Abstract
Intrauterine laparoscopic insemination is diffuse in sheep breeding, yet requires veterinary expertise and expensive equipment. Our aim was to evaluate the time required, reproductive outcome and post-operative complications of mini invasive laparotomic intrauterine insemination, as an alternative approach to laparoscopy. A total of 115 Sopravvissana breed ewes were enrolled, including 75 adults and 40 lamb ewes, after oestrus synchronisation. Ewes were sedated and restrained in dorsal recumbence. Uterine horns were exposed through an abdominal incision of 4–7 cm, cranially to the udder. Thawed semen, containing 100 × 106 progressive motile (70%) and morphologically normal (80%) sperm cells, was inoculated into uterine lumen through an intravenous catheter. The abdominal incision was then routinely closed. The time interval for: (a) laparotomy and uterine exteriorisation, (b) intrauterine insemination, (c) abdominal wall closure and (d) total procedure was recorded. Pregnancy, lambing, twinning rates and sex ratio were submitted to chi-square test, comparing lamb and adult ewes. Time intervals and the duration of laparotomic procedure were compared in lamb and adults through Mann–Whitney U test for independent samples. Median ± standard error (SE) of total time for laparotomic intrauterine insemination was 5.53 ± 0.07 and 4.99 ± 0.11 min, for adult and lamb ewes (p<.05), which was similar to what was reported for laparoscopic methods. At pregnancy check, 60% and 70% of adult and lamb ewes resulted pregnant. Laparotomy is poorly applied due to risk of adherence and decreased fertility. In our study, ewes were naturally bred in the following season with 90% pregnancy rate. Our results showed that time for laparotomy is quite similar to laparoscopic insemination, as for the conception and lambing rates.
Time required for the overall procedure (5.53 ± 0.07 and 4.99 ± 0.11 min, for adult and lamb ewes, respectively, with significant difference (p<.05)) was similar to laparoscopic artificial insemination.
About 60% and 70% of adult and lamb ewes resulted pregnant, respectively.
No decreased fertility was observed in the following breeding season (90% pregnancy rate at natural insemination).
Laparotomic approach is quite similar to laparoscopic insemination, in terms of time required for its complete achievement, for the conception and lambing rates; furthermore it has the advantage of reducing equipment costs.
HIGHLIGHTS
Introduction
Artificial insemination (AI) is well diffused in ruminant industry, allowing a great genetic improvement and simplifying the international trade of males and the conservation of genetic resources. Moreover, it preserves animal health by limiting the diffusion of several infectious diseases. Particularly, in small ruminants, the AI is mostly performed by depositing fresh semen through the cervix with a reported conception rate varying from 40% to 60% (Anel et al. Citation2005; Fair et al. Citation2005). Nevertheless, the use of fresh semen leads to a smaller number of superior rams to be used and requires restrictions due to the shorter vitality of cooled semen during transport. Indeed, this constraint encourages farmers in most intensive farms to progressively use frozen semen. However, lack of efficient methods may reduce the diffusion of AI with frozen semen (Salamon and Maxwell Citation1995a).
The anatomy of ewe’s cervix is complex as it is small, narrow, rigid and tortuous; the long fibrous tubular structure and the internal rings are the most important obstacles for catheterisation by means of pipette and for the deposition of semen into the uterine cavity (Evans and Maxwell Citation1987). Therefore, semen is usually released at the external cervical os trough the vaginal canal (cervical insemination) or it is directly inoculated into the uterine lumen by laparoscopy (laparoscopic insemination).
Halbert et al. (Citation1990) compared four methods of restraint, four vaginal specula, three forceps and four instruments for trans-cervical passage, and developed a method named the Guelph System with variable success in terms of lambing rate (Buckrell et al. Citation1994). Different protocols, techniques, devices and drugs have been proposed by several authors in different conditions in order to improve the ovine cervical catheterisation (Wulster-Radcliffe et al. Citation2004; Rabassa et al. Citation2007; Alvarez et al. Citation2012; Bartlewski and Candappa Citation2015). Pregnancy rate (PR) is different among reports (Sayre and Lewis Citation1996; Husein et al. Citation1998), and some of them are presented as proof of concept even if sometimes those are not followed by validation or field trials with enough number of females, breeds and ages.
The laparoscopic intrauterine insemination has been implemented in sheep since the 1980s (Evans and Maxwell Citation1987). As the fertility of cryopreserved sperm by cervical insemination is extremely poor, the laparoscopic AI is the elective technique (Salamon and Maxwell Citation1995b). Furthermore, intrauterine insemination allows greater PR compared to cervical one even with fresh semen (Santos-Neto et al. Citation2015). Then, intrauterine insemination at prefixed time performed by laparoscopy is diffusely used in several countries. However, although laparoscopy is a minimally invasive procedure, it requires veterinary expertise, implies insufflation of irritant CO2 into the peritoneal cavity, and is more demanding in terms of expensive equipment and labour than other methods. Although further approaches have been attempted to overcome the use of laparoscopy, this technique is still the default method when obtaining greater PR is mandatory. In a preliminary study on 15 ewes subjected to laparotomic intrauterine insemination with frozen semen, we achieved 60% PR (Sylla et al. Citation2014). More recently, a new surgical approach on cervical folds for trans-cervical intrauterine insemination has been reported to achieve 63.7% of PR (Pau et al. Citation2019). As endoscopy equipment is not always available due both to purchase cost and requirement of Practitioner’s specific ability, alternative methods to laparoscopy insemination could optimise costs in sheep breeding management and facilitate the use of AI. However, reports on the time required to accomplish the laparotomic intrauterine insemination are lacking. Thus, the objective of this study was to evaluate the overall time required, the reproductive outcome and post-operative complications obtained by intrauterine insemination through a mini-invasive laparotomic technique, thus evaluating the feasibility and the comparability to laparoscopy of this method in routine in-field AI programmes with frozen semen in sheep.
Materials and methods
This study was carried out in a farm near Perugia, in central Italy, during the period of May–October 2018 and was in accordance with the Institutional Animal Care and Use Committee of the University of Perugia. A total of 115 Sopravvissana breed ewes were enrolled, including 75 adults (mean age of 48.1 ± 17.0 months) and 40 lamb ewes (mean age of 7 ± 0.4 months) with a mean body weight of 45.6 ± 4.6 and 37.15 ± 2.4 kg, respectively. All animals were fed ad libitum with alfalfa hay and free access to water. Any supplement of concentrate or crop was provided. Three weeks before the beginning of the oestrus synchronisation programme, all animals were administered 1 ml/50 kg body weight of ivermectin subcutaneously (Ivomec® 1 g, Boehringer Ingelheim Italia S.p.A.).
Oestrus was synchronised with vaginal progestagen-release devices (Cronogest® 40 mg of cronolone, Intervet Productions S.A., Igoville, France) in a group of 28 ewes for session. After 14 d, pessaries were removed, and 500 IU of PMSG (Folligon®, MSD Animal Health S.r.l., Segrate, Italy) was administered intramuscularly. Ewes were checked for oestrus at 48 h after pessary removal, using a vasectomised ram. Ewes standing firmly to be mounted were considered to be in oestrus; however, only ewes showing an hyperaemic and oedematous vulva were subjected to laparotomic intrauterine insemination.
Commercial Merino ram frozen-thawed semen was purchased from Australia. Semen quality was checked at the moment of thawing, including sperm cell motility, concentration and morphology, by randomly examining one straw from the batch. Each 0.25 ml straw contained 100 × 106 sperm cells; progressive motility was 70% and 80% of sperm cells were morphologically normal. For each ewe, a single semen straw was used. At the time of insemination, a single straw was pulled out from the nitrogen tank and rapidly immersed in a citothaw device at 37 °C for 1 min. After completely drying the straw, semen was extended with 1 ml of 5% glucose solution and filled in 1-ml syringe.
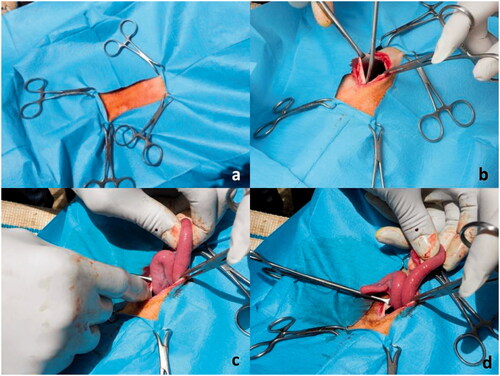
All ewes were fasted for at least 24 h prior to insemination, sedated with acepromazine (Prequillan®, Fatro S.p.a., Ozzano dell’Emilia, Italy) at a dose of 0.05 mg/kg BW IM, treated with carprofen (Rimadyl®, Zoetis Italia, Rome, Italy) at a dose of 1–4 mg/kg BW SQ and restrained in dorsal recumbence in a 30 degree-inclined laparotomy cradle; then they were routinely surgically prepared. The prepubic area, cranially to the mammary glands, was scrubbed with antiseptic iodine 5% solution and chlorexidine. Three millilitres of 2% procaine hydrochloride (Procamidor®, IZO S.r.l., Brescia, Italy) was administered subcutaneously and intramuscularly along the ventral midline, 10 cm cranial to the udder. The uterine horns were manually exposed through an abdominal incision variable from 4 to 7 cm in length, cranially to the udder (Figure ).
Figure 1. Preparation of the patient for artificial insemination. (a) Surgical scrub and delimitation of the incision area, cranially to the udder; (b) incision of 5 cm length of the abdomen wall, along the white line; (c) exposure of the uterine horns; (d) digital palpation of uterine horns for assessing the tone and content.
Before proceeding with insemination, the surface of the ovaries was checked to verify the presence of preovulatory follicles, while the uterine horns were palpated to assess the consistency and the presence of any abnormal collection. The uterine horn corresponding to the side where the graafian follicle was identified, was chosen for catheterisation. A 18-gauge intravenous catheter was inserted through the uterine wall in the cranial third of the uterine horn and an inseminating dose of frozen/thawed semen of 100 × 106 progressively motile spermatozoa was inoculated into the lumen (Figure ).
Figure 2. Artificial intra-uterine insemination. (a) exposure of ovaries and check for pre-ovulatory follicles; (b) insertion of a 18-gauge intravenous catheter into the uterine wall; (c) deposition of thawed semen into the uterine lumen; (d) abdomen wall closure.
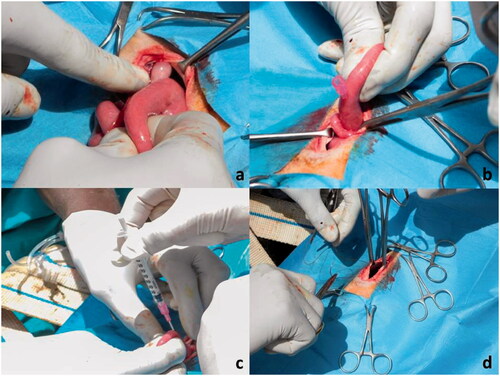
The catheter was slowly removed and the uterine horn was gently massaged and repositioned into the abdominal cavity. The abdominal incision was then routinely closed. An antibiotic suspension of benzylpenicillin procaine + dihydrostreptomycin (Repen®, Fatro S.p.a., Ozzano dell'Emilia, Italy) was administered in a single intramuscular injection at a dose of 0.5 ml/10 kg BW and a local antibiotic spray (Neo Spray Caf®, MSD Animal Health S.r.l., Segrate, Italy) was applied on the wound. Then the ewe was removed from the cradle, returned to a standing position and checked periodically then after for pain (Della Rocca et al., 2017). From the beginning to the end of the surgical procedure, an assistant was in charge of recording the time interval for each step, that is: (a) time to laparotomy and uterine exteriorisation, (b) time of intrauterine insemination, (c) time to abdominal wall closure. Intervals were then summed up for the total duration of the procedure. Time recording was carried out using the stopwatch function of an Android smartphone and each interval was recorded in a table-format in each animal’s dedicated sheet.
Pregnancy diagnosis was carried out at Day 30 post-AI by trans-abdominal ultrasound examination using a portable ultrasound scan equipped with a 5 MHz convex transducer (Mindray M7Vet, Mindray Medical Italy Service srl). At first pregnancy check, uterine aspect and content were examined to evaluate embryo vesicle, heart activity and fluid aspect. Pregnant ewes were rechecked a month later in order to exclude pregnancy loss.
Data were analysed through IBM SPSS version 23 software. Time intervals relatively to overall duration of laparotomic procedure, together with uterine horn exteriorisation, semen deposition and laparotomy closure were expressed as median ± standard error (SE) and compared in lamb and adult groups through Mann–Whitney U test for independent samples. Pregnancy, lambing, twinning rates and sex ratio were submitted to chi-square test, comparing lamb and adult ewes groups.
Differences were considered significant for p<.05.
Results and discussion
Any ewe expelled the intravaginal sponge and any vulvar and vaginal inflammatory process was noticed at the end of synchronisation treatment. At sponge removal, all subjects presented minimal secretion of vaginal mucus. During the following 2 d, a moderate vulvar hyperaemia, oedema and a mucous discharge were considered indicative of pro-oestrus. Five out of 120 (2 lamb ewes and 3 adults) animals failed to show oestrus after synchronisation protocol and were subsequently excluded from the study.
A total of 115 animals, including 75 adult and 40 lamb ewes, were involved in the procedure. Median duration of the entire laparotomic intrauterine insemination, together with elapsed time calculated for each step for both adult and lamb ewes, are reported in Table . Median ± SE total time needed to complete the laparotomic intrauterine insemination was 5.53 ± 0.07 and 4.99 ± 0.11 min., for adult and lamb ewes, respectively (p<.05).
Table 1. Time intervals required for overall procedure and for each step of laparotomic artificial insemination in adult and lamb ewes, expressed ad median ± SE.
At the time of laparotomic exploration of the genital tract, all ewes showed at least one preovulatory follicle. Three ewes presented a haemorrhagic follicle and few subjects showed the presence of two follicles.
All ewes achieved a good state of sedation, as no reaction to scalpel incision was observed; at the end of the surgery they reached immediately the flock by themselves, showing any sign of apparent pain nor compromised physiological functions; half an hour later, all patients started feeding. Any subject showed fever within the following 2 d. No infection of the laparotomic wound and no dehiscence occurred in the following 2 weeks after surgery.
At first pregnancy check, 60% and 70% of adult and lamb ewes resulted pregnant, respectively (Table ). The second pregnancy check revealed that only 1 adult ewe had pregnancy loss. The other ewes lambed physiologically at the term of gestation; twinning rates were 13.64% and 14.29% in adult and lamb ewes, respectively. Male to female sex lamb ratio was 52.00 and 46.88 in adult and lamb ewes, respectively. Chi-square test showed any difference among groups.
Table 2. Reproductive outcomes in adult and lamb ewes after mini-invasive laparotomic artificial insemination.
The objective of this study was to apply an alternative technique of intrauterine AI with frozen-thawed semen in both adult and lamb ewes, suitable to reach an acceptable PR, compared to endoscopy method. Reports on the success rate for both laparotomic and laparoscopic intrauterine insemination exist, although comparative evaluation concerning the time required for the overall procedures is few.
Herein, obtained PR was 60% and 70%, respectively, similar to that reported by other authors applying different approaches. However, pregnancy outcome was greater when compared to intravaginal insemination. In fact, reported results range between 17% and 31.25% (Maxwell and Hewitt Citation1986; Anel et al. Citation2005) for intravaginal insemination, 18.4% and 34.4% for cervical insemination (Maxwell and Hewitt Citation1986; Aybazov et al. Citation2019), 13.7% and 34.8% for TCAI (Halbert et al. Citation1990; Eppleston and Maxwell Citation1995; Salamon and Maxwell Citation1995a). Indeed, by using laparoscopic intrauterine insemination higher PR is obtained, that is around 44.8% (Anel et al. Citation2005), 50.2% (Casali et al. Citation2017) and 63.7% (Pau et al. Citation2019).
Laparotomic approach presents many advantages compared to the laparoscopic one, such as no need of specialised and expensive equipment as endoscope, trocar, air insufflator and the inseminating catheter. The convalescence time is similar to that of other laparotomic surgical techniques. An additional benefit of this approach includes the possibility to fully explore the genital tract before carrying out the insemination, thus checking for the presence of preovulatory follicles and palpating the uterus in order to verify the tone or fluid collection. Uterine cannulation is easier and more reliable than laparoscopic approach, by reducing uterine mobility during the procedure and avoiding the risk of passing through the uterine wall to the other side, before injecting semen. Risk of uterine tissue damage is reduced as well as bleeding or formation of cohalescence between perimetrium and surrounding abdominal organs. Moreover, in case of accidental vessel rupture and haemorrhage, abdominal cavity is already exposed and emergency haemostasis is easier. Azawi and Al-Mola (Citation2011) state that laparotomic technique is poorly applied due to decreased fertility of animals which undergo this procedure. In our study, using a minimally invasive laparotomic method, ewes were naturally bred in the following reproductive season but no decreased PR was noticed (90%).
Inversely, the present laparotomic approach presents some limits such as a greater extension of the incision line (4–7 cm) which is variable according to the ability of the Practitioner; indeed, in case of laparoscopic technique, only two surgical incisions, 1 cm each, are necessary, with no required closure.
The overall time required for the execution of the laparotomic insemination in our study was approximately 5 min in lamb ewes and 5.50 min in adults: this difference results from a larger amount of time (few seconds) required for semen injection and abdomen wall closure in adult, compared to lamb ewes. This difference could be due to a slightly greater difficulty in placing the catheter for semen injection into the uterine lumen of multiparous ewes, which is thicker in adult than lamb ewes. Moreover, in adult ewes, the development of superficial epigastric veins could have impaired the abdomen wall closure for a major tendency to bleeding, compared to nulliparous lamb ewes. This in turn could have delayed the abdomen closure and increased the overall time needed for the completion of the procedure in the two groups. However, even if significant, the average difference of time required for laparotomic intrauterine insemination consisted of 30 s.
In a study conducted by Casali et al. (Citation2017), it is reported that an average of 57 animals per hour were inseminated by laparoscopy, leading to 1 min required at least for each ewe to be inseminated. In the study performed by Azawi and Al-Mola (Citation2011), a similar time (5 min on average) was required for laparoscopic intrauterine insemination, which resembles the mean duration of the procedure as found in our study.
Pau et al. (Citation2019) proposed an innovative techniques which include surgery of ovine cervical folds in order to facilitate the following cervical cannulation and uterine insemination. They reported full patency of the cervical canal in 90.5% and 89.6% of the animals which received 4 and 2 incisions on cervical folds, respectively, at 5 months after intervention. Conception rate after oestrus synchronisation and transcervical AI with frozen-thawed semen was 63.7% and 41.4%, respectively, while lambing with rates were 56.8% and 41.4%, respectively. Compared to our study, PR was similar in animals which received incision of four cervical folds. However, the incision of cervical folds was performed 24 h after first parturition and required surgeons to be trained.
Conclusions
In conclusion, this study showed that intrauterine insemination with frozen semen through a mini-invasive laparotomic approach could overcome with the difficulties imposed by the complex anatomy of the ovine cervix. The time required has been recorded and our results showed that it can be considered similar on the time required for laparoscopic insemination, as for the conception and lambing rates, with the advantage of reduced costs. This technique could be easily applied in intensive sheep farming, whenever lacking a laparoscopic equipment or training for laparoscopic procedure, with good advantages on both time required and PR.
Aknowledgements
The authors aknowledge the Breeders Mac and Francesca Chiacchiarini from "Chiacchiarini Farm"—Pettino, Campello Sul Clitunno, Perugia, Italy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available on a reasonable request from the corresponding author, M.C.
Additional information
Funding
References
- Alvarez M, Chamorro CA, Kaabi M, Opez L, Boixo JC, Anel L, De Paz P. 2012. Design and “in vivo” evaluation of two adapted catheters for intrauterine transcervical insemination in sheep. Anim Reprod Sci. 131( 3–4):153–159.
- Anel L, Kaabi M, Abroug B, Alvarez M, Anel E, Boixo JC, Fuente LF, De Paz P. 2005. Factors influencing the success of vaginal and laparoscopic artificial insemination in churra ewes: a field assay. Theriogenology. 63( 4):1235–1247.
- Aybazov AM, Malmakov NI, Selionova MI, Mamontova TV. 2019. Fertility of ewe following intrauterine laparoscopic insemination with frozen-thawed semen. IOP Conf Earth Environ Sci. 341:012163.
- Azawi OI, Al-Mola MKMA. 2011. A study on the effect of GnRH administration on the ovarian response and laparoscopic intrauterine insemination of Awassi ewes treated with eCG to induce superovulation. Trop Anim Health Prod. 43( 7):1351–1 7.
- Bartlewski PM, Candappa IBR. 2015. Assessing the usefulness of prostaglandin E2 (Cervidil) for transcervical artificial insemination in ewes. Theriogenology. 84( 9):1594–1602.
- Buckrell BC, Buschbeck C, Gartley CJ, Kroetsch T, McCutcheon W, Martin J, Penner WK, Walton JS. 1994. Further development of a transcervical technique for artificial insemination in sheep using previously frozen semen. Theriogenology. 42( 4):601–611.
- Casali R, Pinczak A, Cuadro F, Guillen-Muñoz JM, Mezzalira A, Menchaca A. 2017. Semen deposition by cervical, transcervical and intrauterine route for fixed-time artificial insemination (FTAI) in the ewe. Theriogenology. 103:30–35.
- Della Rocca G, Brondani JT, De Oliveira FA, Crociati M, Sylla L, Elad Ngonput A, Di Salvo A, Luna SPL. 2017. Validation of the Italian version of the UNESP-Botucatu unidimensional composite pain scale for the assessment of postoperative pain in cattle. Vet Anaesth Analg. 44(5):1253–1261. doi:https://doi.org/10.1016/j.vaa.2016.11.008. 28986129
- Eppleston J, Maxwell WM. 1995. Sources of variation in the reproductive performance of ewes inseminated with frozen-thawed ram semen by laparoscopy. Theriogenology. 43(4):777–788.
- Evans P, Maxwell WMC. 1987. Salmon’s artificial insemination of sheep and goats. 1st ed. Waltham (MA): Butterworths.
- Fair S, Hanrahan JP, O’Meara CM, Duffy P, Rizos D, Wade M, Donovan A, Boland MP, Lonergan P, Evans ACO. 2005. Differences between Belclare and Suffolk ewes in fertilization rate, embryo quality and accessory sperm number after cervical or laparoscopic artificial insemination. Theriogenology. 63( 7):1995–2005.
- Halbert GW, Dobson H, Walton JS, Sharpe P, Buckrell BC. 1990. Field evaluation of a technique for transcervical intrauterine insemination of ewes. Theriogenology. 33( 6):1231–1243.
- Husein MQ, Bailey MT, Ababneh MM, Romano JE, Crabo BG, Wheaton JE. 1998. Effect of eCG on the pregnancy rate of ewes transcervically inseminated with frozen-thawed semen outside the breeding season. Theriogenology. 49( 5):997–1005.
- Maxwell WMC, Hewitt LJ. 1986. A comparison of vaginal, cervical and intrauterine insemination of sheep. J Agric Sci. 106( 1):191–193.
- Pau S, Falchi L, Ledda M, Bogliolo L, Ariu F, Zedda MT. 2019. Surgery on cervical folds for transcervical intrauterine artificial insemination with frozen-thawed semen enhances pregnancy rates in the sheep. Theriogenology. 126:28–35.
- Rabassa VR, Tabeleão VC, Pfeifer LFM, Schneider, Ziguer EA, Schossler E. 2007. Efeito das tecnicas transcervical e laparoscopica sobre a taxa de prenhez de ovelhas inseminadas em tempo-fixo. Cien Anim Bras. 8(1):127–133.
- Salamon S and, Maxwell WM. 1995. Frozen storage of ram semen I. Processing, freezing, thawing and fertility after cervical insemination. Anim Reprod Sci. 37( 3–4):185–249.
- Salamon SE, Maxwell WMC. 1995. Frozen storage of ram semen: II. Causes of low fertility after cervical insemination and methods of improvement. Anim Reprod Sci. 38( 1–2):1–36.
- Santos-Neto PC, García-Pintos C, Pinczak A, Menchaca A. 2015. Fertility obtained with different progestogen intravaginal devices using Short-term protocol for fixed-time artificial insemination (FTAI) in sheep. Livest Sci. 182:125–128.
- Sayre BL, Lewis GS. 1996. Cervical dilation with exogenous oxytocin does not affect sperm movement into the oviducts in ewes. Theriogenology. 45( 8):1523–1533.
- Sylla L, Pistolesi A, Biancucci A, Palombi C. 2014. Laparotomic intrauterine insemination with frozen semen in the ewe: preliminary results. LXVIII SISVet Congress, Pisa 16–18th June, p. 340.
- Wulster-Radcliffe MC, Wang S, Lewis GS. 2004. Transcervical artificial insemination in sheep: effects of a new transcervical artificial insemination instrument and traversing the cervix on pregnancy and lambing rates. Theriogenology. 62( 6):990–1002.
