and carcase yield were found in GTE treatments. It was likely due to the impact of different green tea raw material or its extraction processing on the phytochemical composition of the extract. However, further studies need to extend the knowledge about the dietary fat source in the fat deposition and their specific effect on molecular and cellular mechanisms of adipocytes in broiler chicken.
Our data revealed that SO elevated the expression of key gene related to lipogenesis (LPL), while suppressed expressions of the gene was related to lipolysis (ATGL). We also demonstrated that abdominal fat mass and the concentration of blood triglyceride significantly increased, and carcase yield decreased in the SO-supplemented group compared to control group. Our data indicated that some inflammatory genes expression, for example TNF-α was not significantly affected by dietary fat, and the gene expression of IL-6 was not detected in chicken broiler chicken. Also, no significant difference in LMT and relative weight of lymphoid organs were found among treatment groups.
Although the significant results achieved with genes expressions in the current study are encouraging and are consistent with the previous studies (Claire D'Andre et al. Citation2013; Loongyai et al. Citation2018), use of one single reference gene could be a limitation (Busato et al. Citation2019). Therefore, employing more than one reference gene is suggested in future studies.
Conclusions
Our results suggest that abdominal fat mass was affected more by unsaturated fatty acid but a high rate of abdominal fat mass is not responsible for inflammatory in broiler chickens. The supplementation of GTE alleviated the LPL increasing and ATGL-reducing effects of SO diet and ameliorated the effect of the SO on abdominal fat percentage.
Ethical approval
The authors confirm that all applicable international guidelines for the care of animals, experimental procedures and sample collection methods were followed when carrying out the present research.
| Abbreviations | ||
| ATGL | = | Adipose triglyceride lipase |
| IL-6 | = | Interleukin |
| LPL | = | Lipoprotein lipase |
| TNF-α | = | Tumour necrosis factor α |
Disclosure statement
The authors confirm that there are no known conflicts of interest associated with this manuscript, and there has been no significant financial support for this work that could have influenced its outcome.
References
- Alagawany M, Abd El‐Hack ME, Saeed M, Naveed M, Arain MA, Arif M, Tiwari R, Khandia R, Khurana SK, Karthik K, et al. 2020. Nutritional applications and beneficial health applications of green tea and l-theanine in some animal species: A review. J Anim Physiol Anim Nutr (Berl)). 104(1):245–256.
- Al-Zghoul MB, Saleh KM, Ababneh MMK. 2019. Effects of pre-hatch thermal manipulation and post-hatch acute heat stress on the mRNA expression of interleukin-6 and genes involved in its induction pathways in 2 broiler chicken breeds. Poult Sci. 98(4):1805–1819.
- AOAC 2005. Official method of Analysis. 18th Edition, Association of Officiating Analytical Chemists, Washington DC, Method 935.14 and 992.24.
- Azman MA, Konar V, Seven PT. 2004. Effects of different dietary fat sources on growth performances and carcass fatty acid composition of broiler chickens. Review Medicine Veterinary. 156:278–286.
- Bahareldin A, Abdalla JI, Chen E, Nie Q, Zhang X. 2018. Genomic insights Into the Multiple Factors Controlling Abdominal Fat Deposition in a Chicken Model. Front Genet. 9:262–217.
- Bastard JP, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, Vidal H, Hainque B. 2000. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab. 85 (9):3338–3342.
- Bhanja S, Sudhagar M, Goel A, Pandey N, Mehra M, Agarwal SK, Mandal A. 2014. Differential expression of growth and immunity related genes influenced by in- ovo supplementation of amino acids in broiler chickens. Czech J Anim Sci. 59 (No. 9):399–408.
- Bornelov S, Seroussi E, Yosefi S, Benjamini S, Miyara S, Ruzal M, Grabherr M, Rafati N, Molin AM, Pendavis K, et al. 2018. Comparative omics and feeding manipulations in chicken indicate a shift of the endocrine role of visceral fat towards reproduction. BMC Genomics. 19 (1):1–15.
- Busato S, Mezzetti M, Logan P, Aguilera N, Bionsaz M. 2019. What’s the norm in normalization? A frightening note on the use of RT-qPCR in the livestock science. Gene.
- Camargo A, Meneses ME, Perez-Martinez P, Delgado-Lista J, Rangel-Zuniga OA, Marin C, Almaden Y, Yubero-Serrano EM, Gonzalez-Guardia L, Fuentes F, et al. 2014. Dietary fat modifies lipid metabolism in the adipose tissue of metabolic syndrome patients. Genes Nutr. 9 (4):1–9.
- Chavey C, Lazennec G, Lagarrigue S, Clape C, Iankova I, Teyssier J, Annicotte JS, Schmidt J, Mataki C, Yamamoto H, et al. 2009. CXC ligand 5 is an adipose-tissue derived factor that links obesity to insulin resistance. Cell Metab. 9 (4):339–349.
- Chen N, Bezzina R, Hinch E, Lewandowski PA, Cameron-Smith D, Mathai ML, Jois M, Sinclair AJ, Begg DP, Wark JD, et al. 2009. Green tea, black tea, and epigallocatechin modify body composition, improve glucose tolerance, and differentially alter metabolic gene expression in rats fed a high-fat diet. Nutr Res. 29 (11):784–793.
- Claire D'Andre H, Paul W, Shen X, Jia X, Zhang R, Sun L, Zhang X. 2013. Identification and characterization of genes that control fat deposition in chickens. J Anim Sci Biotechnol. 4 (1):43–16.
- Coppack SW. 2001. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 60 (3):349–356.
- Costa CAS, Carlos AS, Santos AS, Monteiro AMV, Moura EG, Nascimento-Saba CCA. 2011. Abdominal adiposity, insulin and bone quality in young male rats fed a high-fat diet containing soybean or canola oil. Clinics. 66 (10):1811–1816.
- Crespo N, Esteve-Garcia E. 2001. Dietary fatty acids profile modifies fat deposition in broiler chicken. Poultr Sci. 80 (1):71–78.
- Deol P, Evans JR, Dhahbi J, Chellappa K, Han DS, Spindler S, Sladek FM. 2015. Soybean oil is more obesogenic and diabetogenic than coconut oil and fructose in mouse: potential role for the liver. PLoS One. 10 (7):e0132672.
- Deol P, Fahrmann J, Yang J, Evans JR, Rizo A, Grapov D, Salemi M, Wanichthanarak K, Fiehn O, Phinney B, et al. 2017. Omega-6 and omega-3 oxylipins are implicated in soybean oil-induced obesity in mice. Sci Rep. 7 (1):12488–12496.
- Hausman GJ, Barb CR, Fairchild BD, Gamble J, Lee-Rutherford L. 2012. Expression of genes for interleukins, neuropeptides, growth hormone receptor, and leptin receptor in adipose tissue from growing broiler chickens. Domest Anim Endocrinol. 43 (3):260–263.
- Huang J, Zhang Y, Zhou Y, Zhang Z, Xie Z, Zhang J, Wan X. 2013. Green tea polyphenols alleviate obesity in broiler chickens through the regulation of lipid-metabolism-related genes and transcription factor expression. J Agric Food Chem. 61 (36):8565–8572.
- Inoue KI, Takano H, Shimada A, Satoh M. 2009. Metallothionein as an anti-inflammatory mediator. Mediators Inflamm. 2009:101659.
- Khoobbakht Z, Roostaei-Ali Mehr M, Mohammadi M, Mohammadghasemi F, Sohani MM. 2020. Supplementation of various zinc sources modify sexual development and testicular IGF family gene expression in pre-pubertal male Japanese quail. Res Vet Sci. 130:87–92.
- Korver DR, Roura E, Klasing KC. 1998. Effect of dietary energy level and oil source on broiler performance and response to an inflammatory challenge. Poult Sci. 77 (8):1217–1227.
- Lee MS, Kim CT, Kim Y. 2009. Green tea (-)-epigallocatechin-3-gallate reduces body weight with regulation of multiple genes expression in adipose tissue of diet-induced obese mice. Ann Nutr Metab. 54 (2):151–157.
- Linde AR, Vazquez EG. 2006. A Simple assay to quantify metallothionein helps to learn about bioindicators and environmental health. Biochem Mol Biol Educ. 34 (5):360–363.
- Loongyai W, Saengsawang N, Danvilai W, Kridtayopas C, Sopannarath P, Bunchasak C. 2018. The Expression of lipoprotein lipase gene with fat accumulations and serum biochemical levels in betong (KU Line) and broiler chickens. Int J Anim Vet Sci. 12 (11):454–457.
- Lumeng CN, Bodzin JL, Saltiel AR. 2007. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 117 (1):175–184.
- Marisa C, Oliveira T, Fernandes R. 2013. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci. 9 (2):191–200.
- Miyoshi H, Perfield JRJW, Obin MS, Greenberg AS. 2008. Adipose triglyceride lipase regulates basal lipolysis and lipid droplet size in adipocytes. J Cell Biochem. 105 (6):1430–1436.
- Pfaffl MW, Horgan GW, Dempfle L. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30(9):e36–10.
- Ramiah SK, Meng GY, Sheau Wei T, Swee Keong Y, Ebrahimi M. 2014. Dietary conjugated linoleic acid supplementation leads to downregulation of PPAR transcription in broiler chickens and reduction of adipocyte cellularity. PPAR Res. 2014:137652.
- Reda FM, El-Kholy MS, Abd El-Hack ME, Taha AE, Othman SI, Allam AA, Alagawany M. 2020. Does the use of different oil sources in quail diets impact their productive and reproductive performance, egg quality, and blood constituents? Poultr Sci. 99(7):3511–3518.
- Sanz M, Lopez-Bote CJ, Menoyo D, Bautista JM. 2000. Abdominal fat deposition and fatty acid synthesis are lower and floxidation is higher in broiler chickens fed diets containing unsaturated rather than saturated fat. J Nutrition. 130(12):3034–3037.
- SAS Institute. 2000. SAS users guide: statistics. Version 9.12. Cary (NC): SAS Institute; p. 126–178.
- Serr J, Suh Y, Lee K. 2009. Regulation of adipose triglyceride lipase by fasting and refeeding in avian species. Poult Sci. 88 (12):2585–2591.
- Shomali T, Mosleh N, Nazifi S. 2012. Two weeks of dietary supplementation with green tea powder does not affect performance, d-xylose absorption, and selected serum parameters in broiler chickens. Comp Clin Pathol. 21 (5):1023–1028.
- Vitali M, Dimauro C, Sirri R, Zappaterra M, Zambonelli P, Manca E, Sami D, Pietro D, Lo F, Davoli R. 2018. Effect of dietary polyunsaturated fatty acid and antioxidant supplementation on the transcriptional level of genes involved in lipid and energy metabolism in swine. PLoS One. 13(10):e0204869.
- Wang YC, Deng JL, Xu SW, Peng X, Zuo ZC, Cui HM, Wang Y, Ren ZH. 2012. Effects of zearalenone on IL-2, IL-6, and IFN-γ mRNA levels in the splenic lymphocytes of chickens. ScientificWorldJournal. 2012:567327.
