 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
We evaluated the preventive effects of the oral administration of chestnut tannins (Castanea sativa) together with its potential metabolic effect on calf diarrhoea. Forty Italian Friesian female calves were included and divided into Group C (control group) and Group T (tannin-treated group). From the third day of life (T0) for the following 56 days (T56), calves from Group C received 2 L of warm water, while 10 g of chestnut tannin powder extract were added to Group T. Calves were weighed at birth and at T56. Daily faecal score evaluation was performed according to the literature. The age at diarrhoea onset (TDE) and the duration of the diarrhoeic episode were recorded. Blood methaemoglobin and liver enzymes were evaluated weekly starting from T0 to T56 by spectrophotometer and clinical chemistry analysis, respectively. The t-Student and chi-square tests were performed. The TDE was higher (p = .04) in Group T than in Group C (12.0 ± 8.2 and 7.7 ± 3.8 days, respectively). There were no differences for ADG between the groups. Group C spent 24.4% of the whole period with diarrhoea, whereas Group T experienced diarrhoea for 18.9% of the period (p = .001). All the blood and serum analytes were within physiological values. The administration of tannins in calves from the third day of life seemed to delay the onset of diarrhoea by almost four days, suggesting effective preventive action of chestnut tannins.
The oral administration of chestnut tannins in calves seemed to delay the onset of diarrhoea by almost four days, thus showing a preventive effect.
The oral administration of chestnut tannins may lead to a better and shorter recovery of the calf, thus decreasing the use of antibiotics.
HIGHLIGHTS
Introduction
Calf diarrhoea is generally caused by infectious agents, leading to substantial economic losses throughout the world due to treatment costs, decreased growth rates, and a higher replacement rate due to culling or death. Rotavirus, Coronavirus, Cryptosporidium parvum and Escherichia coli are the most common pathogens involved in neonatal diarrhoea, especially in animals less than one month old (Cho and Yoon Citation2014; Izzo et al. Citation2015).
Interest in natural products has been growing due to the need to reduce the use of antibiotic molecules in large animal species and in pets (Weese Citation2015). Alternative approaches to prevent and control animal diseases could reduce the wide use of antimicrobials and could improve the health, productivity and welfare of the animals. Several formulations of polyphenols with bacteriostatic, anti-clostridial and astringent functions have been evaluated in livestock (Laudato and Capasso Citation2013; Tosi et al. Citation2013; Mosele et al. Citation2015; Brenes et al. Citation2016). In terms of gastro-intestinal problems such as diarrhoea, gastric ulcers and gastric lesions, several phytotherapic products have been tested in rats, piglets, horses, and calves (Weyl-Feinstein et al. Citation2014; Bonelli et al. Citation2016, Citation2018).
Tannins are a complex group of polyphenolic compounds which are produced by several plants as secondary metabolites in response to stress (Frutos et al. Citation2004). Despite some authors showing that tannins decrease the rate of protein degradation in the rumen, many studies have found that this negative effect varies significantly according to the chemical structure of the tannin and to the amount included in the diet (Buccioni et al. Citation2015a, Citation2015b; Saminathan et al. Citation2019). One controlled clinical trial reported that dietary chestnut extract administered as 0.02% of body mass had a toxic effect on liver function in newborn calves (Wieland et al. Citation2015). However, feeding tannins in a low to moderate concentration appears to improve the average daily gain (ADG) and the health of the gastrointestinal tract in calves of up to two months old (Liepa et al. Citation2018; Soleiman and Kheiri Citation2018), and had no negative effect on liver function (Liepa et al. Citation2018).
In a previous study we showed that feeding calves affected by neonatal diarrhoea with 10 g of chestnut tannins per day (nearly 0.025% of body mass at birth) had astringent and anti-inflammatory effects on the gastro-enteric tract (Bonelli et al. Citation2018). The evaluation of tannins in preventing diarrhoea, and not only in shortening its duration, could therefore be interesting. The aim of the present study was thus to evaluate the preventive effect of the oral administration of chestnut tannins and its potential metabolic effect on calf diarrhoea.
Materials and methods
Animals
The present in vivo blinded study was approved by the Institutional Animal Care and Use Committee (OPBA, Pisa, prot. n. 33479/2016) and was carried out at the University of Pisa’s experimental dairy farm (Centro di Ricerche Agro-Ambientali E. Avanzi).
All the calves underwent the same management condition. Briefly, immediately after birth the calves were identified, weighed (birth weight – BW) (ID 3000, Tru-Test Limited, USA) and housed in a single straw-bedding pen (2.5 × 2 m) according to the current regulations regarding the welfare of calves (2008/11/CE). Two litres of good quality colostrum (≥50 g/L of Ig) evaluated with an optical Brix refractometer (Atago brix N1, Japan) milked from the calves’ dam, or from the colostrum bank, were administered as soon as the calf could drink (30 min − 2 h). Another 2 L were administered within the next 4–8 h in order for a successful transfer of passive immunity (Godden Citation2008). All the calves received a total of 2 L of colostrum, twice a day, until the third day of life. After this they received 3 L of whole milk at 39 °C, twice a day until the third week of life. All the feeding procedures were conducted by an expert operator and using a nipple bucket. Even when there were cases of diarrhoea, no feeding restrictions or changes in feeding regime occurred. From the third day of life, fresh and clean water was provided to each calf ad libitum. Free choice-hay was administered after the first week of life. The calves were moved into a collective pen after the third week of life.
Inclusion criteria
A total of 46 calves were enrolled in the study. The inclusion criteria were female calves born during the period of the study (July 2016–July 2018) that showed a successful transfer of passive immunity 24 hours after birth (assessed by serum total protein measure). We included only female calves because males were sold before the 30th day period after birth. Female calves were excluded from the study if the passive transfer of immunity was inadequate, if they showed diarrhoea before the third day of life, or if they were affected by a disease other than diarrhoea during their first two months of age.
Blood samples
Twenty-four hours after birth, 10 mL of blood was collected from the jugular vein of each calf in order to evaluate the absorption of immunoglobulin. Samples were collected by jugular venepuncture in red-top Vacutainer tubes (10-mL BD Vacutainer glass serum tube, silicone-coated; Becton Dickinson and Co., Franklin Lakes, NJ) and maintained at 4° C until the evaluation. All samples were processed within three hours after collection. The sample was centrifuged (Legend RT, Sorvall; ThermoFisher Scientific Inc., Waltham, MA) at 1,565 × g for 15 minutes in order to collect the serum. Serum total protein (TP) was measured using a temperature-compensating digital refractometer (AR200; Reichert Analytical Instruments, Reichert Inc., Depew, NY) in order to assess the state of passive transfer in each calf (Morrill et al. Citation2012). Calves that showed a serum TP >5.5 g/dL were considered as having a successful passive transfer of immunity (Weaver et al. Citation2000) and were included in the study.
Group formation and chestnut tannin powder administration
Calves were randomly assigned at birth to Group C (control group) (n = 23) or to Group T (tannin-treated group) (n = 23). Starting from the third day of life (T0), all the calves enrolled in group C received 2 L of warm water q24h, while the calves assigned to group T received 2 L of warm water plus 10 g of soluble chestnut tannins (concentration of 5 g/L) as extract powder (750 g/kg of dry matter equivalent of tannic acid; Mauro Saviola Group srl, Radicofani, Siena, Italy) q24h. The chemical composition of the powder is described in Campo et al. (Citation2016). The powder used in the present study was produced in a single batch and analysed by the manufacturer at the beginning of the study. Both solutions were administered at the same time (around 10.00 in the morning) using a graduated calf bottle. The bottle was equipped with a flexible rubber nipple (10 cm of length) specifically designed for calf feeding. First the bottle was filled with warm water, then the soluble powder was added, and the bottle was shaken carefully keeping the nipple hole closed. In order to ensure the entire intake, the bottle was opened and checked each time. No calves refused the solution. Calves received tannin solution until T56, when they were weighed again (weaning-weight – WW).
Clinical examination
Physical examination included evaluation from a distance plus a hands-on examination, focussing on: physical appearance, body weight, body condition, head, mouth, eyes, ears, neck and back, thorax, abdomen, umbilicus, musculoskeletal system, perianal region, body temperature, faeces, urine, and external genitalia (Izzo et al. Citation2015). The manifestation of diarrhoea was defined as a FS ≥ 2 (Renaud et al. Citation2020). The FS represents an evaluation of faecal fluidity as reported in the literature: score 0 = normal consistency; score 1 = semi-formed or pasty; 2 = loose but enough consistency to remain on bedding; 3 = watery faeces that seep through bedding material (McGuirk Citation2008). Each day, before the administration of the tannin solution, all the calves were submitted to a complete physical examination and the individual faecal score (FS) (Renaud et al. Citation2020) and dehydration status were evaluated (Constable et al. Citation1996).
From the first day of diarrhoea, the FS was recorded daily by the same expert operator (LT) by observing fresh faeces in the pen until the calf had completely recovered from the diarrhoea. The duration of a diarrhoeic episode (DDE) was defined as the period (in days) between the first diarrhoea outbreak (faecal score ≥ 2) and the normalising of the FS (faecal score = 0 or 1). The age expressed in days of the first diarrhoea out-break (TDE) and the frequency of diarrhoeic episodes defined as the number of episodes per calf throughout the whole study period were also recorded. Once the diarrhoea had started, a faecal sample was collected from each calf by manual restraint. A gloved, lubricated finger was passed gently through the anus in order to massage the rectal wall and to stimulate rectal evacuation. Fresh faeces were then collected in two different sterile tubes: one aliquot was immediately tested with a rapid ELISA test (test strips for detection of Rotavirus, Coronavirus, E. coli F5 and C. parvum in bovine faeces, Biox Diagnostics, Belgium), while the second aliquot was stored in a refrigerated bag and evaluated within one hour for gastrointestinal parasites, according to Izzo et al. (Citation2015). Milk intake was recorded throughout the diarrhoeic episode. Throughout the diarrhoeic episode, calves in both groups received 1 tablet of Effydral® (Italy Zoetis Ltd.) (sodium chloride 2.34 g, potassium chloride 1.12 g, sodium bicarbonate 6.72 g, citric acid anhydrous 3.84 g, lactose monohydrate 32.44 g, glycine 2.25 g) as an oral rehydration solution q24h. The tablet was added to the 2 L of water administered to Group C or to the 2 L of water plus 10 g of tannins administered to Group T (Izzo et al. Citation2015).
Laboratory analysis
In order to evaluate the potential metabolic effect of the daily administration of tannins, blood methaemoglobin, serum albumin (ALB), gamma-glutamyl transferase (GGT) and aspartate aminotransferase (AST) were weekly evaluated starting from T0 until the end of the experiment (T0-7-14-21-28-35-42-49-56) in all the calves. Briefly, blood samples were drawn from the jugular vein in both EDTA Vacutainer tubes (2 mL) and Vacutainer tubes without EDTA (2.5 mL) (Becton Dickinson and Co., Franklin Lakes, NJ) and immediately refrigerated until the analysis. All samples were processed within three hours after collection. The proportion of haemoglobin circulating as methaemoglobin was measured spectrophotometrically with an in vitro assay test (Paramedical srl, Italy). In brief, 100 µL of whole blood were lysed in phosphate buffer (pH 6.8) and detergent. Lysate was divided into two aliquots of 100 µL. The absorbance of the first aliquot was directly measured at 630 nm using a spectrophotometer before (D1) and after (D2) the addition of sodium azide (70 mM). The absorbance of the second aliquot was measured at 630 nm using a spectrophotometer after the addition of potassium ferrocyanide (50 g/L) (D3) and sodium azide (70 mM) (D4). The percentage methaemoglobin was calculated as follows:
Serum ALB, GGT and AST were evaluated using the clinical chemistry and immunoturbidimetric analyser Liasys© (AMS, Alliance, Italy).
Statistical analysis
The ADG between birth and T56 was calculated for all the calves in both groups. Data on ADG, BW, TDE were analysed with a Shapiro-Wilk normality test in order to assess data distribution. A paired samples t-Student test was performed to verify differences between the two groups. Results concerning the frequency of diarrhoeic episode and faecal score were expressed as median value (minimum and maximum value) and a Mann-Whitney test was performed to evaluate differences between the two groups. A Chi-square test was performed to compare the total number of days the calves experienced diarrhoea throughout the whole study period (56 days) between the two groups. Data on ALB, GGT and AST were analysed by a mixed linear model including the fixed effect of the treatment (control and tannins), the fixed effect of the sampling time (nine levels from T0 to T56), the fixed effect of the interaction between treatment and sampling time, the random effect of the calf nested within the treatment (n = 40) and the random effect of the general error of the model. Results were expressed as LSmean ± standard error of mean. The Tukey test for multiple comparison was performed to evaluate the differences between means. Data on methaemoglobin were expressed as the median value (minimum and maximum value). A Friedman test for repeated measures, with Dunn's test for multiple comparisons were performed to evaluate the differences during time in both groups. Values with p ≤ .05 were considered statistically significant. SAS 8.0 was used for the statistical analysis.
Results
Forty-six calves met the inclusion criteria and were enrolled in Group C (n = 23 calves) and in Group T (n = 23). However, a total of six calves (3 calves per group) were excluded during the study period because they showed signs of severe pneumonia. Thus, each group consisted of a total of 20 calves. No calves refused the tannin solution. All the calves showed at least one episode of diarrhoea during the study period.
No alterations were found upon physical examination, and the hydration status was normal for all calves throughout the study period, even during the diarrhoeic episode. No calves showed a decrease in milk intake due to the diarrhoea, and thus no pharmacological treatments or fluid therapy were needed. Forty % of calves belonging to group C and 55% of calves belonging to group T were found to be positive for Cryptosporidium parvum. Five % of calves belonging to group C and 10% of calves belonging to group T were positive for Rotavirus. Fifty-five % of calves belonging to group C and 35% belonging to group T were negative to the rapid ELISA test. Faecal flotation did not show intestinal parasites.
The average BW was similar between the two groups (38.7 ± 6 and 39.5 ± 3.5 kg for groups C and T, respectively). The groups were thus considered similar concerning the weight at birth. The average WW was 72.25 ± 10.93 kg and 69.79 ± 9.60 kg for groups C and T, respectively. The ADG was 0.569 ± 0.153 kg/day, and 0.544 ± 0.111 kg/day, for groups C and T, respectively. Differences between the two groups were not significant. The mean age at TDE was 7.7 ± 3.8 days for group C, and 12.0 ± 8.2 days for group T, with a statistically significant difference between the two groups (p = .04) (Figure ). Considering the whole observation period, the Chi-square test showed that calves in group C spent 24.4% of the whole period with diarrhoea (nearly 15 days), whereas calves in group T had a diarrhoea episode for 18.9% of the period (nearly 10 days). A statistically significant difference was found between the two groups (p = .001) (Figure ). Data regarding the frequency of diarrhoeic episodes and the median faecal score are reported in Table .
Figure 1. The mean age, expressed in days, at the diarrhoea onset (TDE) for group C (n = 20 calves) and for group T (n = 20 calves). C: control group or group C; T: tannin-treated group or group T. Different letters denote a significant difference (a ≠ b: p < .05).
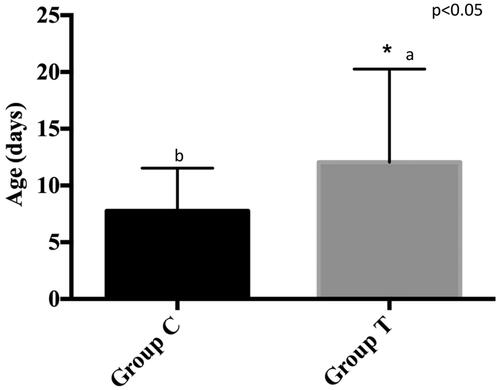
Figure 2. Days with diarrhoea as the relative percentage of the total observation period (60 days). C: control group or group C; T: tannin-treated group or group T. D: diarrhoea; ND: no diarrhoea; Chi-square test. Different letters denote a significant difference (a ≠ b: p = .001).
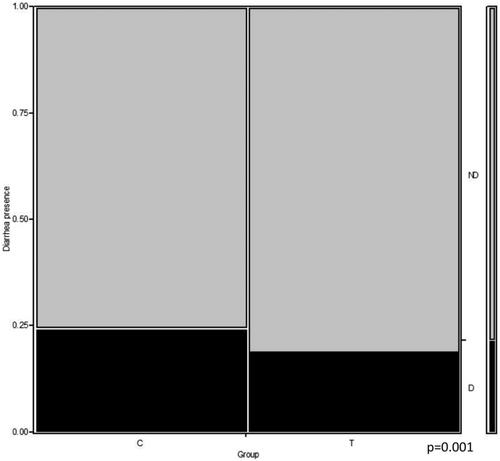
Table 1. The median, minimum (m) and maximum (M) values regarding the frequency of diarrhoeic episodes expressed as the number of diarrhoeic episodes per calf during the study period and the median faecal score for Group C and Group T.
Data on GGT values at each time for both groups are reported in Table , while data on ALB and AST values at each time for both groups are reported in Tables and . All the values were within the reference ranges (Klinkon and Jezek, Citation2012). Data on methaemoglobin were within the reference ranges (Lee and Beauchemin Citation2014) and no statistically significant differences were found between times.
Table 2. Results of the gamma-glutamyl transferase (GGT) concentration expressed as LSmean ± standard error for group C (n = 20 calves) and group T (n = 20 calves).
Table 3. Results of albumin (ALB) concentration expressed as LSmean ± standard error for group C (n = 20 calves) and group T (n = 20 calves).
Table 4. Results of aspartate aminotransferase (AST) concentration expressed as LSmean ± standard error for group C (n = 20 calves) and group T (n = 20 calves).
Discussion
The pathogen that was isolated the most in our population of diarrhoeic calves was Cryptosporidium parvum, in line with literature (Cho and Yoon Citation2014; Weyl-Feinstein et al. Citation2014; Izzo et al. Citation2015). The mean age for the onset of diarrhoea for all calves included was also in line with the literature (Cho and Yoon Citation2014; Izzo et al. Citation2015).
Tannins have numerous biological properties with protein precipitation capacity, anti-microbial, anti-parasitic and antioxidant activities being the most important in terms of their use in ruminants (Huang et al. Citation2018). Regarding the properties concerning neonatal diarrhoea in calves, the most interesting are the anti-microbial and the anti-parasitic activities. The microbial cell membrane is the primary site of inhibitory action by tannins (Mcallister et al. Citation2005; Liu et al. Citation2013). Depending on the chemical composition and structure, tannins have been shown to be most effective on Gram-negative or Gram-positive bacteria (Wang et al. Citation2013). Tannins have shown both ‘direct’ and ‘indirect’ actions on parasite cells. The direct mechanisms include the reduction in the establishment of the infective third-stage larvae, the excretion of nematode eggs by adult worms, and the development of eggs to third-stage larvae (Brunet et al. Citation2008; Hoste et al. Citation2012). On the other hand, an indirect mechanism is the improvement in the host’s resistance to nematodes (Pathak et al. Citation2016). Tannins given at the same inclusion level as the present study, have also shown an anti-protozoal activity against Criptosporidium parvum (Bonelli et al. Citation2018) and Eimeria (Landau et al. Citation2010; Markovics et al. Citation2012; Burke et al. Citation2013). Tannins appear to directly decrease the viability of the larval stage and disrupt egg hatching, as previously observed for nematodes (Brunet et al. Citation2008; Hoste et al. Citation2012).
The onset of diarrhoea in calves in group T was four days later than calves in group C. This suggests a preventive effect of the chestnut tannin administered orally from the third day of life. Preventive neonatal calf diarrhoea is a major disease that negatively affects the cattle industry, leading to a significant economic impact (Reddy et al. Citation2020). Of the 5% mortality rate in pre-weaned calves, 56% is attributed to digestive problems and diarrhoea with the rest caused by respiratory problems (USDA Citation2016). Diarrhoea is very stressful and debilitating for calves and a later onset of diarrhoea may lead to a better and shorter recovery of the neonate (Cho and Yoon Citation2014). Treated animals showed a later onset of diarrhoea and the degree of diarrhoea was less severe than the non-treated groups. Our results are in line with studies evaluating the administration of tannins in calves affected by neonatal diarrhoea (Kekana Citation2014; Bonelli et al. Citation2018; Liepa et al. Citation2018; Ranaut et al. Citation2018; Soleiman and Kheiri Citation2018; Gupta et al. Citation2020). The preventive administration of tannins from the first days of the calf’s life improved the faecal consistency score (Soleiman and Kheiri Citation2018) and the onset of diarrhoea was later than in the control group (Liepa et al. Citation2018). Other studies have shown no effect on prevention (Oliveira et al. Citation2010; Gupta et al. Citation2020) however a dose-dependent effect in improving the diarrhoea episode was found (Gupta et al. Citation2020). This difference may be related to the different biochemical composition of the product used. We assume that the beneficial effect of the tannin was due to the prevention of water loss through the mucous membranes, and the anti-inflammatory, bacteriostatic and antiprotozoal effects reported for this compound (Reddy et al. Citation2020; Smulski et al. Citation2020).
Despite the delay in the onset of diarrhoea in the treated group, no statistically significant differences were found concerning the number of episodes, the average faecal score during the diarrhoeic episode and the ADG between the two groups. On the other hand, considering the whole observation period, the percentage number of days the calves experienced a diarrhoea episode for group T was significantly lower than for group C (18.9% vs. 24.4%, Figure ). As a consequence, on average, calves in group T had diarrhoea for nearly 10 days, whereas calves from group C had it for 15 days. In veterinary medicine, an astringent effect during a diarrhoeic episode is considered to have a negative impact on the animals’ health. In fact, limiting the faecal output leads to a decrease in excretion of bacteria and bacterial products, thus enhancing the absorption of toxins (Constable Citation2009). However, the tannins did not influence the acute phase of diarrhoea and the faecal output, but seemed to reduce the time the calves experienced diarrhoea, thus enhancing the welfare of the calves and increasing the chances of a prompt recovery. Neonatal calf diarrhoea has been shown to affect the growth rate, milk production productive and reproductive performance (Aghakeshmiri et al. Citation2017). Moreover, diarrhoea in neonates can lead to toxin spread through the bloodstream, severe dehydration and lactic acidosis, which need an intensive care approach, aggressive fluid therapy and use of antimicrobial. Decreasing the number of days that calves experience diarrhoea, can reduce medical complications, thus involving fewer costs for the farmer and a better future performance (Lorenz et al. Citation2011). These results were in line with findings reported in calves (Bonelli et al. Citation2018; Liepa et al. Citation2018; Gupta et al. Citation2020) where the shortening of the diarrhoeic episode was found in tannin-treated patients. The lack of differences concerning the ADG between the two groups suggests that the tannin powder does not affect the animals’ growth. This result is in contrasts with some studies in which the tannins seemed to affect the growth performance because of the decrease in food palatability, protein digestibility and the voluntary intake (Gai et al. Citation2009; Liu et al. Citation2009; Dalle Zotte et al. Citation2012). However, a study on concentrated pomegranate peel extract (containing a high concentration of both condensed and hydrolysable tannins) reported no effects of tannins on ADG in the first 60 days of life in dairy calves (Weyl-Feinstein et al. Citation2014). In our study, the chestnut tannin powder was administered at the same dose throughout the whole study period despite the calf’s growth rate. This might have influenced some results since previous studies used tannins as a ‘treatment’ and for a relatively short period of time, and not as prevention in the first two months of life (Weyl-Feinstein et al. Citation2014; Katsoulos et al. Citation2017; Bonelli et al. Citation2018). Further studies are needed to evaluate the preventive effect of chestnut tannins administered by calculating the dose in grammes per kilogramme and adjusting it in line with the daily weight gain of the calves.
In mammals, ingested hydrolysable tannins are metabolised to gallic acid, ellagic acid and pyrogallol. The oral administration of gallic acid in the rat results in urinary excretion of the unchanged compound, 3,5-dihydroxy-4-methoxybenzoic acid, and the decarboxylated product, pyrogallol. Acid-labile conjugates of these compounds have also been observed (Smeriglio et al. Citation2017). The harmful effects of tannins in ruminants and monogastric animals range from chronic to systemic effects (subacute intoxication) (Mueller-Harvey Citation2006). In large quantities, tannins are toxic to the liver and kidneys, causing centrilobular necrosis, tissue degeneration, and glomerulonephritis (Jerónimo et al. Citation2016). The enzyme GGT is mainly located in bile ducts and the kidney. The circulating GGT levels can be increased by cholestasis and bile duct damage, or by colostrum feeding in ruminants (Klinkon and Jezek, Citation2012). Albumin levels can be influenced by nutrition and the liver functioning. They are predominantly synthesised in the liver, thus their amount depends on the maturity and functional ability of the liver (Steinhardt and Thielscher Citation2000). The AST enzyme is an indicator of tissue necrosis and in the case of liver damage its serum levels can be increased. Calves included in group T presented higher concentrations of AST and lower levels of ALB compared to those in group C. No differences were found for GGT and methaemoglobin. Both the increase in serum AST concentrations and the decrease in ALB levels could be the effect of the ingested hydrolysable tannins on the liver. Changes in the albumin and AST levels may be due to conditions not involving the liver. However, they are considered to be a sensitive biomarker of liver cell injury in bovines (Sattler and Fürll Citation2004). Our findings could suggest an initial hepatocellular damage caused by the administration of chestnut tannins in calves, as already reported in calves (Wieland et al. Citation2015). Despite the differences found between the two groups, the serum AST, ALB and GGT and blood methaemoglobin results fell within the normal range, confirming previous findings in neonatal calves (Liepa et al. Citation2018). In our opinion, given an oral dose of 10 g/calf q24h, of chestnut tannins could be a safe procedure for calves of up to two months old.
Conclusions
Calfhood diseases are one of the most significant livestock health issues faced by veterinarians and farmers. The recent literature has focussed on investigating alternative therapies for the most common neonatal calf problems. We found that chestnut tannins seemed to be effective in shortening and delaying the onset of neonatal diarrhoea in calves. A dose of 10 g per day of chestnut tannins did not show any adverse metabolic effects. No preventive effect was observed for the etiologic agents found in our population at a fixed dosage. Further studies could evaluate the preventive aspect by administering an adjusted dose in line with the daily weight gain of the calves.
In conclusion, chestnut tannins can be used for managing diarrhoea in calves of up to 2 months old.
Ethical Approval
The present study was approved by the Institutional Animal Care and Use Committee (OPBA, Pisa, prot. n. 33479/2016).
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial relationships that could be construed as a potential conflict of interest.
Additional information
Funding
References
- Aghakeshmiri F, Azizzadeh M, Farzaneh N, Gorjidooz M. 2017. Effects of neonatal diarrhea and other conditions on subsequent productive and reproductive performance of heifer calves. Vet Res Commun. 41(2):107–112.
- Bonelli F, Busechian S, Meucci V, Caporrino G, Briganti A, Rueca F, Zappulla F, Ferini E, Ghiandai L, Sgorbini M. 2016. pHyloGASTRO in the treatment of equine gastric ulcer lesions. J Equine Vet Sci. 46:69–72.
- Bonelli F, Turini L, Sarri G, Serra A, Buccioni A, Mele M. 2018. Oral administration of chestnut tannins to reduce the duration of neonatal calf diarrhea. BMC Vet Res. 14(1):227
- Brenes A, Viveros A, Chamorro S, Arija I. 2016. Use of polyphenol-rich grape by-products in monogastric nutrition: a review. Anim Feed Sci Technol. 211:1–17.
- Brunet S, Martinez-Ortiz-De-Montellano C, Torres-Acosta JFJ, Sandoval-Castro CA, Aguilar-Caballero AJ, Capetillo-Leal CM, Hoste H. 2008. Effect of the consumption of Lysiloma, latisilliquumon the larval establishment of parasitic nematodes in goats. Vet Parasitol. 157(1–2):81–88.
- Buccioni A, Pauselli M, Viti C, Minieri S, Pallara G, Roscini V, Rapaccini S, Trabalza Marinucci M, Lupi P, Conte G, et al. 2015a. Milk fatty acid composition, rumen microbial population, and animal performances in response to diets rich in linoleic acid supplemented with chestnut or quebracho tannins in dairy ewes. J Dairy Sci. 98(2):1145–1156.
- Buccioni A, Serra A, Minieri S, Mannelli F, Cappucci A, Benvenuti D, Rapaccini S, Conte G, Mele M. 2015b. Milk production, composition, and milk fatty acid profile from grazing sheep fed diets supplemented with chestnut tannin extract and extruded linseed. Small Rum Res. 130:200–207.
- Burke JM, Miller JE, Terrill TH, Orlik ST, Acharya M, Garza JJ, Mosjidis J. 2013. Sericea lespdeza as an aid in the control of Emeria spp. in lambs. Vet Parasitol. 193(1-3):39–46.
- Campo M, Pinelli P, Romani A. 2016. Hydrolyzable tannins from sweet chestnut fractions obtained by a sustainable and eco-friendly industrial process. Nat Prod Commun. 11(3):409–415.
- Cho Y, Yoon KJ. 2014. An overview of calf diarrhea – infectious etiology, diagnosis, and intervention. J Vet Sci. 15(1):1–17.
- Constable PD, Gohar HM, Morin DE, Thurmon JC. 1996. Use of hypertonic saline-dextran solution to resuscitate hypovolemic calves with diarrhea. Am J Vet Res. 57(1):97–104.
- Constable PD. 2009. Treatment of Calf Diarrhea: antimicrobial and ancillary treatments. Vet Clin North Am Food Anim Pract. 25(1):101–120.
- Dalle Zotte A, Matics ZE, Bohatir P, Sartori A, Gerncsée ZS, Szendrö ZS, 2012. Effect of dietary supplementation of chestnut hydrolysable tannin on digestive efficiency, growth performance and meat quality in growing rabbits. Proceedings of the10th World Rabbit Congress; Sep 3–6; Sharm El-Sheikh – Egypt. p. 961–965.
- Frutos P, Hervas G, Giraldez FJ, Mantecon AR. 2004. Review. Tannins and ruminant nutrition. Span J Agric Res. 2(2):191–202.
- Gai F, Gasco L, Liu HW, Lussiana C, Brugiapaglia A, Masoero G, Zoccarato I. 2009. Effect of diet chestnut tannin supplementation on meat quality, fatty acid profile and lipid stability in broiler rabbits. Ital J Anim Sci. 8(sup2):787–789.
- Godden S. 2008. Colostrum management for dairy calves. Vet Clin North Am Food Anim Pract. 24(1):19–39.
- Gupta N, Rajora VS, Gupta KK, Arora N. 2020. Evaluation of novel polyherbal formulation for the clinico-therapeutic management of diarrhea in calves. J Entomol Zool Stud. 8(6):193–199.
- Hoste HC, Martinez-Ortiz-De-Montellano F, Manolaraki S, Brunet N, Ojeda-Robertos I, Fourquaux JF, Torres-Acosta JFJ, Sandoval-Castro CA. 2012. Direct and indirect effects of bioactive tannin-rich tropical and temperate legumes against nematode infections. Vet Parasitol. 186(1–2):18–27. e
- Huang Q, Liu X, Zhao G, Hu T, Wang Y. 2018. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim Nutr. 4(2):137–150.
- Izzo M, Gunn AA, House JK. 2015. Neonatal diarrhea. In: Smith BP, editor. Large animal internal medicine. St. Louis, MS: Elsevier; p. 314–335.
- Jerónimo E, Pinheiro C, Lamy E, Dentinho MT, Sales-Baptista E, Lopes O, Capela FS. 2016. Tannins in ruminant nutrition: impact on animal performance and quality of edible products. In: Combs CA, editor. Tannins: biochemistry, food sources and nutritional properties. New York, USA: Nova Science Publishers Inc; p. 121–168.
- Katsoulos PD, Karatzia MA, Dovas CI, Filioussis G, Papadopoulos E, Kiossis E, Arsenopoulos K, Papadopoulos T, Boscos C, Karatzias H. 2017. Evaluation of the in-field efficacy of oregano essential oil administration on the control of neonatal diarrhea syndrome in calves. Res Vet Sci. 115:478–483.
- Kekana T. 2014. Effects of supplemental garlic (Allium sativum) powder and probiotics on diarrhea and immunoglobulin response in pre-weaned dairy calves. J Dairy Sci. 97(S1):565.
- Klinkon M, Jezek J. 2012. Values of blood variables in calves. In: Perez-Marin MM, editor. A Bird’s-Eye view of veterinary medicine. Croatia, MS: InTech; p. 301–320.
- Klinkon M, Jezek J. 2012. Values of blood variables in calves. In: Perez-Marin CC, editor. A Bird’s-eye view of veterinary medicine. Rijeka, Croatia: InTech; p. 301–320.
- Landau S, Azaizeh H, Muklada H, Glasser T, Ungar ED, Baram H, Abbas N, Markovics A. 2010. Anthelmintic activity of Pistacia lentiscus foliage in two middle eastern breeds of goats differing in their propensity to consume tannin-rich browse. Vet Parasitol. 173(3–4):280–286.
- Laudato M, Capasso R. 2013. Useful plants for animal therapy. OA Altern Med. 1(1):1.
- Lee C, Beauchemin KA. 2014. A review of feeding supplementary nitrate to ruminant animals: nitrate toxicity, methane emissions, and production performance. Can J Anim Sci. 94(4):557–570.
- Liepa L, Zolnere E, Dūrītis I, Krasnova I, Segliņa D. 2018. Effects of Hippophae rhamnoides L. leaf and Marc extract with reduced tannin concentration on the health and growth parameters of newborn calves. Eur J Med Plants. 22(1):1–11.
- Liu HW, Gai F, Gasco L, Brugiapaglia A, Lussiana C, Guo KJ, Tong JM, Zoccarato I. 2009. Effects of chestnut tannins on carcass characteristics, meat quality, lipid oxidation and fatty acid composition of rabbits. Meat Sci. 83(4):678–683.
- Liu XL, Hao YQ, Jin L, Xu ZJ, McAllister TA, Wang Y. 2013. Anti-Escherichia coli O157: H7 properties of purple prairie clover and sainfoin condensed tannins. Molecules. 18(2):2183–2199. e
- Lorenz I, Fagan J, More S. 2011. Calf health from birth to weaning. II. Management of diarrhoea in pre-weaned calves. Ir Vet J. 64(1):9.
- Markovics A, Cohen I, Muklada H, Glasser TA, Dvash L, Ungar ED, Azaizeh H, Landau SY. 2012. Consumption of Pistacia lentiscus foliage alleviates coccidiosis in young goats. Vet Parasitol. 186(3–4):165–169.
- Mcallister TA, Martinez T, Bae HD, Muir AD, Yanke LJ, Jones GA. 2005. Characterization of condensed tannins purified from legume forages: chromophore production, protein precipitation, and inhibitory effects on cellulose digestion. J Chem Ecol. 31(9):2049–2068.
- McGuirk SM. 2008. Disease management of dairy calves and heifers. Vet Clin North Am Food Anim Pract. 24(1):139–153.
- Morrill KM, Conrad E, Polo J, Lago A, Campbell J, Quigley J, Tyler H. 2012. Estimate of colostral immunoglobulin G concentration using refractometry without or with caprylic acid fractionation. J Dairy Sci. 95(7):3987–3996.
- Mosele JI, Macià A, Motilva MJ. 2015. Metabolic and microbial modulation of the large intestine ecosystem by non-absorbed diet phenolic compounds: a review. Molecules. 20(9):17429–17468.
- Mueller-Harvey I. 2006. Unravelling the conundrum of tannins in animal nutrition and health. J Sci Food Agric. 86(13):2010–2037.
- Oliveira RA, Narciso CD, Bisinotto RS, Perdomo MC, Ballou MA, Dreher M, Santos JEP. 2010. Effects of feeding polyphenols from pomegranate extract on health, growth, nutrient digestion, and immunocompetence of calves. J Dairy Sci. 93(9):4280–4291.
- Pathak AK, Dutta N, Banerjee PS, Goswami TK, Sharma K. 2016. Effect of condensed tannins supplementation through leaf meal mixture on voluntary feed intake, immune response and worm burden in Haemonchus contortus infected sheep. J Parasit Dis. 40(1):100–105.
- Ranaut NDS, Sharma P, Ravikanth K, Ganguly B. 2018. Comparative efficacy of herbal anti-diarrheal products for treatment of diarrhea in calves. Pharm Biol Eval. 5(1):10–13.
- Reddy PRK, Elghandour MMMY, Salem AZM, Yasaswini D, Reddy PPR, Reddy AN, Hyder I. 2020. Plant secondary metabolites as feed additives in calves for T antimicrobial stewardship. Anim Feed Sci Technol. 264:114469.
- Renaud DL, Buss L, Wilms JN, Steele MA. 2020. Technical note: is fecal consistency scoring an accurate measure of fecal dry matter in dairy calves? J Dairy Sci. 103(11):10709–10714.
- Saminathan M, Ramiah SK, Gan HM, Abdullah N, Wong CMVL, Ho YW, Idrus Z. 2019. In vitro study on the effects of condensed tannins of different molecular weights on bovine rumen fungal population and diversity. Ital J Animal Sci. 18 (1):1451–1462.
- Sattler T, Fürll M. 2004. Creatine kinase and aspartate aminotransferase in cows as indicators for endometritis. J Vet Med A Physiol Pathol Clin Med. 51(3):132–137.
- Smeriglio A, Barreca D, Bellocco E, Trombetta D. 2017. Proanthocyanidins and hydrolysable tannins: occurrence, dietary intake and pharmacological effects. Br J Pharmacol. 174(11):1244–1262.
- Smulski S, Turlewicz-Podbielska H, Wylandowska A, Włodarek J. 2020. Non-antibiotic possibilities in prevention and treatment of calf diarrhoea. J Vet Res. 64(1):119–126.
- Soleiman P, Kheiri F. 2018. The effect of different levels of tannic acid on some performance traits in holstein dairy calves. Iran J Appl Anim Sci. 8(1):19–23.
- Steinhardt M, Thielscher HH. 2000. Tiergerechte Haltung und physiologische Funktionen von Tieren. TieräRztliche Umschau. 55(4):189–198.
- Tosi G, Massi P, Antongiovanni M, Buccioni A, Minieri S, Marenchino L, Mele M. 2013. Efficacy test of a hydrolysable tannin extract against necrotic enteritis in challenged broiler chickens. Italian J Anim Sci. 12(3):386–389.
- USDA. 2016. Dairy 2014: Dairy Cattle Management Practices in the United States, 2014 [Fort Collins, CO, USA]: USDA-Animal and Plant Health Inspection Service (APHIS)-Veterinary Services (VS)-Center for Epidemiology and Animal Health (CEAH); [accessed 2021 Mar 19]. https://www.aphis.usda.gov/animal_health/nahms/dairy/downloads/dairy14/Dairy14_dr_PartI.pdf.
- Wang Y, Jin L, Ominski KH, He M, Xu Z, Krause DO, Acharya SN, Wittenberg KM, Liu XL, Stanford K, et al. 2013. Screening of condensed tannins from canadian prairie forages for anti-Escherichia coli O157:H7 with an emphasis on purple prairie clover (Dalea purpurea vent). J Food Prot. 76(4):560–567.
- Weaver DM, Tyler JW, VanMetre DC, Hostetler DE, Barrington GM. 2000. Passive transfer of colostral immunoglobulins in calves. J Vet Intern Med. 14(6):569–577.
- Weese JS. 2015. Antimicrobial use and antimicrobial resistance in horses. Equine Vet J. 47(6):747–749.
- Weyl-Feinstein S, Markovics A, Eitam H, Orlov A, Yishay M, Agmon R, Miron J, Izhaki I, Shabtay A. 2014. Short communication: effect of pomegranate-residue supplement on Cryptosporidium parvum oocyst shedding in neonatal calves. J Dairy Sci. 97(9):5800–5805.
- Wieland M, Weber BK, Hafner-Marx A, Sauter-Louis C, Bauer J, Knubben-Schweizer G, Metzner M. 2015. A controlled trial on the effect of feeding dietary chestnut extract and glycerol monolaurate on liver function in newborn calves. J Anim Physiol Anim Nutr. 99(1):190–200.
