Abstract
Date palm pollen (DPP) has been widely tested in vitro in preservation of spermatozoa of different farm animals except in rabbits. The objective of this study was to evaluate the effect of various concentrations (20, 40, 80 mg/mL) of DPP aqueous extract on epididymal and ejaculated rabbit spermatozoa during in vitro incubation at 37 °C during 120 min. NaCl Pollen Extender (NPE) and TRIS Base Extender (TPE) were respectively used in epididymal and ejaculated sperm preservation. Sperm motility parameters were objectively analysed by a Computer Aided Sperm Analyser (CASA). The results revealed that in epididymal sperm at 0 minute, sperm motility showed significant higher values (p ≤ 0.05) for all doses of DPP in comparison to the control group. After 30, 60 and 120 min, the motility rate was higher (p > .05) in all the experimental groups compared to the control. Concerning velocity traits (VCL and VAP), and trajectories parameters (LIN, ALH, BCF), the doses of 40 mg/mL and 80 mg/mL showed the highest values than Control. In ejaculated samples, the motility rate at 0 minute improved only with the dose of 80 mg/mL and DPP showed protective effect at all doses (20, 40, 80 mg/mL) up to 60 min of incubation. DPP enhanced spermatozoa velocity (VCL and VAP) and the trajectory of sperm (LIN, ALH, BCF) even from 20 mg/mL at any points of storage. In conclusion, aqueous extract of date palm pollen is a suitable supplement to the extender for rabbit sperm by protecting and improving sperm motility parameters.
The addition of DPP extract improves the quality of epididymal and ejaculated rabbit sperm.
The extender supplemented with 80 mg/mL of DPP in NPE has a beneficial effect on kinetic traits of epididymal sperm. Nevertheless, in TPE extender, 20 mg/mL of DPP was enough to improve sperm motility traits in ejaculated rabbit semen.
HIGHLIGHTS
Introduction
Rabbit meat is increasingly recognised as a healthy meat with high nutritional value (Petracci et al. Citation2009), as being low in fat, sodium and cholesterol and rich in protein (Dalle Zotte and Szendrő Citation2011). Rabbit meat contains also high levels of essential amino acids with easy digestibility (Hernández and Dalle Zotte Citation2010). In this context, the use of reproductive biotechnologies could be a promising way to enhance rabbit meat production and improve rabbit breeding.
Artificial insemination (AI) has become a consolidated practice on rabbit farms; it optimises human resources and increases animals’ reproductive performance (Dal Bosco et al. Citation2011). Semen preservation is one of the main problems for a wide use of AI in rabbitries (Alvariño Citation2000). A decrease in fertility of rabbit sperm with cell structure and function damage are caused after storage process longer than 24–48 h (Rosato and Iaffaldano Citation2011; Di Iorio et al. Citation2014). Moreover, the cryopreserved rabbit sperm is associated with a reduction in motility, viability and a low fertility rates or prolificacy, consequently artificial insemination is usually performed using fresh or cooled sperm (Castellini et al. Citation2006; Rosato and Iaffaldano Citation2011). Indeed, unlike other species, hypertonic solution, and cryoprotective agents containing hydroxyl groups such as glycerol (Ndors et al. Citation2015) affect negatively rabbit spermatozoa.
Spermatozoa are particularly vulnerable to oxidative damage owing to their specific cellular plasma membranes composition especially the large amount of long chain polyunsaturated fatty acids (Alvarez and Storey Citation1995), while their cytoplasm contains low concentrations of scavenging enzymes (Tvrdá et al. Citation2011). This could cause overexpression of Radical Oxidative Substances (ROS) with a subsequent decrease in motility and viability (De Lamirande and Gagnon Citation1992). This Oxidative damage may ultimately lead to infertility by increasing midpiece morphological defects with deleterious effects on sperm capacitation and acrosome reaction (Agarwal et al. Citation2014). Therefore, the diluents used as extenders play a significant role in the success of artificial insemination in maintaining suitable sperm motility, viability, membrane integrity, fertilising ability and antioxidant protection (Al-Daraji Citation2004) . Therefore, storage of fresh sperm without affecting the fertilising capability is a high demand in rabbit commercial farms.
The use of natural products, like plant derivatives in several diseases treating is as old as humankind itself (Silva and Fernandes Citation2010). Recently, there is growing interests in plant based alternatives in the preservation of sperm quality. Some medicinal plants are rich in natural antioxidant compounds such as vitamins C and E, folate, zinc, selenium, carnitine and carotenoids (Fatma et al. Citation2009). They are scavengers of ROS and have protective effects against oxidative stress damage by reversing the adverse impact of high ROS concentrations on semen parameters (Ross et al. Citation2010).
Several natural extracts and infusions are used to preserve animal spermatozoa in different extenders (Sansone et al. Citation2000). Likewise, many studies have shown that several compounds of natural herbal antioxidants (Piomboni et al. Citation2008; Taepongsorat et al. Citation2008) can enhance sperm quality, motility and potentially sperm-fertilising capacity. Accordingly, plant based diluent for fresh sperm could be employed as an alternative to the common antioxidants. Thus, the development of new herbal medicine for improving the quality of sperm (mobility and vitality) is therefore an interesting goal.
Phoenix dactylifera pollen (Date palm pollen: DPP) is the male germ powder of palm flowers. In traditional medicine, suspension of DPP has been widely used for curing male infertility as a folk remedy, to improve reproductive performances in men and women, from ancient times (Bahmanpour et al. Citation2006). In fact, in Algerian Sahara, DPP is used as food and sexual booster in both men and women, by preparing the mixture of pollen powder with bee honey eaten daily after fasting. Women take it during the ovulation period by sprinkling pollen grains mixed to herbal extracts upon a sanitary towel during the fertile phase of menstrual cycle to improve ovulation and fertilisation. The beneficial role of this preparation has been reported specially to clean the uterus and induce its wetness (Selmani et al. Citation2017).
DPP is known to contain a variety of compounds such as a high concentration of total phenolic content, flavonoids and anthocyanins as well as the presence of significant quantity of selenoproteins (Baliga et al. Citation2011) which makes it an excellent candidate for antioxidant activity associated to low negative side effects (Fallahi et al. Citation2015). Thus it is a very suitable supplement in infertility particularly by reducing free radicals and enhancing sperm motility (Fallahi et al. Citation2015). Therefore, synergic beneficial effect of bioactive compounds along with antioxidant activity of DPP extract might result to improved properties of spermatozoa.
Additionally, various in vitro and in vivo experimental studies, and clinical trials, have evaluated the effects of DPP on the reproductive system. All research indicates that there are significant improvements in the sperm parameters, such as motility, viability, acrosome reaction, and lipid peroxidation (Fallahi et al. Citation2015). Al-Samarrai et al. (Citation2017) have found a higher fertility on rabbit bucks after orally administration of DPP suspension, which enhance the production of testosterone and increase the spermatogenesis.
Few in vitro studies have shown the beneficial effect of DPP extract supplementation to sperm extender in preserving and maintaining semen quality during cryoconservation especially in human, bulls buffalo and stallion (Al-Dujaily et al. Citation2012; El-Sheshtawy et al. Citation2014, Citation2016; El-Sisy et al. Citation2018; Mohamed and Talal Citation2020).
To the best of our knowledge, there are no data on the in vitro impact of DPP on rabbit semen. Therefore, the aim of the present study was to evaluate the effect of various concentrations of DPP aqueous extract on epididymal and ejaculated rabbit sperm. For this reason, we evaluated the sperm motility using CASA system during various times (0–120 min) of in vitro incubation at 37 °C.
Materials and methods
Sampling of plant material
Pollen grains of date palm (Phoenix dactylifera L.) (Dokkars) were collected from Touggourt located about 660 km south of Algiers in March 2019 air dried for 48 h under dark condition then stored at 4 °C until use.
Preparation of different sperm extenders
For aqueous extraction of DPP, we used different extenders, TRIS Base Extender for ejaculated sperm and NaCl (0.9%) for epididymal sperm to maintain the physiological characteristics of spermatozoa. TRIS buffer could be considered to have similar proprieties of the seminal plasma components (sugar, antibacterial agents), whereas saline solution consist in a base washing solution for epididymal sperm, which otherwise do not have a storage liquid.
TRIS base extender (TBE)
A Tris-based extender was used to prepare treatment for ejaculated semen. It contained Tris (hydroxymethylaminomethane) (3.79 g), citric acid (2.16 g), glucose (0.59 g), Penicillin G (0.1 g), dihydrostreptomycin (0.1 g) and distilled water (100 mL), (300 mOsm/l; pH 7.1).
TRIS pollen extender (TPE)
Pollen grain extract was prepared in (TBE). 20, 40, and 80 mg of DPP was soaked in 3 test tubes each containing 1 mL of TBE, after vortex all tubes were stored in a refrigerator (adjusted at 4 °C) for 24 h and finally centrifuged to obtaint the supernatant representing the used extender (TPE).
NaCl pollen extender (NPE)
DPP extract was prepared in saline solution (NaCl 0.9%, 308 mOsm/L, pH 7.2) for epididymal sperm. 20, 40, and 80 mg of DPP was soaked in 3 test tubes each containing 1 mL of NaCl, after vortex all tubes were stored in refrigerator (adjusted at 4 °C) for 24 h and finally centrifuged to obtain the supernatant representing the used extender (NPE).
Sperm collection and initial evaluation
The ejaculated sperm was collected from 10 New Zealand White mature bucks (8 months old) housed in individual cages; water and food were provided ad libitum. Rabbits were trained to serve the artificial vagina before study. The collection was performed after sexual stimulation by a rabbit doe using an artificial vagina pre-warmed as described by Boiti et al. (Citation2005). After semen collection, any gel plug was removed; the sperm was examined macroscopically to assess volume, colour and pH level, and routed to the laboratory in a water bath at 35–37 °C. In order to obtain the epididymal sperm, six samples of rabbit testis from 6 different animals were obtained from a local slaughter house and transported at a cooler temperature in ice chest to the laboratory. The sperm collection was performed by the retrograde flushing method of the tail of the epididymis as previously described by Martinez-Pastor et al. (Citation2006) by applying pressure to the vas deferens by injecting fluid (media) using a syringe until the contents of the cauda emerge from a cut made near the junction with the epididymis body.
The sperm taken from each rabbit bucks and testis were mixed to obtain a sperm pool to avoid individual differences. After sperm collection, the sperm concentration was adjusted to the concentration of 1 × 108/mL by the addition of an adequate volume of different sperm extenders (TBE extender for the ejaculated sperm and NaCl extender to epididymal sperm) using Thomahemocytometer slide and microscopic evaluation of the pooled sperm was carried out before the sperm processing.
Sperm processing
Both types of pooled sperm (ejaculated and epididymal) were divided into 4 samples; they were diluted with TBE extender without DPP (control) and other aliquots with the different concentrations of TPE extenders for the ejaculated sperm. For the epididymal sperm NaCl extender without DPP was used as control and the other aliquots were diluted in the different concentrations of NPE extender.
Assessment of sperm motility
Sperm motility was evaluated for ejaculated and epididymal sperm using the Computer assisted sperm analysis (CASA) method (PROiSER R + D Systems, Valencia, Spain). Each sample 10 μL was placed into Makler counting chamber (Makler Counting chamber, Sefi-Medical Instruments ltd., Biosigma S.r.l., Italy). Analyses were realised at different incubation times (0, 30, 60, 120 minutes) of incubation using a previously established rabbit specific set-up (Castellini et al. Citation2011). Samples were stored in incubator at 37 °C during the whole experiment. In each sample, spermatozoa kinematics was analysed at 10X field using a negative phase contrast microscope, namely Sperm motility (M), Velocity Curved Line (VCL), Average Path Velocity (VAP), Linearity (LIN), Amplitude of Lateral Movement of the Head (ALH) and Beat Cross Frequency (BCF).
Statistical analysis
The control group was compared to the experimental groups for both types of semen. The data were analysed using the Statview 4.02 software (Abacus Concepts Inc., Berkeley, CA, USA). The results are expressed as mean ± SEM. Differences on motility parameters were determined using a one-way ANOVA followed by Fisher’s test. Values were considered significant when (p ≤ .05).
Results
Epididymal sperm
Sperm motility
The effects of different concentrations of DPP aqueous extract on epididymal sperm motility evaluated at different time of incubation (0, 30, 60, 120 min) are represented in Figure . As evidently seen, as soon as sperm was incubated with the extenders including DPP (NPE group), remarkable differences in motility parameters was recorded.
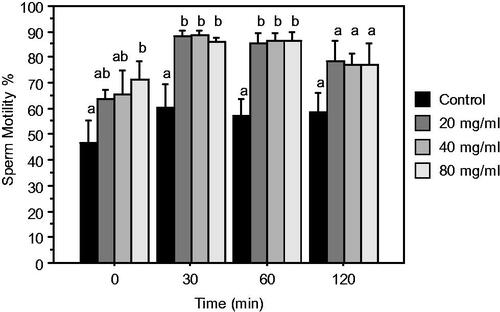
Figure 1. Sperm motility (Mean ± SEM) at 0, 30, 60, 120 min of incubation of epididymal rabbit sperm diluted with control or NPE extender (control + 20, 40, 80 mg/mL DPP extract). Different letters indicate significant differences (p ≤ .05).
As expected, during the storage time progress the motility rate lightly declined in all the groups. At time 0, the sperm motility showed higher values (p ≤ .05) for any doses of DPP in comparison to the control group (e.g. 90.71%, 79.56%, and 94.37%, respectively vs. 41.22%).
After 30, 60 and 120 min of in vitro incubation, the motility rate was higher (p > .05) in all the experimental groups compared to the control. However, the differences between groups were not significant due to the high variability of values.
Speed traits of sperm (VCL and VAP)
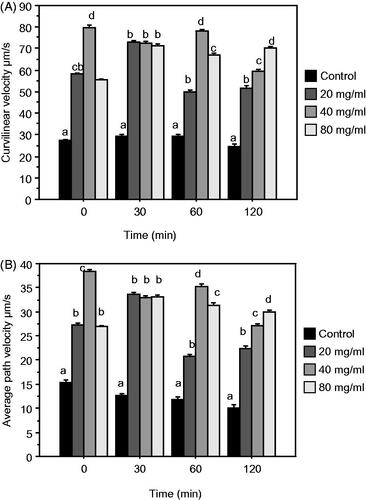
When compared to the control, the addition of the various DPP doses, significantly increase velocity traits (VCL and VAP) at any time points of storage (Figure ). The doses of 40 mg/mL and 80 mg/mL generally showed the highest values than Control.
Figure 2. Mean ± SEM of Curvilinear Velocity (VCL) (A) and Average Path Velocity (VAP) (B), after 0, 30, 60, 120 min of incubation of epididymal rabbit sperm diluted in control and NaCl Pollen Extender (NPE) extender (control + 20, 40, 80 mg/mL of Date palm pollen (DPP) extract). Different letters indicate significant differences (p ≤ .05).
Trajectoiry traits of sperm (LIN, ALH, BCF)
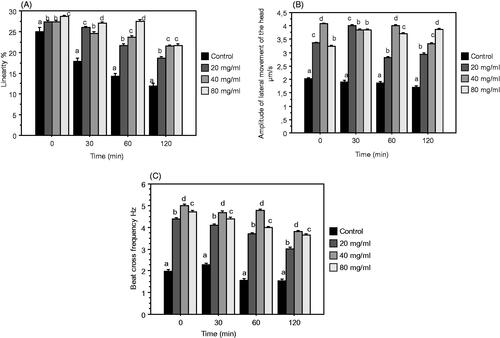
Figure shows that the addition of DPP significantly enhanced the trajectories parameters (LIN, ALH, BCF) compared to the control. Also for all these traits, the highest values were obtained with amount of DPP 40 and 80 mg/mL.
Figure 3. Mean ± SEM of Linearity (LIN) (A), Amplitude of Lateral Head displacement (ALH) (B), and Beat Cross Frequency (BCF) (C) after 0, 30, 60, 120 min of incubation. Epididymal rabbit semen was diluted with control or NPE extender (control +20, 40, 80 mg/mL DPP extract). Different letters indicate significant differences (p ≤ .05).
Ejaculated sperm
Sperm motility
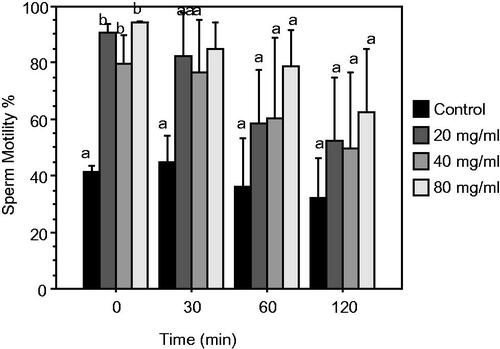
The effect of different dose of DPP aqueous extract (20, 40, 80 mg/mL; TPE extender) on the motility of ejaculated sperm at different times of incubation is represented in Figure . Differences in motility rate of samples treated with the aqueous extract of DPP compared to the control were recorded up to 60 min of incubation. After 120 min of in vitro incubation, no significant effect was noted between groups due to the higher variability.
Speed traits of sperm (VCL and VAP)
DPP treatment significantly improved spermatozoa velocity (VCL and VAP) compared to the control and this at all times points (Figure ), 20 mg/mL of DPP was enough for enhancing both VCL and VAP.
Figure 5. Mean (±SEM) of Curvilinear Velocity (VCL) (A), Average Path Amplitude (VAP) (B), after 0, 30, 60, 120 min of incubation of ejaculated sperm rabbit diluted in control and TPE extender (control + 20, 40, 80 mg/mL DPP extract). Different letters indicate significant differences (p ≤ .05).
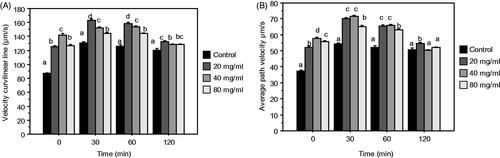
At the end of incubation (120 min), the differences respect to control tended to be lower than before (≤60 min), for every DPP doses.
Trajectoiry traits of sperm (LIN, ALH, BCF)
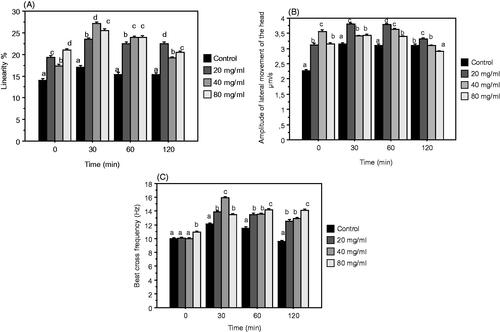
The incubation of spermatozoa with DPP extract (Figure ) generally caused significant increase on trajectories traits compared to the control with all doses at any time points of storage. The only exception is ALH wich showed values similar to control at the end of incubation period; a part samples supplemented with 80 mg/mL.
Figure 6. Mean (±SEM) of linearity (LIN) (A), Amplitude of lateral movement of the head (ALH) (B) and Beat cross frequency (BCF) (C) after 0, 30, 60, 120 min of incubation of ejaculated sperm rabbit diluted in control and TPE extender (control + 20, 40, 80 mg/mL DPP extract). Different letters indicate significant differences (p ≤ .05).
Discussion
It is widely known that immediately after sperm collection, the production of free radicals decreased sperm viability, motility and fertilisation potential (Agarwal et al. Citation2006). Moreover, during in vitro sperm storage the antioxidants present in the seminal plasma are no longer available. Therefore, spermatozoa are exposed to lipid peroxidation and become vulnerable to oxidative damage (Kim and Parthasarathy Citation1998).
During sperm storage, the membrane lipid peroxidation caused by an excess level of ROS, negatively affect negatively sperm lipids stability, proteins, nucleic acids and also sugars (Bansal and Bilaspuri Citation2011; Kim et al. Citation2011). Rabbit sperm is particularly rich in long chain PUFA (Castellini et al. Citation2019; Rodríguez et al. Citation2019) and consequently shows a higher susceptibility to the peroxidation.
For this reason, artificial insemination in rabbit, generally takes place within 24–48 hours after sperm collection (Carluccio et al. Citation2010) and the cooled storage of rabbit spermatozoa is the most diffused and recommended technique for rabbit semen.
Therefore, extending the storage time of sperm without reducing fertility parameters is an important goal for the AI achievement.
In the last fifteen years, to improve spermatozoa properties, some protective additives were tested and used, such as vitamin C, vitamin E, zinc, taurine, hypotaurine, and glutathione addition (Agarwal et al. Citation2005; Bansal and Bilaspuri Citation2009; Carluccio et al. Citation2010).
To the best of our knowledge, there is no study available on adding in vitro DPP extract to rabbit semen. Therefore, this study was performed to assess the effects of various concentrations of DPP extract on epididymal and ejaculated rabbit spermatozoa during in vitro incubation (up to 120 min) at 37 °C.
Sperm motility traits, evaluated by CASA (Massányi et al. Citation2008), was used as objective indicators of sperm quality as they are important parameters in evaluating the fertilising ability of sperm (Vijayaraghavan Citation2003). Several quantitative parameters, including VCL, VAP, VSL, STR, LIN, ALH, WOB and BCF, have been considered as potential indicators of sperm quality and vigour (Schettgen et al. Citation2002; Duty et al. Citation2004).
Our study showed a dose-dependent effect on sperm traits with addition of 20, 40, 80 mg/mL of DPP aqueous extract to epididymal and ejaculated sperm through 120 min of incubation.
In epididymal sperm, at T0, all the tested doses showed a higher motility rate compared to the control, and the dose of TPE 80 mg/mL was the most effective for ejaculated semen. The reasons of the different behaviours of epididymal and ejaculated sperm were probably to ascribe to the extender used. The TPE contained a higher quantity of energising molecules (sugar) and damage protectors (antibiotic and stabilizers) which enhanced and protected the spermatozoa. In agreement, the positive effects on motility, due to the DPP addition, was visible only at a higher concentration (80 mg/mL). On the contrary the NPE, being devoided of anyother beneficial molecule, showed a motility improvement already at low NPP concentrations (from 20 mg/mL), as it was the only energy substrate. The positive effect of TPE on motility rate in ejaculated sperm was recorded up to 60 min of incubation with all doses.
These findings agree with previous studies that investigated the effects of DPP aqueous extract addition to sperm extender on sperm during cryopreservation. El-Sheshtawy et al. (Citation2014) observed that sperm motility in bull is improved by the aqueous extract of DPP added to the Tris-citrate-fructose extender (with or without egg yolk), proving its good preserving capacity at 30 mg/mL of chilled sperm and at and at 10, 50 mg/mL for frozen-thawed sperm. Similar results were reported by El-Sheshtawy et al. (Citation2016) in improving sperm motility of chilled and thawed buffalo bull sperms by adding aqueous extract of DPP to Tris-citrate extender (10–50 mg/mL for chilled sperm and 30–50 mg/mL for thawed sperm). Furthermore, DPP had a beneficial effect on the chilling and freezing process of Arabian stallion sperm after the inclusion of 20 mg/mL of pollen grain extract in modified INRA-82 extender (El-Sisy et al. Citation2018). Recently, Mohamed and Talal (Citation2020) also found that adding DPP extract at 0.4 mg/mL extender had a crucial role in improving sperm motility at thawing as well as 1, 2- and 3-months post-cooling periods in Holstein bulls.
In human sperm, Al-Dujaily et al. (Citation2012) observed that in vitro sperm motility was improved when 20% of DPP extract is added to the extender.
In our study,the increase in sperm motility was also associated with a significant improvement in velocity traits (VCL, VAP) in both epididymal and ejaculated spermatozoa with all tested concentrations of DPP extract.
Numerous studies suggested a correlation of kinematic parameters with the fertilising ability of spermatozoa. According to Verstegen et al. (Citation2002) sperm samples, which produce an in vitro fertilisation index above 50%, had significantly higher VCL, VAP and VSL values. Nagy et al. (Citation2015) found that VAP is the most useful sperm motility characteristic, which has clinical relevance in the prediction of fertility. However, Larsen et al. (Citation2000) observed that VCL was the most significant parameter correlated with the fertilisation rate in human sperm. In mice, Olds-Clarke (Citation1996) demonstrated the importance of VCL for the formation of reservoir at the utero-tubal junction.
Significant improvements were also obtained in our study for the others sperm trajectory parameters (LIN, BCF and ALH) both in ejaculated and epididymal sperm, with all DPP concentrations compared to the control sample.
The higher dose of NPE 80 mg/mL was the most effective for epididymal sperm, nevertheless, in TPE extender, 20 mg/mL of DPP was enough to improve kinematic parameters in ejaculated semen.
According to some authors, BCF and LIN are able to add useful information on the fertilising potential of sperm because they have a positive correlation with gestation rate (Farrell et al. Citation1998; Verstegen et al. Citation2002; Ahmed et al. Citation2017).
DPP beneficial effect on spermatozoa could be attributed to its antioxidant properties (El-Sisy et al. Citation2018) and its powerful free radical scavenging capacity (El-Kashlan et al. Citation2015). Indeed, DPP seems able to protect sperm cells against oxidative damage (Agarwal and Rao Citation2000) by scavenging ROS through high content of polyphenols such as flavonoids (Wettasinghe and Shahidi Citation2000; Le Blanc et al. Citation2009), vitamins C, B1, B2, E, nicotinic acid (Niacin) and vitamin A (Hassan Citation2011), carotenoids and phytosterols (Broadhurts Citation1999). Moreover, they are good source of protein, amino acids, dietary fibre, fatty acids that assumes a key role in the sperm membrane fluidity and susceptibility to lipid peroxidation, enzymes, hormones (estrone, oestradiol and estriol) and minerals (Stubbs and Smith Citation1984; Kroyer and Hegedus Citation2001).
DPP also contains alkaloids, carbohydrates, glycosides, tannins, terpenoids, saponins, coumarins, lignin (Al-Samarai et al. Citation2016). All these compounds would help to furnish energy for the sperm metabolism and protect sperm damage by strengthening plasma membrane stability and compensating the reduction in the endogenous antioxidants of seminal plasma due to dilution as well as to counteract excess production of ROS.
Otherwise, some authors reported that DPP has a potent antimicrobial, antifungal, anti-toxicant and anti-inflammatory activities (Aba Al-Khail et al. Citation2003; Shakiba et al. Citation2011; Lee et al. Citation2015; Daoud et al. Citation2019). All these effects could improve sperm quality through reduction of bacterial growth. In the current study, the NPE extender for epididymal sperm was prepared without antibiotics; therefore, the positive effects of DPP on sperm quality during the incubation may also be related to its anti-microbial activity. All these mechanisms should be experimentally verified, but could explain the higher motility and kinetic parameters observed in both epididymal and ejaculated sperm with DPP extract.
Additionally, different in vivo trials on human and animals reported the beneficial effects of DPP on spermatogenesis and sperm parameters, including motility, sperm count, morphology, acrosome reaction, lipid peroxidation and DNA integrity with increasing testicular and epididymal weights and sexual hormones (luteinizing hormone, follicle stimulating hormone, testosterone; El-Sheshtawy et al. Citation2014; Iftikhar et al. Citation2011; Abdi et al. Citation2017; Fallahi et al. Citation2015). Selmani et al. (Citation2017) showed in rats that oral administration (120 mg/kg b.w.) of Algerian DPP after 30 days, improved some reproductive endpoints (testicle weight and serum testosterone level). Also, Al-Samarrai et al. (Citation2017) found in rabbit a higher fertility, which may be explained by the enhanced production of testosterone and spermatogenesis by stimulating the growth of primary and secondary spermatocytes, when DPP was orally administered to mature bucks.
Conclusion
Based on these preliminary results, we may conclude that higher dose of DPP 80 mg/mL in NPE presented a beneficial effect helping to maintain a good kinetic traits of epididymal sperm. Nevertheless, in TPE extender, 20 mg/mL of DPP was enough to improve sperm motility traits in ejaculated rabbit semen.
These results revealed that DPP aqueous extract is a suitable supplement for sperm dilution; it might open new promising opportunities for the development of a new rabbit spermatozoa media efficient and safe for in vitro fertilisation and artificial insemination that can improve sperm quality and provide a better protection against oxidative damages.
As such, further studies are needed to confirm this result and evaluate the conservation of chilled rabbit semen with the supplementation of DPP aqueous extract to extender and determine the optimal concentration and the possible effect on in vivo fertility.
Ethical approval
All experimental procedures were carried out in compliance with the requirements of ethical committee of SAAD DAHLAB University of Blida (U.Blida1), Algeria
Acknowledgments
The authors express their thanks to the laboratory team of Bejaia and Perugia Universities for their availability and the support granted, we wish to thank Dr Paolo Lattaioli for his helpful assistance throughout our internship and Mr Azzeddine BENGHANIMA for his valuable aid for pollen collection in oasis.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Abdi F, Roozbeh N, Mortazavian AM. 2017. Effects of date palm pollen on fertility: research proposal for a systematic review. BMC Res Notes. 10(1):363.
- Agarwal A, Gupta S, Sharma RK. 2005. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 3:28.
- Agarwal S, Rao AV. 2000. Tomato lycopene and its role in human health and chronic diseases. CMAJ. 163(6):739–744.
- Agarwal A, Sharma R, Nallella K, Thomasjr A, Alvarez J, Sikka S. 2006. Reactive oxygen species as an independent marker of male factor infertility. Fertil Steril. 86(4):878–885.
- Agarwal A, Tvrda E, Sharma R. 2014. Relationship amongst teratozoospermia, seminal oxidative stress and male infertility. Reprod Biol Endocrinol. 12(1):45.
- Ahmed H, Andrabi SMH, Anwar M, Jahan S. 2017. Use of post-thaw semen quality parameters to predict fertility of water buffalo (Bubalusbubalis) bull during peak breeding season. Andrologia. 49(4):e12639:1-6.
- Al-Daraji HJ. 2004. Diluent supplementation with vitamins A, C and E for improving fertilizing ability of indigenous roosters’ semen. Patent No. 3195.Issued from C.O.S.Q.C Iraq.
- Al-Dujaily SS, Al-Shahery NJ, Zabbon AA. 2012. Effect of Phoenix dactylifera pollen on in vitro sperm activation of infertile men. Al-Mustansiriyah J Sci. 23:27–34.
- Al-Samarai AH, Al-Salihi FG, Al-Samarai RR. 2016. Phytochemical constituents and nutrient evaluation of date palm (Phoenix dactylifera, L.) pollen grains. Tikrit J Pure Sci. 21(1):56–62.
- Al-Samarrai RRH, Al-Samarrai ASM, Al-Samarrai AMH. 2017. Effect evaluation of Iraqi Date Palm pollen on sex hormones level of male local rabbits. Chem Adv Mater. 2(4):53–59.
- Alvarez JG, Storey BT. 1995. Differential incorporation of fatty acids into and peroxidative loss of fatty acids from phospholipids of human spermatozoa. Mol Reprod Dev. 42(3):334–346.
- Alvariño JMR. 2000. Reproductive performance of male rabbits. Proceedings 7th World Rabbit Congress, Valencia, Spain. p. 13–35.
- Bahmanpour S, Talaei T, Vojdani Z, Panjehshahin MR, Poostpasand A, Zareei S, Ghaeminia M. 2006. Effects of Phoenix dactylifera pollen on sperm parameters and reproductive system of adult male rats. Iran J Med Sci. 31(6):208–212.
- Baliga MS, Baliga BRV, Kandathil SM, Bhat HP, Vayalil PK. 2011. A review of the chemistry and pharmacology of the date fruits (Phoenix dactylifera L.). Food Res Int. 44(7):1812–1822.
- Bansal AK, Bilaspuri GS. 2009. Antioxidant effect of vitamin E on motility, viability, and lipid peroxidation of cattle spermatozoa under oxidative stress. Anim Sci Rep. 1:5–14.
- Bansal AK, Bilaspuri GS. 2011. Impacts of oxidative stress and antioxidants on semen functions. Vet Med. 2011(7):1–7.
- Boiti C, Castellini C, Besenfelder U, Thau-Clément M, Liguori L, Renieri T, Pizzi F. 2005. Guidelines for the handling of rabbit bucks and semen. World Rabbit Sci. 13:71–91.
- Broadhurts CL. 1999. Bee products: medicine from the hive. Nutr Sci News. 4:366–368.
- Carluccio A, Robbe D, De Amicis I, Contri A, Tosi U, Russo F, Paoletti M. 2010. Artificial insemination in rabbits: laboratory and field trial with three different semen extenders. World Rabbit Sci. 12(2):65–79.
- Castellini C, Dal Bosco A, Ruggeri S, Collodel G. 2011. What is the best frame rate for evaluation of sperm motility in different species by computer-assisted sperm analysis? Fertil Steril. 96(1):24–27.
- Castellini C, Dal Bosco A, Cardinali R. 2006. Long term effect of post-weaning rhythm on the body fat and performance of rabbit doe. Reprod Nutr Dev. 46(2):195–204.
- Castellini C, Mattioli S, Signorini C, Signorini C, Cotozzolo E, Noto D, Moretti E, Brecchia G, Dal Bosco A, Belmonte G, et al. 2019. Effect of dietaryn-3 source on rabbit male reproduction. Oxid Med Cell Longev. 2019:3279670.
- Dal Bosco A, Rebollar PG, Boiti C, Zerani M, Castellini C. 2011. Ovulation induction in rabbit does: current knowledge and perspectives. Anim Reprod Sci. 129(3–4):106–117.
- Dalle Zotte A, Szendrő Z. 2011. The role of rabbit meat as functional food. Meat Sci. 88(3):319–331.
- Daoud A, Malika D, Bakari S, Hfaiedh N, Mnafgui K, Kadri A, Gharsallah N. 2019. Assessment of polyphenol composition, antioxidant and antimicrobial properties of various extracts of Date Palm Pollen (DPP) from two Tunisian cultivars. Arab J Chem. 12(8):3075–3086.
- De Lamirande E, Gagnon C. 1992. Reactive oxygen species and human spermatozoa I: effect on the motility of intact spermatozoa and on sperm axonemes. J Androl. 13:368–378.
- Di Iorio M, Manchisi A, Rocco M, Chrenek P, Laffaldano N. 2014. Comparison of different extenders on the preservability of rabbit semen stored at 5 °C for 72 hours. Ital J Anim Sci. 13:710–714.
- Duty SM, Calafat AM, Silva MJ, Brock JW, Ryan L, Chen Z, Overstreet J, Hauser R. 2004. The relationship between environmental exposure to phthalates and computer-aided sperm analysis motion parameters. J Androl. 25(2):293–302.
- El-Kashlan AM, Nooh MM, Hassan WA, Rizk SM. 2015. Therapeutic potential of date palm pollen for testicular dysfunction induced by thyroid disorders in male rats. PLoS One. 10(10):e0139493.
- El-Sheshtawy RI, El-Nattat WS, Ali AH, Sabra HA. 2014. The effect of Phoenix dactylifera pollen grains tris-infusion on semen preservability of local bull breeds. Glob Vet. 13:728–732.
- El-Sheshtawy RI, El-Nattat WS, Shalaby SI, Shahba MI, Al-Se’dawy IE. 2016. Chilled and post-thawed semen characteristics of buffalo semen diluted in tris extender enriched with date palm pollen grains (TPG). Asian Pac J Reprod. 5(3):252–255.
- El-Sisy GA, El-Badry DA, El-Sheshtawy RI, El-Nattat WS. 2018. Effects of Phoenix dactylifera pollen grains extract supplementation on post-thaw quality of Arabian stallion semen. Bulg J Vet Med. 21(1):40–49.
- Fallahi S, Rajaei M, Malekzadeh K, Kalantar SM. 2015. Would Phoenix dactyflera pollen (palm seed) be considered as a treatment agent against Males’ infertility? A systematic review. Electron Physician. 7(8):1590–1596.
- Farrell PB, Presicce GA, Brockett CC, Foote RH. 1998. Quantification of bull sperm characteristics measured by computer-assisted sperm analysis (CASA) and the relationship to fertility. Theriogenol. 49(4):871–879.
- Fatma BA, Nozha CF, Ines D, Hamadi A, Basma H, Leila AK. 2009. Sperm quality improvement after date seed oil in vitro supplementation in spontaneous and induced oxidative stress. Asian J Androl. 11(3):393–398.
- Hassan HMM. 2011. Chemical composition and nutritional value of palm pollen grains. Global J Biotech Biochem. 6:1–7.
- Hernández P, Dalle Zotte A. 2010. Influence of diet on rabbit meat quality. In: The nutrition of the rabbit. de Blas and Wiseman editors. Oxon (UK): CABI Publishing; p. 163–178.
- Iftikhar S, Bashir A, Anwar MS, Mastoi SM, Shahzad M. 2011. Effect of date palm pollen (dpp) on serum testosterone levels in prepubertal albino rats. Pak J Med Health Sci. 6:639–644.
- Kim S, Lee YJ, Kim YJ. 2011. Changes in sperm membrane and ROS following cryopreservation of liquid boar semen stored at 15 °C. Anim Reprod Sci. 124(1–2):118–124.
- Kim JG, Parthasarathy S. 1998. Oxidation and the spermatozoa. Semin Reprod Endocrinol. 16(4):235–239.
- Kroyer G, Hegedus N. 2001. Evaluation of bioactive properties of pollen extracts as functional dietary food supplement. Innov Food Sci Emerg. 2(3):171–174.
- Larsen L, Scheike T, Jensen TK, Bonde JP, Ernst E, Hjollund NH. 2000. Computer-assisted semen analysis parameters as predictors for fertility of men from the general population. The Danish First Pregnancy Planner Study Team. Hum Reprod. 15(7):1562–1567.
- Le Blanc BW, Davis OK, Boue S, DeLucca A, Deeby T. 2009. Antioxidant activity of Sonoran Desert bee pollen. Food Chem. 115(4):1299–1305.
- Lee HJ, Park JS, Yoon YP, Shin YJ, Lee SK, Kim YS, Hong JH, Son KH, Lee CJ. 2015. Dioscin and methylprotodioscin isolated from the root of Aspara-guscochinchinensis suppressed the gene expression and production of airway MUC5AC mucin induced by phorbol ester and growth factor. Phytomedicine. 22(5):568–572.
- Martinez-Pastor F, Garcia-Macias V, Alvarez M, Chamorro C, Herraez P, Paz P. d, Anel L. 2006. Comparison of two methods for obtaining spermatozoa from the cauda epididymis of Iberian red deer. Theriogenology. 65(3):471–485.
- Massányi P, Chrenek P, LukáCˇ N, Makarevich AV, Ostro A, Zˇivcˇak J, Bulla J. 2008. Comparison of different evaluation chambers for analysis of rabbit spermatozoa motility parameters using CASA system. Slovak J Anim Sci. 2:60–66.
- Mohamed OA, Talal AA. 2020. Some post-cryopreserved semen characteristics of holstein bulls as influenced by adding aquoeus extract of urticadioica and date palm pollen powder to tris extender. Plant Arch. 1:461–467.
- Nagy Á, Polichronopoulos T, Gáspárdy A, Solti L, Cseh S. 2015. Correlation between bull fertility and sperm cell velocity parameters generated by computer-assisted semen analysis. Acta Vet Hung. 63(3):370–381.
- Ndors L, Ajuogu PK, Nyeche VN. 2015. The assessment of artificial breeding pattern on fertility in rabbit does in the humid tropics. IOSR-JAVS. 8:81–85.
- Olds-Clarke P. 1996. How does poor motility alter sperm fertilizing ability. J Androl. 17(3):183–186.
- Petracci M, Bianchi M, Cavani C. 2009. Development of rabbit meat products fortified with n-3 polyunsaturated fatty acids. Nutrients. 1(2):111–118.
- Piomboni P, Gambera L, Serafini F, Campanella G, Morgante G, De Leo V. 2008. Sperm quality improvement after natural anti-oxidant treatment of asthenoteratospermic men with leukocytospermia. Asian J Androl. 10(2):201–206.
- Rodríguez MG, Rebollar P, Mattioli S, Castellini C. 2019. n-3 PUFA sources (precursor/products): a review of current knowledge on rabbit. Animals. 9(10):806.
- Rosato M, Iaffaldano N. 2011. Effect of chilling temperature on the long-term survival of rabbit spermatozoa held either in a tris-based or a jellified extender. Reprod Domest Anim. 46(2):301–308.
- Ross C, Morriss A, Khairy M, Khalaf Y, Braude P, Coomarasamy A, El-Toukhy T. 2010. A systematic review of the effect of oral antioxidants on male infertility. Reprod Biomed Online. 20:11–723.
- Sansone G, Nastri MJF, Fabbrocini A. 2000. Storage of buffalo (Bubalusbubalis) semen. Anim Reprod Sci. 62(1–3):55–76.
- Schettgen T, Koch HM, Drexler H, Angerer J. 2002. New gas chromatographic-mass spectrometric method for the determination of urinary pyrethroid metabolites in environmental medicine. J Chromatogr. B. 778(1–2):121–130.
- Selmani C, Chabane D, Bouguedoura N. 2017. Ethnobotanical survey of Phoenix dactylifera L. Pollen used for the treatment of infertility problems in Algerian oases. Afr J Tradit Complement Altern Med. 14(3):175–186.
- Shakiba M, Kariminik A, Parsia P. 2011. Antimicrobial activity of different parts of Phoenix dactylifera. Int J Mol Clin Microbiol. 1:107–111.
- Silva NCC, Fernandes Jr A. 2010. Biological properties of medicinal plants: a review of their antimicrobial activity. J Venom Anim Toxins Incl Trop Dis. 16(3):402–413.
- Stubbs CD, Smith AD. 1984. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta. 779(1):89–137.
- Taepongsorat L, Tangpraprutgul P, Kitana N, Malaivijitnond S. 2008. Stimulating effects of quercetin on sperm quality and reproductive organs in adult male rats. Asian J Androl. 10(2):249–258.
- Tvrdá E, Kňažická Z, Bárdos L, Massányi P, Lukáč N. 2011. Impact of oxidative stress on male fertility – a review. Acta Vet Hung. 59(4):465–484.
- Verstegen J, Iguer-Ouada M, Onclin K. 2002. Computer assisted semen analyzers in andrology research and veterinary practice. Theriogenology. 57(1):149–179.
- Vijayaraghavan S. 2003. Sperm motility: patterns and regulation. In: Introduction to Mammalian reproduction. Tulsiani D. editors. Boston (USA): Kluwer Academic Publishers; p. 79–91.
- Wettasinghe M, Shahidi F. 2000. Scavenging of reactive- oxygen species and DPPH free radicals by extracts of borage and evening primrose meals. Food Chem. 70(1):17–26.