Abstract
There are few data about early thermal conditioning (TC) and its effect on later life in rabbits. Therefore, the present study aimed to investigate the impact of exposing two rabbit breeds to early age short-term heat stress (EA-STHS) on physiological parameters and hepatic expression levels of some genes. Rabbits from New Zealand White (NZW) and Baladi Black (BB) rabbit breeds were reared in a controlled environmental chamber (28 ± 2 °C and 40 ± 2% RH). At 42 days of age, rabbits from both breeds were equally divided into two groups; control (untreated) and heat exposed groups (kept at 36 ± 1 °C and 62% RH for 6 h). At 13 weeks of age, rabbits of all groups were re-exposed to acute heat stress (36 ± 1 °C and 62% RH for 6 h). At 13 weeks of age, plasma CORT, plasma MDA, and hepatic MDA had significantly lower levels in the rabbits from the two breeds subjected to EA-STHS than untreated control groups. plasma TAC and the relative hepatic expression of all the studied genes like Hsp70, HspA9, Hsp90α and UCP2 had significantly higher levels in EA-STHS treated NZW and BB rabbits compared with the control groups. In conclusion, the results suggest that EA-STHS has a long-lasting effect on the later life response to acute heat stress in NZW and BB breeds, as indicated by the hepatic expression profile of several genes, corticosterone, and antioxidant parameters. Additionally, each breed had characteristic patterns for the tested parameters at each time point and treatment.
Rabbits are susceptible to thermal stress, which is associated with adverse effects on production and reproduction traits.
Early-age thermal conditioning is one of the suggested protective treatments to reduce the heat stress adverse effects.
Early age short-term thermal stress may induce thermotolerance ability to later time heat exposure in both rabbit breeds.
HIGHLIGHTS
Introduction
Rabbits are susceptible to thermal stress, which is associated with adverse effects on the thermoregulatory system, antioxidant defense system, physiological parameters, production, and reproduction traits and could lead to death in extreme conditions resulting in economic losses (Marai et al. Citation2002; Pei et al. Citation2012; Ei-Badry et al. Citation2015; Ezzat et al. Citation2019; Sakr et al. Citation2019). With global warming, heat stress in the summer (especially in the hot region of the world) represents one of the biggest challenges for the rabbit industry (Marai et al. Citation2002). Early-age thermal conditioning is one of the suggested protective treatments to reduce the heat stress adverse effects. The animals are exposed to thermal stress for a short period during the early stage of their life. Several studies confirmed that the rabbits exposed to heat stress for a short period during earlier age exhibit improved physiological parameters, productivity performance, and heat tolerance during the exposure to later heat stress conditions (Ei-Badry et al. Citation2015; Ezzat et al. Citation2019; Sakr et al. Citation2019; Madkour et al. Citation2020; Madkour et al. Citation2021).
Several cellular and molecular mechanisms aim to protect the eukaryotes from the harmful physiological and cellular effects resulting from exposure to heat and/or oxidative stress. For example, thermal stress has detrimental effects since it leads to protein un- and mis-folding. To deal with thermal stress, eukaryotic cells activate conserved cell protective mechanisms called heat shock response (HSR), to maintain protein homeostasis. Heat shock factor 1 (HSF1) has an essential role in HSR since it acts as a transcription factor that upregulates the gene expression of the heat shock proteins (HSPs) (Barna et al. Citation2018). HSPs act as molecular chaperones that contribute to refolding or degrading the misfolded proteins and facilitating the newly synthesised protein's accurate folding. Moreover, HSPs suppress several apoptotic signals and support the intracellular pathways critical for cell survival and function (Dubrez et al. Citation2020).
Stress stimulates the hypothalamus–pituitary–adrenal axis, which increases corticosteroids secretion from the adrenal cortex. Depending on this, corticosterone is used as stress biomarkers (Gong et al. Citation2015; de Bruijn and Romero Citation2018). Glucocorticoid receptor overexpression enhances mice's ability to tolerate injury and hyperthermia-induced by heat stress (Chen and Yu Citation2018).
Insulin-like growth factors-1 (IGF-1) is a polypeptide acting downstream of growth hormone (GH), known as somatotropic axis, to induce anabolism and tissue growth. IGF-1 possesses several bioactivities, such as induction of maturation or differentiation of target cells, cell survival, and cell functions (Wrigley et al. Citation2017). Uncoupling proteins 2 (UCP2) is an ion/anion transporter protein in the inner membrane of mitochondria, and it is expressed in numerous animal tissues. UCP2 has a vital role in several biological processes such as metabolism, apoptosis, and cell division (Li et al. Citation2019). UCP2 also has an antioxidant role since it inhibits the reactive oxygen species (ROS) generation in mitochondria and protects the cells from oxidative damage due to excessive superoxide production (Cadenas Citation2018).
Although several studies suggested early life thermal conditioning (TC) as a technique to promote poultry resistance to heat stress, little are known about the molecular and physiological changes associated with early age short-term thermal stress in rabbits. Therefore, the current study aimed to characterise the effect of exposure to early age short-term thermal stress on both hepatic expression of several genes and some blood parameters following future exposure to acute heat stress at marketing age. This investigation was performed on New Zealand white (NZW) and the local strain Baladi Black (BB) rabbits to compare each bread's response to the studied parameters. At 42 days of age, we aimed to study the effect of exposure to early age short-term thermal stress on the target parameters in 2 breads at this time point. At 13 weeks of age, we aimed to explore the effect of exposure to early age short-term thermal stress (at 42 days of age) on both of hepatic expression of several genes and some blood parameters following future exposure to acute heat stress at marketing age (13 weeks of age).
Materials and methods
Experimental design
New Zealand white (NZW) and the local strain Baladi Black (BB) rabbits were used in this investigation. A total number of 120 male 35-days old rabbits (60 from each breed) were obtained from a commercial farm. Rabbits were housed in galvanised wire cages (40 cm height × 30 cm width × 50 cm length) and reared under normal conditions (28 ± 2 °C and 40 ± 2% RH) for seven days until the 42 of age to adapt to the local condition and reduce transportation stress. At 42 days of age, both breeds' rabbits were equally divided into two groups; control groups (NZWC and BBC) and heat exposed groups, which kept at 36 ± 1 °C and 62% RH for 6 h (NZWT and BBT). Six rabbits from each group were randomly taken and slaughtered then blood samples were collected in heparinised sterile tubes. Liver samples were removed immediately, were dissected, snap-frozen in liquid nitrogen, and stored at −80 °C until further processing. We examined the target parameters in a control untreated group from each breed which are New Zealand white control (NZWC) and Baladi Black Control (BBC) compared to their treated groups which are New Zealand white treated (NZWT) and Baladi Black treated (BBT), respectively.
The remaining rabbits from all groups were kept up to 13 weeks in an automatically controlled environmental chamber at 28 ± 1 °C and 40% RH. At 13 weeks of age all the experimental rabbit groups NZWC, BBC, NZWT and BBT were subjected to acute heat stress (36 °C 62% RH for 6 h) and renamed as NZWC2, BBC2, NZWT2 and BBT2, respectively. Six rabbits were randomly taken, slaughtered, liver samples were taken as previously mentioned at seven weeks of age for other traits. Depending on this, we examined the target parameters following exposure to acute heat stress at 13 weeks of age in the control groups NZWC2 and BBC2 (did not exposed to early age short-term thermal stress at 42 days of age) compared to thier treated groups NZWT2 and BBT2, respectively (exposed to early age short-term thermal stress at 42 days of age). The rabbits were offered feed with a commercial diet for ad libitum consumption.
Measurement of corticosterone, total antioxidant capacity and malondialdehyde levels in plasma
The collected blood samples were centrifuged at 1700 ×g for 15 min at 4°C for plasma collection. Plasma samples were preserved at −80 °C for further analysis. Plasma CORT was determined using ELISA commercial kits (IBL International GmbH, CatLog NO. RE52211), while the total antioxidant capacity (TAC) and malondialdehyde (MDA) were measured using specific colorimetric based kits (Bio-diagnostics, Giza, Egypt).
Measurement of malondialdehyde level in liver
Liver samples were dissected out and washed immediately with 0.9% NaCl solution, and then were homogenised on ice using phosphate-buffered saline (PBS, pH 7.4). The homogenates were centrifuged at 10,000 × g for 15 min at 4 °C, and the resulting supernatants were stored at −80 °C. MDA was measured in the supernatants using the specific colorimetric based kit (Bio-diagnostics, Giza, Egypt).
RNA isolation and real-time quantitative RT-PCR
Total RNA was extracted as previously (Kang et al. Citation2017) from quick-frozen liver samples using TRIzol® reagent (Invitrogen Life Technologies, Palo Alto, CA, USA) according to the manufacturer instructions and was treated by DNase I, RNase-free (Thermo Fisher Scientific, Cat. #EN0521) and the total RNA was purified by RNeasy mini kit (Qiagen, Valencia, CA, USA). The RNA quality and quantity were determined using agarose gel electrophoresis and NanoDrop 1000 (Thermo Scientific, Wilmington, DE, USA). According to the manufacturer instructions, four micrograms of total RNA from were converted into cDNA using Revert Aid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Cat. # K1621) as described previously (Mahrous, Aboelenin, Abd El-Kader, et al. Citation2020; Mahrous, Mabrouk, et al. Citation2020; Sroor et al. Citation2020; Mahrous et al. Citation2021). The cDNA reactions were diluted 10-fold using DNase/RNase-Free water.
A specific primer pair for the mRNA of the selected genes was designed (Table ) using Primer-BLAST software (www.ncbi.nlm.nih.gov/tools/primer-blast). The annealing temperature of each pair was optimised using a conventional PCR to exclude the presence of unspecific products or primer dimer and the PCR products were analysed by 2% agarose gel electrophoresis as described previously (Mahrous, Aboelenin, Rashed, et al. Citation2020). Each qPCR reaction had a final volume of 15 µL of reaction mixture which consisted of 7.5 µL 2X SYBR Green PCR master mix kit (Thermo Fisher Scientific, Cat. #4344463), DNase/RNase-Free water, 0.3 μM forward and reverse specific primers for each gene (Table ) and 2 µL of cDNA template. The qPCR analyses were performed in Rotor-Gene Q (Qiagen, Hilden, Germany) system. Thermal cycling profiles were initiated by one denaturation step of 10 min at 95 °C, followed by 40 cycles with the following parameters: denaturation at 95 °C for 30 s, annealing at the specific annealing temperature of each primer pair (Table ) for 15 s and extension at 72 °C for 30 s. Fluorescence data were acquired at the end of the extension step. A dissociation protocol with a gradient from 65 °C to 95 °C (0.5 °C every 30 s) was used to investigate the qPCR reaction's specificity and primer dimers' presence. Rabbits glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and β-actin were used as internal controls. All qRT-PCR experiments were performed in triplicate. The average cycle threshold (Ct) was determined, and Delta-Ct scores for gene transcripts in each sample were normalised using Delta-Ct scores for GAPDH/β-actin and expressed as the fold change in gene expression using the equation, 2−ΔΔCT.
Table 1. A list of the primer’s sequences, annealing temperature, and amplicon size which used in the present study.
Statistical analyses
Statistical differences between each group were assessed by a two-way analysis of variance (ANOVA) using the SAS 8.0 software (SAS Institute Inc., Cary, NC, USA). Comparisons between the control group’s mean values and those of each experimental group were performed using the Duncan test for multiple comparisons. P values of less than .05 were regarded as statistically significant.
Results
Effects of early life thermal stress on plasma corticosterone, lipid peroxidase, total antioxidant capacity, and hepatic MDA
At 42 days of age
Several changes were observed in plasma and liver parameters due to short heat stress treatment at day 42. The NZWT and BBT groups compared to NZWC and BBC groups had a significant increase (p < .05) in plasma CORT, MDA, and hepatic MDA levels by about 130 and 169%, 144 and 93% and 171 and 163%, respectively (Figure ). Plasma TAC was significantly decreased (p < .05) in the NZWT and BBT groups compared to NZWC and BBC groups by about 63% in both breeds (Figure ).
Figure 1. Effect of early age short-term thermal stress (36 ± 1ºC for 6 h) at day 42 of age on plasma corticosterone CORT (A), plasma malondialdehyde (MDA) (B), plasma plasma total antioxidant capacity (TAC) (C) and hepatic malondialdehyde (MDA) (D) of NZW and BB rabbits. The four groups (n = 6 rabbit/group) include New Zealand white control (NZWC), New Zealand white treated (NZWT), Baladi Black Control (BBC), and Baladi Black treated (BBT). Different lower-case letters above the bars indicate significant differences (p < .05) among groups.
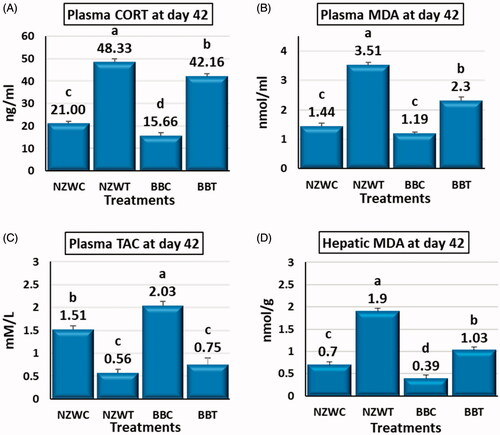
Numerous differences were detected between the NZW and BB breeds in the value of the plasma and hepatic parameters. The NZWC and NZWT groups compared to BBC and BBT groups had significantly higher (p < .05) plasma CORT and hepatic MDA levels by about (34, 15) and (79. 84) %, respectively (Figure ). Plasma MDA had a significantly higher level (p < .05) by about 53% in NZWT compared to BBT, although there was no significant difference between the control groups of both breeds (Figure ). Compared to BBC group, the TAC value significantly lower (p < .05) by about 25% in NZWC group but there was no significant difference between the heat-stressed groups of the two breeds (Figure ).
At 13 weeks of age
The EA-STHS at day 42 leads to significant changes in the estimated values for the target tests at week 13 of age. Plasma CORT, MDA and hepatic MDA significantly lower (p < .05) in the EA-STHS exposed groups NZWT2 and BBT2 compared to their control groups NZWC2 and BBC2 by 43, 34 and 52% and 34, 47 and 63%, respectively (Figure ). Both NZWT2 and BBT2 groups compared to NZWC2 and BBC2 control groups showed significantly higher (p < .05) total antioxidant capacity by about 141, 81%, respectively (Figure ).
Figure 2. Effect of early age short-term thermal stress at day 42 of age on plasma corticosterone (CORT) (A), plasma malondialdehyde (MDA) (B), plasma total antioxidant capacity (TAC) (C) and hepatic malondialdehyde (MDA) (D) of NZW and BB rabbits which re-exposed to acute heat stress (36±1° C for six hours) at 13 weeks of age. All the early (42 days of age) heat-stressed (NZWT and BBT) and unstressed (NZWC and BBC) groups were exposed to acute heat stress (36±1° C for six hours) at 13 weeks of age. Upon the acute heat stress at week 13 of age, NZWC, NZWT, BBC and BBT groups were renamed as New Zealand white control exposed (NZWC2), New Zealand white treated (NZWT2), Baladi Black Control exposed (BBC2) and Baladi Black treated (BBT2), respectively. Different lower-case letters above the bars indicate significant differences (p < 0.05) among groups.
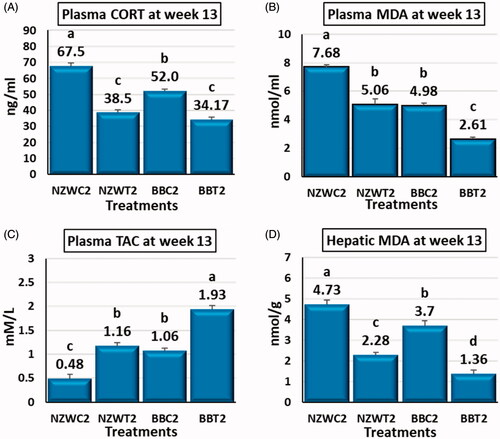
The results of the investigated parameters were varied between NZW and BB breeds at 13 weeks of age. Plasma corticosterone concentration was significantly higher (p < .05) in the NZWC by 30% compared to the BBC group. In contrast, the differences between both breeds EA-STHS exposed groups were not significant (Figure ). Both plasma and hepatic MDA were significantly higher (p < .05) in NZWC2 and NZWT2 compared to BBC2 and BBT2 by 54 and 28% and 94 and 67%, respectively (Figure ). In comparison to BBC2 and BBT2 groups. TAC showed that NZWC2 and NZWT2 had significantly lower (p < .05) total antioxidant capacity by 55 and 40%, respectively (Figure ).
Effects of early life thermal stress on hepatic mRNA expression of HSF1, GR, Hsp70, HspA9, IGF-1, Hsp90α and UCP2
At 42 days of age
The exposure to short-term heat stress at day 42 associated with an alteration in gene expression profile of a Set of diverse genes within the liver of New Zealand white and Baladi Black breeds. The short term heat stress treatment for NZW rabbits – in comparison with their control – downregulated (p < .05) the hepatic expression of HSF1, GR, Hsp70, HspA9, IGF-1, and UCP2 genes by 40, 47, 14, 25, 16, and 55%, respectively (Figure ). The heat stress did not significantly affect the expression of Hsp90α in NZW breed or both of HSF1 and GR genes in BB breed (Figure ). Comparable to BBC groups, the heat stress upregulated (p < .05) the expression Hsp70, HspA9, IGF-1, and Hsp90α genes by 23, 39, 26 and 65%, respectively, while the same treatment downregulated UCP2 expression by 19% (Figure ).
Figure 3. Effect of early age short-term thermal stress (36 ± 1ºC for 6 h) at day 42 of age on mRNA expression : heat shock transcription factor 1 (HSF1) (A), glucocorticoid receptor (GR) (B), heat shock 70kDa protein 2 (Hsp70) (C), heat shock protein family A (Hsp70) member 9 (HspA9) (D), Insulin-like growth factors-1 (IGF-1) (E), heat shock protein 90 alpha family class A member 1 (Hsp90α) (F) and Uncoupling proteins 2 (UCP2) (G) genes in the liver of NZW and BB rabbits. The four groups (n = 6 rabbit/group) include New Zealand white control (NZWC), New Zealand white treated (NZWT), Baladi Black Control (BBC), and Baladi Black treated (BBT). Different lower-case letters above the bars indicate significant differences (p < .05) among groups.
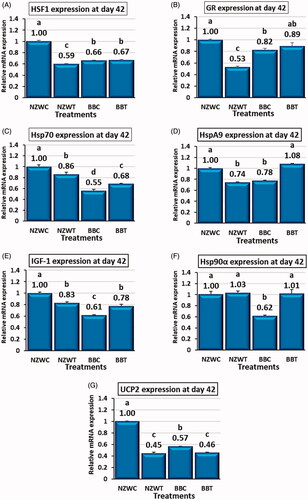
New Zealand white and Baladi Black breeds had unique hepatic expression profile for the control and heat-stressed groups at day 42 of age. HSF1, GR, Hsp70, HspA9, IGF-1, Hsp90α and UCP2 expression were higher (p < .05) in NZW control group in compare with BB control group by 51, 21, 80, 28, 62, 62 and 76%, respectively (Figure ). Heat stressed group NZWT had lower (p < .05) gene expression level for HSF1, GR, and HspA9 compared to BBT group by 10, 40, and 31%, respectively, while the expression of Hsp70 gene was higher in NZWT group compared to BBT group by 26% (Figure ). No significant differences were observed between the expression of IGF-1, Hsp90α, and UCP2 genes in heat-stressed groups from both breeds (Figure ).
At 13 weeks of age
The EA-STHS at 42 days of rabbit age modified the hepatic expression pattern of various genes in New Zealand white and Baladi Black breeds as a response to acute heat stress at week 13 of age. In comparison with the control groups NZWC2 and BBC2, both of NZWT2 and BBT2, which exposed to EA-STHS had significantly greater (p < .05) transcription level of HSF1, GR, Hsp70, HspA9, IGF-1, Hsp90α and UCP2 genes by 371 and 26%, 173 and 24%, 252 and 121%, 494 and 57%, 156 and 78%, 28 and 208% and 145 and 56%, respectively (Figure ).
Figure 4. Effect of early age short-term thermal stress at day 42 of age on mRNA expression heat shock transcription factor 1 (HSF1) (A), glucocorticoid receptor (GR) (B), heat shock 70kDa protein 2 (Hsp70) (C), heat shock protein family A (Hsp70) member 9 (HspA9) (D), Insulin-like growth factors-1 (IGF-1) (E), heat shock protein 90 alpha family class A member 1 (Hsp90α) (F) and Uncoupling proteins 2 (UCP2) (G) genes in the liver of NZW and BB rabbits which re-exposed to acute heat stress (36±1° C for six hours) at 13 weeks of age. All the early (42 days of age) heat-stressed (NZWT and BBT) and unstressed (NZWC and BBC) groups were exposed to acute heat stress (36±1° C for six hours) at 13 weeks of age. Upon the acute heat stress at week 13 of age, NZWC, NZWT, BBC and BBT groups were renamed as New Zealand white control exposed (NZWC2), New Zealand white treated (NZWT2), Baladi Black Control exposed (BBC2) and Baladi Black treated (BBT2), respectively. Different lower-case letters above the bars indicate significant difference (p < 0.05) among groups.
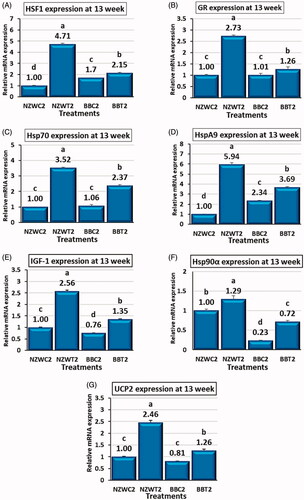
At 13 weeks of age, the control and EA-STHS treated group exhibits gene expression profiles that differ between New Zealand white and Baladi Black breeds. The expression of HSF1 and HspA9 genes was significantly lower (p < .05) in NZWC2 compared with BBC2 by 41 and 57%, respectively (Figure ). The IGF-1 and Hsp90α expression in NZWC2 was higher (p < .05) than BBC2 by 31 and 331, respectively (Figure ). No significant variances in the expression of GR, Hsp70, and UCP2 genes were found between NZWC2 and BBC2 (Figure ). NZWT2 group is higher than (p < .05) BBT2 group in term of HSF1, GR, Hsp70, HspA9, IGF-1, Hsp90α and UCP2 gene expression by 118, 117, 48, 61, 90, 80 and 94%, respectively (Figure ).
Discussion
Plasma corticosterone, lipid peroxidase, total antioxidant capacity and hepatic MDA responses to early thermal stress
Upon heat stress, many neuroendocrine changes induced, through the activation of hypothalamic-pituitary-adrenal (HPA) axis, including release corticosterone in blood stream (Jasnic et al. Citation2010). elevation corticosterone due to elevated ambient temperature is a physiological adaptation manner to cope with stress and enhance life-saving activities for endurance (Sapolsky et al. Citation2000; Wingfield et al. Citation2001). In the current study, increased plasma corticosterone due to thermal conditioning is in agreement with previous studies in rabbits (Sakr et al. Citation2019), chicken (Kang et al. Citation2019; Baxter et al. Citation2020), and mice (Dou et al. Citation2019). Reduction of plasma CORT in conditioned rabbits (NZWT2 and BBT2) than unconditioned (NWZC2 and BBC2) groups at 13 weeks means heat-conditioned rabbits are less stressed than others, in other words, these rabbits success to habituate to heat stress (Sakr et al. Citation2019) and these results in agreement with (Star et al. Citation2009; Nagwa et al. Citation2012; Morsy Citation2013). Thermal stress is considered a major cause of oxidative stress through the overproduction of ROS, and finally, MDA is used as an indicator for oxidative stress (Yang et al. Citation2010; Madkour et al. Citation2015; Akbarian et al. Citation2016). The current results showed that thermal conditioning increases the plasma level of MDA (NZWT and BBT) and decreases TAC. These results were following (Abd El-Hack et al. Citation2019; Sakr et al. Citation2019). However, at 13 weeks of age, the MDA level decreased, and TAC increased in conditioned groups (NZWT2 and BBT2) compared to unconditioned groups (NZWC2 and BBC2). These results confirmed that early life thermal conditioning has a positive effect on antioxidative properties and minimising lipid peroxidation (Nagwa et al. Citation2012; El-Wardany et al. Citation2016; Morsy Citation2018; Sakr et al. Citation2019) hepatic MDA results confirmed and supported aforementioned results that early thermal conditioning enhance the antioxidative capacity through decrease MDA level in liver.
Contrasting responses of NZW and BB rabbits due to thermal conditioning at 42 day of age
Several studies recently narrated the role of early thermal conditioning (TC) for alleviating the detrimental impacts of heat stress on chickens. However, there are very limited data about early thermal conditioning in rabbits and its effect on heat tolerance in later life. Ondruska et al. (Citation2011) found that NZW rabbits' exposure to elevate ambient temperature (36 ± 3 °C for 12 h.), negatively affected their internal homeostasis. At 42 days of age, the current study results showed that TC significantly decreased the gene expression of HSF1, GR and Hsp70 in NZWT rabbits compared to control NZWC (Figure ). During severe stress, the heat shock response fails to resolve stress, that kinases like p38 and JNK rapidly inactivates HSF1 through decrease expression of Hsp70 (Chu et al. Citation1996; Anckar and Sistonen Citation2011). Besides, (Kang et al. Citation2017) suggest that GR expression's downregulation during acute nutritional stress might be a negative-feedback adaptive mechanism to prevent the potentially damaging induced in broiler liver. Other studies (Jones et al. Citation2004; Wadekar et al. Citation2004) documented a complicated functional relationship between GR, HSF1, and Hsp70. Also (Li and Sanchez Citation2005) reported that the activity of HSF1 is an essential part of the mechanism by which stress potentiates GR transcriptional activity. Moreover, one proposed molecular mechanism has been suggested, during conditions of heat stress, HSPs expression increased to a specific temperature limit. Upon reaching this limit, then HSPs expression begins to decline, resulting from the transcription system's dysfunction (Diller Citation2006). Taken all fore mentioned mechanisms together, we can presume that in NZWT could not cope with high temperature (36 °C for 6 h.), so the GR, HSF1, and Hsp70 were suppressed. In contrast to the pattern of hepatic expression of GR and HSF1 in NWZT, TC induces a slight increase (p > .05) in the BBT group. The different responses between the two breeds might be attributable to the heat tolerance ability of the BBT rabbits. BBT had a significant (p < .05) increase in hepatic Hsp70 gene expression compared to BBC. This activation may be a kind of cytoprotective mechanism that further permits the cells to survive. Previous studies have confirmed this finding in rabbits (Pei et al. Citation2012), mice (Wang et al. Citation2014), chickens (Zulkifli et al. Citation2014; Cedraz et al. Citation2017) and fish (Benítez-Dorta et al. Citation2017).
A possible role of hepatic HSF1, GR and Hsp70 in thermotolerance acquisition at 13 weeks of age
Accordingly, we found that at 13 weeks of age, both thermally conditioned breeds (Figure ) showed a significantly (p < .05) higher level of gene expression for HSF1, GR, Hsp70 compared to their relative control. This enhancement of Hsp70 and HSF1 may be an indicator of improving thermotolerance acquisition in the rabbit in later life due to early life TC. This suggestion agrees with former studies (Al-Zghoul and El-Bahr Citation2019) that reported that the enhancement of HSPs and HSFs gene expression was associated with the acquisition of enhanced thermotolerance in thermally conditioned chicks. HSF1 mediates cytoprotection induced by conditioned animals at a mildly increased temperature (Kourtis et al. Citation2012). Furthermore, overexpression of HSF1 leads to increased thermoresistance and increased longevity in the nematode (Kumsta and Hansen Citation2017). Thermotolerance is a process that resulted from elevating the content of HSPs especially Hsp70 family (Amorim et al. Citation2015). Also, overexpression of Hsp70 was associated with an amelioration of thermotolerance acquisition in chickens (Al-Zghoul et al. Citation2013), enhance embryo heat tolerance in a reptile (Gao et al. Citation2014), and suppress apoptosis of cardiomyocytes in heat-stressed mice (Wang et al. Citation2014).
A possible role of hepatic HspA9 and IGF-1 in the thermal conditioning process in rabbits
The HspA9 was initially identified as a 75 kDa protein and was categorised as one of the Hsp70 families (Wadhwa et al. Citation2002). In the present study, HspA9 expression follows the same trend as in Hsp70, whether downregulation in NWZT or upregulation in BBT at seven weeks of age (Figure ). However, at 13 weeks of age, the pattern of expression has been shifted to be upregulated in NWZT2 (Figure ). According to the previous studies, the present finding states that HspA9 prevents oxidative damage in hepatic rats by ameliorating mitochondrial function (Qiukai et al. Citation2013). Furthermore, overexpression of HspA9 in both thermally conditioned breeds is evidence for reducing apoptosis (Peng et al. Citation2013).
There are scarce of information about the effect of heat stress on the expression of hepatic IGF-1. The results related to IGF-1 were shown in Figure ; the pattern of hepatic IGF-1 expression follows the same trend of expression as in Hsp70 and HspA9 whether downregulation in NWZT or upregulation in BBT group at seven weeks of age or 13 weeks of age (Figure ). Bonilla et al. (Citation2011) confirmed that IGF1 increases transcript abundance of several genes involved in cellular protection, including those involved in cytoprotective effects against free radicals and apoptosis regulation in a bovine embryo. Furthermore, (Satrapa et al. Citation2013) suggested that the IGF system might induce thermotolerance in heat-stressed bovine embryos' culture media.
The Insulin/IGF-1-like signalling (IIS) has been known to have a cellular mechanism against stress, suggesting a potential mechanism at the molecular level that binds IIS and HSF-1 pathways together (Chiang et al. Citation2012). The current result indicates that IGF-1 belongs to the same regulatory modulator with Hsp70 and HspA9, which might be HSF-dependent.
A possible role of hepatic Hsp90α and UCP2 in the thermal conditioning process in rabbits
Hsp90 family has a critical role in cellular homeostasis and one of the most substantial chaperone families (Hahn et al. Citation2011). The upregulation of Hsp90 mRNA in rabbit testes after chronic stress plays a protective role in repairing the damage caused by heat stress (Pei et al. Citation2012). Over-expression of Hsp90 is a mechanism through which cells recover from stress and guard it against subsequent offence (Wu et al. Citation2012). These results agree with previous studies since heat conditioning at 42 days of age (Figure ) significantly increased (p < .05) hepatic Hsp90α mRNA level in BBT compared to its control BBC. However, in the NZWT group, there was a slight increase (p > .05) in hepatic Hsp90α mRNA level compared to its control NZWC. Lei et al. (Citation2009) reported that the concentrations Hsp90α mRNA in the liver of acute heat-stressed broilers exhibit an oscillatory pattern. They concluded that the rate of Hsp90 appears to be related to the standing time and the strength of heat stress. They added that with increasing heat up to a critical temperature and birds can tolerate, the Hsp90 synthesis also increased up to this critical temperature. In their study, there was upregulation of hepatic Hsp90 due to exposure to high temperature for 2 h, following a slight reduction after 3 and 5 h. In the current study, expression of Hsp90α was evaluated at a single-time-point (after 6 h of thermal conditioning), and maybe this is the reason since NZWT could not tolerate the high temperature for a long time so they could not induce Hsp90α synthesis to cope heat stress. At 13 weeks of age, both thermally conditioned breeds (Figure ) showed a significantly (p < .05) higher level of gene expression for Hsp90α than their relative control. A rapid increase in Hsp90 could account for thermotolerance acquisition's amelioration to cope with thermal stress in the heat conditioned rabbits (Al-Zghoul and El-Bahr Citation2019).
In terms of hepatic UCP2, as predicted at 42 day of age (Figure ), acute heat stress downregulated gene expression for UCP2, in both rabbit strains. This finding in agreement with Mujahid et al. (Citation2006) who found that acute heat stress stimulates mitochondrial superoxide production in broiler skeletal muscle possibly via downregulation of UCP2. However, at 13 weeks of age (Figure ) thermally conditioned rabbit in both breeds had overexpressed hepatic UCP2 compared to their control groups indicating a protective function of this overexpression and improve thermotolerance acquisition resulted from thermal conditioning at early life. The substantial role of UCP2 is to monitor mitochondria‐derived ROS production (Sreedhar and Zhao Citation2017) and the upregulation of UCP2 may reduce ROS production due to its antioxidative protective effects (Huang et al. Citation2019; Migliaccio et al. Citation2019). Besides, UCP2 overexpression may enhance cytoprotection by alleviating oxidative stress (Mattiasson et al. Citation2003) and attenuating superoxide production to protect hepatic damage in mice due to oxidative stress (Zhang et al. Citation2003). Taken these results with plasma and hepatic MDA together, we could conclude that early thermal stress improves antioxidant capacity through upregulation of UCP2 and reflects on MDA levels in plasma and liver.
Conclusions
The current results indicated that early thermal conditioning at 36 °C for 6 h at 42 days of age in rabbit induce overexpression of hepatic HspA9 and UCP2 and reduce plasma and hepatic MDA at later life is an indicator for improving mitochondrial function to prevent oxidative damage in the liver, moreover overexpression of hepatic HSPs and HSF-1 at later life, this may be an indicator for improving thermotolerance acquisition in thermally conditioned rabbits. From previous results, we could conclude that early life thermal conditioning may be used as a potential tool to mitigate heat stress in rabbit, especially with the global warming problem.
Animal welfare statement
The authors confirm that they have followed EU standards for the protection of animals used for scientific purposes.
Ethical approval
The experimental design and all the research protocols were approved by the Medical Research Ethics Committee (MREC) of the National Research Centre with ethical approval code 20/108.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Abd El-Hack ME, Alagawany M, Abdelnour S. 2019. Responses of growing rabbits to supplementing diet with a mixture of black and red pepper oils as a natural growth promoter. J Anim Physiol Anim Nutr. 103(2):509–517.
- Akbarian A, Michiels J, Degroote J, Majdeddin M, Golian A, De Smet S. 2016. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J Anim Sci Biotechnol. 7:37.
- Al-Zghoul M-B, Al-Zhgoul M-B, Dalab AES, Ababneh MM, Jawasreh KI, Al Busadah KA, Ismail ZB. 2013. Thermal manipulation during chicken embryogenesis results in enhanced Hsp70 gene expression and the acquisition of thermotolerance. Res Vet Sci. 95(2):502–507.
- Al-Zghoul MB, El-Bahr SM. 2019. Basal and dynamics mRNA expression of muscular HSP108, HSP90, HSF-1 and HSF-2 in thermally manipulated broilers during embryogenesis. BMC Vet Res. 15(1):83.
- Amorim FT, Fonseca IT, Machado-Moreira CA, Magalhães FCJT. 2015. Insights into the role of heat shock protein 72 to whole-body heat acclimation in humans. Temperature. 2(4):499–505.
- Anckar J, Sistonen L. 2011. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu Rev Biochem. 80:1089–1115.
- Barna J, Csermely P, Vellai T. 2018. Roles of heat shock factor 1 beyond the heat shock response. Cell Mol Life Sci. 75(16):2897–2916.
- Baxter MFA, Greene ES, Kidd MT, Tellez-Isaias G, Orlowski S, Dridi S. 2020. Water amino acid-chelated trace mineral supplementation decreases circulating and intestinal HSP70 and proinflammatory cytokine gene expression in heat-stressed broiler chickens. J Anim Sci. 98(3):skaa049.
- Benítez-Dorta V, Caballero MJ, Betancor MB, Manchado M, Tort L, Torrecillas S, Zamorano MJ, Izquierdo M, Montero DJG, Endocrinology C. 2017. Effects of thermal stress on the expression of glucocorticoid receptor complex linked genes in Senegalese sole (Solea senegalensis): acute and adaptive stress responses. Gen Comp Endocrinol. 252:173–185.
- Bonilla AQ, Oliveira LJ, Ozawa M, Newsom EM, Lucy MC, Hansen PJ. 2011. Developmental changes in thermoprotective actions of insulin-like growth factor-1 on the preimplantation bovine embryo. Mol Cell Endocrinol. 332(1–2):170–179.
- Cadenas S. 2018. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim Biophys Acta Bioenerg. 1859(9):940–950.
- Cedraz H, Gromboni JGG, Garcia AAPJ, Farias Filho RV, Souza TM, Oliveira ER, Oliveira EB, Nascimento CSD, Meneghetti C, Wenceslau AA. 2017. Heat stress induces expression of HSP genes in genetically divergent chickens. PLoS One. 12(10):e0186083.
- Chen Y, Yu T. 2018. Glucocorticoid receptor activation is associated with increased resistance to heat-induced hyperthermia and injury. Acta Physiol. 222(4):e13015.
- Chiang WC, Ching TT, Lee HC, Mousigian C, Hsu AL. 2012. HSF-1 regulators DDL-1/2 link insulin-like signaling to heat-shock responses and modulation of longevity. Cell. 148(1–2):322–334.
- Chu B, Soncin F, Price BD, Stevenson MA, Calderwood SK. 1996. Sequential phosphorylation by mitogen-activated protein kinase and glycogen synthase kinase 3 represses transcriptional activation by heat shock factor-1. J Biol Chem. 271(48):30847–30857.
- de Bruijn R, Romero LM. 2018. The role of glucocorticoids in the vertebrate response to weather. Gen Comp Endocrinol. 269:11–32.
- Diller KR. 2006. Stress protein expression kinetics. Annu Rev Biomed Eng. 8:403–424.
- Dou J, Montanholi YR, Wang Z, Li Z, Yu Y, Martell JE, Wang YJ, Wang Y. 2019. Corticosterone tissue-specific response in Sprague Dawley rats under acute heat stress. J Therm Biol. 81:12–19.
- Dubrez L, Causse S, Borges Bonan N, Dumetier B, Garrido C. 2020. Heat-shock proteins: chaperoning DNA repair. Oncogene. 39(3):516–529.
- Ei-Badry AE-A, Easa F, Ragab A, Hekal A, Ali KA, Badri F. 2015. Role of early neonatal heat acclimation in alleviate hyperthermia-induced oxidative stress in rabbits. Egypt J Rabbit Sci. 25(1):83–101.
- El-Wardany I, Shourrap MI, Madkour M, Abd El-Azeem NA. 2016. Effect of Age at mating and silver nanoparticles administration on progeny productive performance and some blood constituents in Japanese quail. Int J ChemTech Res. 9(8):21–34.
- Ezzat S, Elwerdany IE, Galal AE, Madkour M. 2019. Effect of early age heat stress on growth performance and some blood parameters of two strains of rabbits. Arab Univ J Agric Sci. 27(5):2621–2629.
- Gao J, Zhang W, Dang W, Mou Y, Gao Y, Sun BJ, Du WG. 2014. Heat shock protein expression enhances heat tolerance of reptile embryos. Proc Biol Sci. 281(1791):20141135.
- Gong S, Miao YL, Jiao GZ, Sun MJ, Li H, Lin J, Luo MJ, Tan JH. 2015. Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLoS One. 10(2):e0117503.
- Hahn A, Bublak D, Schleiff E, Scharf K-D. 2011. Crosstalk between Hsp90 and Hsp70 chaperones and heat stress transcription factors in tomato. Plant Cell. 23(2):741–755.
- Huang J, Peng W, Zheng Y, Hao H, Li S, Yao Y, Ding Y, Zhang J, Lyu J, Zeng Q. 2019. Upregulation of UCP2 expression protects against LPS-induced oxidative stress and apoptosis in cardiomyocytes. Oxid Med Cell Longev. 2019:2758262.
- Jasnic N, Korac A, Velickovic K, Golic I, Djordjevic J, Djurasevic S, Djordjevic I, Vujovic P, Cvijic G. 2010. The effect of acute heat exposure on rat pituitary corticotroph activation: the role of vasopressin. Folia Histochem Cytobiol. 48(4):507–512.
- Jones TJ, Li D, Wolf IM, Wadekar SA, Periyasamy S, Sanchez ER. 2004. Enhancement of glucocorticoid receptor-mediated gene expression by constitutively active heat shock factor 1. Mol Endocrinol. 18(3):509–520.
- Kang D, Park J, Shim K. 2019. Heat treatment at an early age has effects on the resistance to chronic heat stress on broilers. Animals. 9(12):1022.
- Kang SW, Madkour M, Kuenzel WJ. 2017. Tissue-specific expression of DNA methyltransferases involved in early-life nutritional stress of chicken. Front Genet. 8:204.
- Kourtis N, Nikoletopoulou V, Tavernarakis N. 2012. Small heat-shock proteins protect from heat-stroke-associated neurodegeneration. Nature. 490(7419):213–218.
- Kumsta C, Hansen M. 2017. Hormetic heat shock and HSF-1 overexpression improve C. elegans survival and proteostasis by inducing autophagy. Autophagy. 13(6):1076–1077.
- Lei L, Yu J, Bao E. 2009. Expression of heat shock protein 90 (Hsp90) and transcription of its corresponding mRNA in broilers exposed to high temperature. Br Poult Sci. 50(4):504–511.
- Li D, Sanchez ER. 2005. Glucocorticoid receptor and heat shock factor 1: novel mechanism of reciprocal regulation. Vitam Horm. 71:239–262.
- Li J, Jiang R, Cong X, Zhao Y. 2019. UCP2 gene polymorphisms in obesity and diabetes, and the role of UCP2 in cancer. FEBS Lett. 593(18):2525–2534.
- Madkour M, Aboelenin MM, Aboelazab O, Elolimy AA, El-Azeem NA, El-Kholy MS, Alagawany M, Shourrap M. 2021. Hepatic expression responses of DNA methyltransferases, heat shock proteins, antioxidant enzymes, and NADPH 4 to early life thermal conditioning in broiler chickens. Ital J Anim Sci. 20(1):433–446.
- Madkour M, Aboelenin MM, Younis E, Mohamed MA, Hassan H, Alagawany M, Shourrap M. 2020. Hepatic acute-phase response, antioxidant biomarkers and DNA fragmentation of two rabbit breeds subjected to acute heat stress. Ital J Anim Sci. 19(1):1558–1566.
- Madkour M, Ali HM, Yassein SA, Abdel-Fatt SA, El-Allawy HM. 2015. Effect of dietary organic selenium supplement on growth and reproductive performance of Japanase quail breeders and their progeny and its relation to antioxidation and thyroid activity. Inter J Poultry Sci. 14(6):317–324.
- Mahrous KF, Aboelenin MM, Abd El-Kader HAM, Mabrouk DM, Gaafar AY, Younes AM, Mahmoud MA, Khalil WKB, Hassanane MS. 2020. Piscidin 4: genetic expression and comparative immunolocalization in Nile tilapia (Oreochromis niloticus) following challenge using different local bacterial strains. Dev Comp Immunol. 112:103777.
- Mahrous KF, Aboelenin MM, Rashed MA, Sallam MA, Rushdi HE. 2020. Detection of polymorphism within leptin gene in Egyptian river buffalo and predict its effects on different molecular levels. J Genet Eng Biotechnol. 18(1):6.
- Mahrous KF, Mabrouk DM, Aboelenin MM, Abd El-Kader HAM, Gaafar AY, Younes AM, Mahmoud MA, Khalil WKB, Hassanane MS. 2020. Molecular characterization and immunohistochemical localization of tilapia piscidin 3 in response to Aeromonas hydrophila infection in Nile tilapia. J Pept Sci. 26(11):e3280.
- Mahrous KF, Mabrouk DM, Aboelenin MM, Abd El Kader HAM, Hassanane MS. 2021. Identification and characterization of antimicrobial peptide genes in Clarias gariepinus and Chelon ramada. Jordan J Biol Sci. 14(1):51–64.
- Marai IF, Habeeb AA, Gad AE. 2002. Rabbits’ productive, reproductive and physiological performance traits as affected by heat stress: a review. Livest Prod Sci. 78(2):71–90.
- Mattiasson G, Shamloo M, Gido G, Mathi K, Tomasevic G, Yi S, Warden CH, Castilho RF, Melcher T, Gonzalez-Zulueta M, et al. 2003. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat Med. 9(8):1062–1068.
- Migliaccio V, Gregorio ID, Putti R, Lionetti L. 2019. Mitochondrial involvement in the adaptive response to chronic exposure to environmental pollutants and high-fat feeding in a rat liver and testis. Cells. 8(8):834.
- Morsy AS. 2013. Effect of heat shock exposure on the physiological responses and semen quality of male chickens under heat stress conditions. Egypt Poult Sci. 33(1):143–161.
- Morsy AS. 2018. Hematological parameters and productive performance of heat-stressed laying hens as influenced by early heat shock program, sodium bicarbonate and/or vitamin C supplementation. Res J Ani & Vet Sci. 10(2):1–8.
- Mujahid A, Sato K, Akiba Y, Toyomizu M. 2006. Acute heat stress stimulates mitochondrial superoxide production in broiler skeletal muscle, possibly via downregulation of uncoupling protein content. Poult Sci. 85(7):1259–1265.
- Nagwa AA, Amal MH, GM, M KRE. 2012. Effect of using heat shock programs on thermoregulation responses and performance of laying hens under desert conditions. Egypt Poult Sci. 32(4):777–780.
- Ondruska L, Rafay J, Okab AB, Ayoub MA, Al-Haidary AA, Samara EM, Parkanyi V, Chrastinova L, Jurcik R, Massanyi P, et al. 2011. Influence of elevated ambient temperature upon some physiological measurements of New Zealand White rabbits. Veterinarni Medicina. 56(4):180–186.
- Pei Y, Wu Y, Qin Y. 2012. Effects of chronic heat stress on the expressions of heat shock proteins 60, 70, 90, A2, and HSC70 in the rabbit testis. Cell Stress Chaperones. 17(1):81–87.
- Peng C, Yang P, Cui Y, He M, Liang L, Di Y. 2013. HSPA9 overexpression inhibits apoptin-induced apoptosis in the HepG2 cell line. Oncol Rep. 29(6):2431–2437.
- Qiukai E, Liu X, Liu Y, Liu W, Zuo J. 2013. Over-expression of GRP75 inhibits liver injury induced by oxidative damage. Acta Bioch Bioph Sin. 45(2):129–134.
- Sakr OG, Mousa BH, Morsy AS, Emam KRS, Morsy AS, Ahmed NA. 2019. Effect of early heat shock exposure on physiological responses and reproduction of rabbits under hot desert conditions. JWPR. 9(2):90–101.
- Sapolsky RM, Romero LM, Munck AU. 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 21(1):55–89.
- Satrapa R, Razza E, Castilho A, Simões R, Silva C, Nabhan T, Pegorer M, Barros C. 2013. Differential expression of IGF family members in heat-stressed embryos produced in vitro from OPU-derived oocytes of Nelore (Bos indicus) and Holstein (Bos taurus) cows. Reprod Domest Anim. 48(6):1043–1048.
- Sreedhar A, Zhao Y. 2017. Uncoupling protein 2 and metabolic diseases. Mitochondrion. 34:135–140.
- Sroor FM, Aboelenin MM, Mahrous KF, Mahmoud K, Elwahy AHM, Abdelhamid IA. 2020. Novel 2-cyanoacrylamido-4,5,6,7-tetrahydrobenzo[b]thiophene derivatives as potent anticancer agents. Arch Pharm. 353(10):e2000069.
- Star L, Juul-Madsen HR, Decuypere E, Nieuwland MG, de Vries Reilingh G, van den Brand H, Kemp B, Parmentier HK. 2009. Effect of early life thermal conditioning and immune challenge on thermotolerance and humoral immune competence in adult laying hens. Poult Sci. 88(11):2253–2261.
- Wadekar SA, Li D, Sanchez ER. 2004. Agonist-activated glucocorticoid receptor inhibits binding of heat shock factor 1 to the heat shock protein 70 promoter in vivo. Mol Endocrinol. 18(3):500–508.
- Wadhwa R, Taira K, Kaul SC. 2002. An Hsp70 family chaperone, mortalin/mthsp70/PBP74/Grp75: what, when, and where? Cell Stress Chaper. 7(3):309.
- Wang X, Yuan B, Dong W, Yang B, Yang Y, Lin X, Gong GJPO. 2014. Induction of heat-shock protein 70 expression by geranylgeranylacetone shows cytoprotective effects in cardiomyocytes of mice under humid heat stress. PLoS One. 9(4):e93536.
- Wingfield JC, Lynn S, Soma KK. 2001. Avoiding the ‘costs’ of testosterone: ecological bases of hormone-behavior interactions. Brain Behav Evol. 57(5):239–251.
- Wrigley S, Arafa D, Tropea D. 2017. Insulin-like growth factor 1: at the crossroads of brain development and aging. Front Cell Neurosci. 11:14.
- Wu CX, Zhao FY, Zhang Y, Zhu YJ, Ma MS, Mao HL, Hu CY. 2012. Overexpression of Hsp90 from grass carp (Ctenopharyngodon idella) increases thermal protection against heat stress. Fish Shellfish Immunol. 33(1):42–47.
- Yang L, Tan G-Y, Fu Y-Q, Feng J-H, Zhang M-H. 2010. Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp Biochem Physiol C Toxicol Pharmacol. 151(2):204–208.
- Zhang G, Nichols RD, Taniguchi M, Nakayama T, Parmely MJ. 2003. Gamma interferon production by hepatic NK T cells during Escherichia coli infection is resistant to the inhibitory effects of oxidative stress. Infect Immun. 71(5):2468–2477.
- Zulkifli I, Najafi P, Nurfarahin AJ, Soleimani AF, Kumari S, Aryani AA, O'Reilly EL, Eckersall PD. 2014. Acute phase proteins, interleukin 6, and heat shock protein 70 in broiler chickens administered with corticosterone. Poult Sci. 93(12):3112–3118.
