Abstract
The search for host isolated probiotic bacteria from animal intestine may discover new probiotic candidates with promising health properties. This study evaluated the safety and functional probiotic potential of the lactic acid bacteria (LAB) isolated from the Iranian native ruminants intestine under in vitro assays. The isolates were selected according to criteria including survivability in low pH, bile salts, pancreatic enzymes, different temperatures, NaCl concentrations, antibacterial activities, presence of adhesion genes and safety characteristics. The selected LAB were then identified to species level using 16S rRNA gene sequencing. The results showed that out of one hundred and eighty-seven LAB isolates, only six strains (NABRII50, NABRII51, NABRII52, NABRII53, NABRII54 and NABRII55) were tolerant to low pH, bile salt, pancreatin enzyme, 45 °C temperature and 2% sodium chloride. The six selected isolates belonged to Lactobacillus mucosae. Two of the adhesion genes (mub and map) were detected in all strains except NABRII53. The virulence factors were observed in NABRII50, NABRII53 and NABRII55. The tetracycline resistance gene (tet (S)) was detected in NABRII55. This study was the first effort to select Lb. mucosae strains with the probiotic potential from the Iranian ruminants intestine. These results revealed that the ruminant intestinal ecosystem could be considered as a valuable origin of probiotic candidates and all the selected LAB strains except NABRII50, NABRII53 and NABRII55 could be considered as promising probiotics.
Lactobacillus mucosae strains isolated from Iranian native ruminants intestine including NABRII51, NABRII52 and NABRII54 showed probiotic potential under in vitro assays.
The Lb. mucosae strains including NABRII51, NABRII52 and NABRII54 were able to survive in intestinal physiological conditions, and carried the adhesion genes, such as mub and map.
In vitro biosafety assays confirmed that Lb. mucosae strains (NABRII51, NABRII52 and NABRII54) were safe to further studies.
HIGHLIGHTS
Introduction
Currently, there is a great interest in probiotics application for health and improve livestock performance (Li et al. Citation2020). The application of probiotic products can improve immune function, digestion and feed efficiency. Lactic acid bacteria (LAB) have been used as probiotics to modulate the composition of intestinal microbiota to promote the health of the host intestine (de Moraes et al. Citation2017). LAB isolates have been isolated from a variety of sources, including fermented and dairy products, plants, soil and various organs of poultry, cattle or fish (Kuppusamy et al. Citation2020). In addition, large amounts of LAB with vital function have been found in the ruminant’s digestive tract (Timmerman et al. Citation2006).
Based on the selection criteria for probiotic strains, a bacterial strain should be able to withstand low gastric pH and bile salts of the intestine, adhere to the intestinal mucosa and exceed safety criteria, such as the absence of haemolytic activity and sensitivity to antimicrobials of human and veterinary importance (Musikasang et al. Citation2009; Iñiguez-Palomares et al. Citation2011). Besides, resistance to osmolytes and temperature is considered as notable features that lead to the successful performance of probiotic bacteria (Salas-Jara et al. Citation2016; Aleksandrzak-Piekarczyk et al. 2019) and those candidates which meet the established criteria for probiotic can be used to produce probiotic supplements.
The genus Lactobacillus is one of the beneficial LAB. Most of the studies have shown that Lactobacillus sp. are able to survive in harsh conditions of the gastrointestinal tract (GIT). The organic acids produced by LAB form an acidic environment that can inhibit the viability of pathogenic bacteria (Dunne et al. Citation2001; Bernardeau et al. Citation2008). Among LAB species, Lactobacillus mucosae is one of the highly mucosa-associated subpopulations closely related to the animal and human intestine and other mucosal niches (Etzold et al. Citation2014; Drobná et al. Citation2017). The results of various studies have shown that Lb. mucosae strains can promote host resistance against pathogens and improves mucosal immunity by increasing epithelial impermeability and barrier function, producing secondary metabolites and antimicrobial compounds (Pajarillo et al. Citation2017).
The indigenous livestock population of Iran is the unique reserves adapted to the various climatic conditions of this vast country. Adaptation to different climates has led to developing a specific microbial community in the GIT of ruminants (Ebrahimi et al. Citation2018; Naeemi et al. Citation2019). Given these points, this study aimed to investigate the functional probiotic properties and safety characteristics of autochthonous Lb. mucosae strains from the duodenal mucosal layer of native goat, sheep and cattle from west and north areas of Iran, using molecular and microbial approaches.
Material and methods
Initial isolation, purification and phenotypic characterisation
The LAB colonies were isolated from duodenum mucosal layer suspensions of cows (n = 3), sheeps (n = 3) and goats (n = 3) belonging to the North Providence, Guilan, and the west providences, Kermanshah and Hamedan of Iran. The samples were received from a livestock slaughterhouse located in the north of Iran (Deylam Sanat Shargh Livestock Industrial Slaughterhouse CO-OP CO (37°08′37.4′′N 49°38′38.1′′E)). The experimental protocols were carried out according to the principles of the Declaration of Helsinki (World Medical Association [WMA] 2008). The mucosal layer suspensions were spread on de Man, Rogosa and Sharpe (MRS) agar medium (Merck, Darmstadt, Germany), which were supplemented with 0.1% (w/v) L-cysteine (Merck, Darmstadt, Germany). After incubation at 37 °C for 48 h, the selected colonies were identified by Gram stain and catalase activity tests. The Gram-positive and catalase-negative isolates were stored in the MRS broth containing 10% (w/v) skimmed milk, and 60% (1:1) glycerol at −80 °C for further investigations.
Resistance to different acidic conditions, bile salts and pancreatin
Rapid preliminary screening for the acid tolerance of the LAB isolates was performed according to the method described by Ehrmann et al. (Citation2002) with some modifications. Acid tolerance assay of each selected isolate from previous step was performed according to the method described by Yamazaki et al. (Citation2012) and Grispoldi et al. (Citation2020). Tolerance to the acidic conditions was determined by comparing number of bacterial colonies before (in neutralised PBS, as control) and after exposure to acidic conditions including pH = 2.5 and pH = 3 adjusted by 1 N HCl for 3 h.
The bacterial resistance to bile salts was performed according to the method described by Kumar and Kumar (Citation2015) through measuring suspensions optical density at 630 nm (OD630) before and after incubation in MRS broth containing 0.3% (w/v) Oxgall for 8 h. The suspensions without Oxgall were considered as control. At last, the LAB isolates which showed resistance to 0.3% (w/v) Oxgall more than 50% were considered as bile-resistant.
For evaluation of the bacterial cell resistance to pancreatin, 10 μL of each selected isolates suspension was inoculated into 170 μL MRS broth supplemented with 1.9 mg/mL of pancreatin (Sigma-Aldrich, St. Louis, MO), adjusted to pH = 8.0. Also, MRS broth (pH = 8.0) without pancreatin was considered as control. After incubating at 37 °C for 3 h, OD630 was recorded and the result was expressed based on growth rate (Hosseini et al. Citation2009; Sharifuzzaman et al. Citation2018).
Molecular identification
DNA extraction was performed with the Gram-positive bacteria DNA extraction kit (Cinaclone, Tehran, Iran) . The bacterial 16S ribosomal RNA-based polymerase chain reaction was carried out using universal primers 27f (5′AGAGTTTGATCMTGGCTCAG-3′) and 1492r (5′ TACGGYTACCTTGTTACGACTT-3′) (Plessas et al. Citation2017). After purification, PCR products were sequenced by the automated DNA sequencing system (Macrogen, Seoul, Korea). The sequences were edited by Bioedit software version 7 (Hall Citation1999). A comparison of the sequence similarity was made by the Basic Local Alignment Search Tool (BLAST) of the National Centre of Biotechnology Information (NCBI). CLUSTALW program of the Bioedit software version 7 was used to sequences alignment. A phylogenetic tree was built using the neighbour-joining tree method based on the 16S rRNA gene sequence analysis by MEGA software version 6 (Tamura et al. Citation2013). In the phylogenetic tree construction, nucleotide sequences, including six isolated LAB sequences derived from this study and five sequences of the Lactobacillus species derived from Genbank, were involved. The Lactococcus lactis strain NCDO 604 was used as an out group.
Resistance assay of Lb. mucosae strains under temperature stress and sodium chloride concentrations
The effects of different temperatures and sodium chloride concentrations on bacterial survivability were examined according to the procedure described by Mortezaei et al. (Citation2020) and Aleksandrzak‐Piekarczyk et al. (Citation2019), respectively. During temperature treatments, each selected isolate was exposed to different temperatures (37, 45 and 50 °C) for 48 h and formation of bacterial colonies was evaluated. For salinity resistance test, each selected isolate was inoculated on MRS-agar plates supplemented with or without (control) NaCl (2, 4, 6 or 8% (w/v)). After incubation at the 37 °C for 48 h, presence and quality developed colonies were compared with control plates. The experiments were carried out in triplicate and the results were expressed by qualitative data.
Antimicrobial activity of Lb. mucosae strains
The inhibitory activity of the selected strains against four pathogenic bacteria (Salmonella typhimurium (ATCC 14028), Salmonella enteritidis (ATCC13076), Escherichia coli (o157) and Staphylococcus aureus (ATCC 25923)) was determined by the double agar layer method (Touré et al. Citation2003; Gaudana et al. Citation2010). Briefly, 2 μL of each selected Lactobacillus strain grown overnight culture was spotted onto MRS agar plates. Then plates incubated at 37 °C for 18 h in CO2 incubator (5%). After colony development, the plates were overlaid with soft agar (containing 0.7% (w/v) agar and Trypticase Soy Agar (TSA) kept at 50 °C), seeded with 1% (v/v) of an active overnight culture of each pathogen, and incubated aerobically at 37 °C. After one night of incubation, the growth inhibition zones around LAB colonies were determined. The test was performed in triplicate.
Assessment of adhesion properties of Lb. mucosae strains
The adhesion properties of the selected Lb. mucosae strains were preliminarily determined by Congo red staining. Then, isolates were screened by PCR to investigate the presence of genes encoding adhesion proteins (msa, map, mub and ef-tu).
Congo red staining
The hydrophobicity of the Lb. mucosae strains was determined by Congo red staining (Leyva-Madrigal et al. Citation2011). The bacterial colonies were streaked on MRS agar plates containing 0.03% (w/v) Congo red (Merck, Darmstadt, Germany) and incubated at 37 °C for 24 h anaerobically. Subsequently, red colonies were considered as hydrophobic strains, and white or transparent colonies were considered as non-hydrophobic.
PCR detection of adhesion encoding genes
The selected Lb. mucosae strains were screened for adhesion encoding genes according to the PCR protocol described by de Moraes et al. (Citation2017) after bacterial DNA extraction was done by the Gram-positive bacteria DNA extraction kit (Cinaclone, Iran). The primers were employed for the amplification of msa, map, mub and ef-tu presented in Table . The amplified products were then separated by electrophoresis in 1.0% (w/v) agarose gels.
Table 1. Primer sequences utilised in the investigation of adhesion properties of the selected isolates.
Safety assessments of Lb. mucosae strains
Haemolytic activity
The haemolytic activity of selected isolates was performed on blood agar (Quelab, Montréal, Canada), supplemented with 5% (v/v) of sheep blood. After 48 h incubation at 37 °C in CO2 incubator (0.5%), the plates were then examined for the halo of haemolysis. The bacterial isolates without displaying the signs of β-haemolysis around the colonies were classified as non-haemolytic (without β-haemolysis) (Maragkoudakis et al. Citation2009).
Antibiotic susceptibility
The assay for antibiotic susceptibility of the six Lb. mucosae strains were performed in 96-well plates using the broth-microdilution method for the eight antibiotics of human and veterinary importance (ampicillin, clindamycin, gentamicin, streptomycin, tetracycline, erythromycin, kanamycin and chloramphenicol) according to the European Food Safety Authority (EFSA Citation2012). Antibiotic susceptibility was expressed as the minimum inhibitory concentration (MIC, μg/mL) necessary for inhibition bacteria visible growth and compared with the lactobacilli MIC breakpoint values recommended by EFSA (Citation2012).
PCR detection of genes for tetracycline resistance, virulence factors and biogenic amines production
The presence of tetracycline resistance genes, virulence factors genes, such as gelE (gelatinase), hyl (hyaluronidase), asa1 (aggregation substance), esp (enterococcal surface protein), cylA (cytolysin), efaA (endocarditis antigen), ace (adhesion of collagen) and genes encoding biogenic amines production, such as histidine decarboxylase (hdc1), histidine decarboxylase (hdc2), tyrosine decarboxylase (tdc) and ornithine decarboxylase (odc) were evaluated according to the PCR protocols (Muñoz et al. Citation2014; Perin et al. Citation2014; de Moraes et al. Citation2017). The primers employed for amplifying the encoding genes are presented in Table . Briefly, extracted DNA using the Gram-positive bacteria DNA extraction kit was used for the PCR amplification according to the PCR protocol described by Muñoz et al. (Citation2014). The amplified products were then separated by electrophoresis in 0.8 to 2.0% (w/v) agarose gels in 0.5× TAE buffer.
Table 2. Primer sequences utilised in the investigation of genes encoding for tetracycline resistance, virulence factors and biogenic amines.
Statistical analysis
The data were expressed as the mean values (or log values) ± standard error (SE). All in vitro assays were performed in triplicate. p Values of less than .05 were considered statistically significant. Significant differences between means were determined by Duncan’s multiple range tests after analysis of variance (ANOVA) with SPSS version 16.0 software (SPSS Inc., Chicago, IL).
Results and discussion
Bacterial initial isolation and phenotypic characterisation
The bacterial isolation and phenotypic characterisation resulted in isolation of one hundred and eighty-seven Gram-positive, non-spore-forming, catalase-negative and non-motile bacteria from the duodenum mucosal layer of native Iranian cows, sheep and goats. The LAB colonies were rod-shaped or coccoid form and the cells were shown in pairs or short chains.
Survival assays under acidic conditions, bile salts and pancreatin resistance
Based on the previous studies, in vitro assessments, such as acid, bile and pancreatic enzymes tolerance, and survey some physiological characteristics (such as optimum growth temperature and salt sensitivity) have been considered as a good indicator to evaluate the probiotic properties of a bacterial isolates (Kim et al. Citation2019; Kuppusamy et al. Citation2020). After preliminary growth assays under acidic condition, seventy LAB isolates exhibited good survivability to pH = 3 (data not shown).
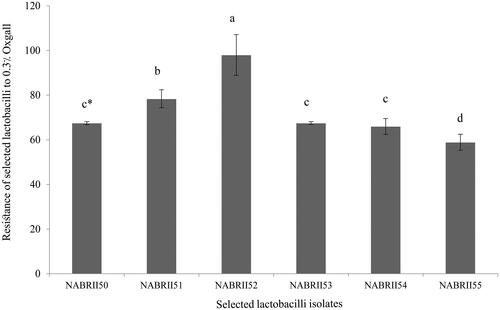
In the small intestine, the presence of bile is the most crucial stress agent for ingested bacteria. According to the results, the six selected lactobacilli isolates showed an ability to grow over 50% at 0.3% Oxgall (Figure ). Among them, NABRII52 and NABRII55 showed significantly higher (97.97%) and lower (58.88%) ability than other isolates in this regard, respectively (p < .05). 0.3% bile salt concentration is a critical concentration for evaluating the ability of LAB to tolerance bile salts, and those with resistance more than 50% at this range considered as bile resistant isolates (Sahadeva et al. Citation2011; Kumar and Kumar Citation2015).
Figure 1. Bile tolerance for six selected lactobacilli exposure to 0.3% Oxgall. Data were presented as means ± SE, in three replicates. The lowercase letters show significant differences between values after 8 h (p < .05).
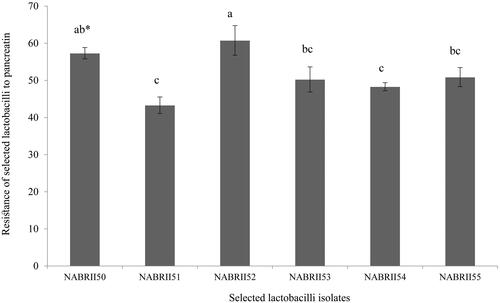
In the ruminants, pH values can vary between 5.7 and 7.3 in the rumen and 2–3 in the abomasum (Gentile et al. Citation2004). The secretion of hydrochloric acid by the gastric cells is an important defence mechanism to protect the host body against the ingested pathogens (Smith Citation2003). The results of acid tolerance for all six Lactobacillus strains are shown in Table . All of the selected isolates showed a significant reduction in pH = 2.5 than control (0 h) (p < .05), But their survival rate remained more than 90% after exposure to pH = 2.5 for 3 h. In addition, all isolates’ survivability in pH = 3 was similar to the control (0 h) expect NABRII55 (p < .05). Jensen et al. (Citation2012) observed a reduction in cell viability of Lb. reuteri strains when incubated at pH = 3 for 3 h. Similarly, de Moraes et al. (Citation2017) reported a reduction in the initial viability of Lb. mucosae strains population, after gastric simulation at pH = 2.5 for 3 h. Generally, the selected LAB showed different levels of resistance to acid and bile salt which probably could be due to the strain-dependent mechanisms (Li et al. Citation2020).
Table 3. Viability of six selected lactobacilli (log cfu/mL) after 3, h exposure to pH 2.5 and 3 after 3, h compared with control (0, h).
Furthermore, all six selected Lactobacillus isolates exhibited strong tolerance to pancreatic enzymes (Figure ). Among them, NABRII52 showed significantly higher tolerance to 1.9 mg/mL of pancreatin (60.78%) after 3 h exposure in comparison to the other selected isolates (p < .05) except NABRII50. The results of tolerance to pancreatin are in agreement with the results obtained by Maragkoudakis et al. (Citation2009) and Mahmoudi et al. (Citation2016).
Molecular identification using 16S rRNA gene sequence
The results of comparative 16S rRNA gene analysis showed that the six selected LAB belonged to the genus Lactobacillus and were 99–100% similar to Lb. mucosae. The 16S rRNA gene sequences of the six Lb. mucosae strains were deposited in the GenBank database under the accession numbers MH595979.1 to MH595980.1 for isolates NABRII50 to NABRII55, respectively (Table ). In this study, a phylogenetic tree (Figure ) depicts the phylogenetic relationships between the six Lb. mucosae strains and five type strains obtained from the Genbank based on 16S rRNA gene sequence analysis. Lc. lactis (NCDO 604) was used as the outgroup. The phylogenetic tree depicted that the six Lb. mucosae strains grouped into one leading group.
Figure 3. Phylogenetic tree based on the neighbour-joining method of 16S rRNA gene sequences. Bootstrap values above 50% are indicated at the nodes of the tree. The scale bar represents 0.02-nucleotide substitutes per position.
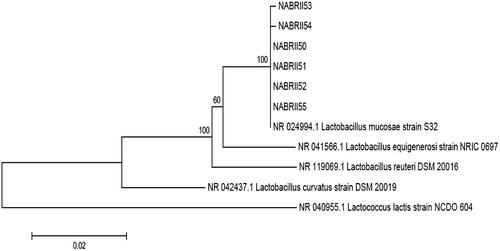
Table 4. Molecular identification of six selected isolates.
The Lb. mucosae was first isolated from the intestine of piglets as a new species (Iñiguez-Palomares et al. Citation2007). To our knowledge, the present research is the first report on the isolation of Lb. mucosae strains from the duodenum of Iranian sheep, goat and cow. Taxonomic studies have shown a close relation between Lb. mucosae and Lb. reuteri (Wang et al. Citation2016).
Resistance to temperatures and sodium chloride
Temperature also plays a vital role in LAB growth (Yang et al. Citation2018). The optimum growth temperature of lactobacilli lies between 30 and 40 °C, but depending on their species; they can grow at different temperatures from 5 to 53 °C (Ahmed et al. Citation2006). This study also revealed that a temperature of 50 °C has a negative effect on the survivability of the isolated Lb. mucosae strains, and their respective optimum temperatures were 37 and 45 °C (Table ). Also, osmotic stress may be a significant inhibitor of bacterial growth and causes structural and functional damage to strains (Ge et al. Citation2011; Zhang et al. Citation2014). Furthermore, the Lb. mucosae strains in this study were salt sensitive and could not grow at more than 2% salt concentration (Table ). Silva et al. (Citation2019) found that Lb. reuteri strains had different growth rates towards 6% NaCl, while Lb. mucosae CRL 1508 was not resistant to the same NaCl concentration.
Table 5. The six selected isolates resistance to temperatures and sodium chloride.
Antimicrobial activity of the selected Lb. mucosae strains
The Antimicrobial activity of the selected Lb. mucosae strains were evaluated using various Gram-positive (S. aureus) and Gram-negative (S. typhimurium, S. enteritidis, and E. coli) pathogenic bacteria (Table ). Results of this study showed that NABRII52 and NABRII53 significantly inhibited the growth of the S. typhimurium (ATCC 14028) and S. enteritidis, respectively (p < .05). Our results are consistent with the results of the study by Bian et al. (Citation2011), which reported that Lb. reuteri DPC16 cell-free supernatants significantly inhibited the growth of selected Gram-negative food-borne pathogens (S. Typhimurium and E. coli) compared to Gram-positive pathogens (Listeria monocytogenes and S. aureus). Some previous studies have suggested that the production of bacteriocin-like metabolites by Lb. mucosae strain may be the reason (Maldonado et al. Citation2018).
Table 6. Antimicrobial activity of six selected lactobacilli.
Adhesion properties of the six selected Lb. mucosae strains
Adhesive ability and effective colonisation in GIT is a desirable feature for probiotic bacteria because it can inhibit pathogens’ growth in the lumen through competitive exclusion (London et al. Citation2014). Various mechanisms, such as the presence of some adhesins, fimbriae, pili or cell surface proteins may be related to these phenomena (Devi and Halami Citation2017).
The hydrophobic nature of the outer surface may also play a role in the binding of bacteria to the host tissue. The evaluation hydrophobicity test using Congo red stain can confirm this nature (Leyva-Madrigal et al. Citation2011). A positive result indicates that the bacteria would not repel from the intestinal epithelium and have the ability to bind non-specifically to the intestinal epithelium by hydrophobic interactions (Leyva-Madrigal et al. Citation2011). In this study, all the Lb. mucosae strains had hydrophobic structures in the cell wall.
Previous studies have shown that mucus-targeting proteins or mucus-binding proteins (mub) and mucus adhesion-promoting protein (map), which were well characterised among Lactobacillus species, mediate the adherence of them to the intestinal mucosal layer (Buck et al. Citation2005; Devi and Halami Citation2017; Chatterjee et al. Citation2018). The presence of these genes strengthens the probiotic potential and mucus-binding ability of Lb. mucosae (Roos et al. Citation2000). Previously, it has been reported that this species usually carries the mub gene and can attach to the intestinal mucosal layer with this feature (Roos et al. Citation2000). The mub and map genes encode for extracellular mucus-binding proteins (mub) and a mucous adhesion-promoting protein (map), respectively (Buck et al. Citation2005). The presence of mub and map genes in the studied Lb. mucosae strains strengthens the probiotic potential of these strains. In this study, all the isolated Lb. mucosae strains except Lb. mucosae NABRII53, carried both genes (Table ).
Table 7. Presence of genes associated with adhesion properties in six selected lactobacilli.
Safety assessments of the six selected Lb. mucosae strains
Haemolytic activity
The absence of haemolytic activity is one of the safety issues to select new potential probiotic strains because it indicates that the isolated bacteria do not have one of the virulence factors and potential possible negative effect on humans and animals (De Vuyst et al. Citation2003). The isolated strains displaying haemolytic activity (β or α-haemolysis) can produce toxins that induce erythrocyte lysis. The results of the haemolytic activity in this study showed no harmful effects under in vitro assays. Our results confirm the findings of Repally et al. (Citation2018) and Adetoye et al. (Citation2018), which reported that other Lb. mucosae strains isolated from sheep milk and cattle faeces had no haemolytic activity.
Antibiotics susceptibility
Acquired antibiotic resistance remains a serious concern due to the high risk of horizontal spread of resistance genes when a viable microorganism is used as an active agent in probiotic supplements (EFSA Citation2012; Frieri et al. Citation2017). According to the EFSA (Citation2012), when a bacterial strain is inhibited by a concentration of a specific antimicrobial agent equal to or lower than the specified cut-off values, considered as a susceptible strain and no further antibiotic resistance studies are required (EFSA Citation2012). In this study, we investigated the antibiotic susceptibility profiles of the six Lb. mucosae strains from the Iranian native cows, sheep and goats. The results of the antibiotic susceptibility profiles showed that all Lb. mucosae strains were sensitive to clinically relevant antibiotics such as ampicillin, gentamicin, kanamycin, streptomycin, erythromycin, clindamycin and chloramphenicol. However, only Lb. mucosae NABRII53 and Lb. mucosae NABRII55 were phenotypically resistant to tetracycline (MIC > 8 μg/mL) (Table ).
Table 8. Antibiotic susceptibility of six selected Lb. mucosae strains (MIC, µg/mL).
PCR detection of genes encoding tetracycline resistance
The tetracycline-resistant strains were screened by PCR for the presence of tetracycline resistance genes (tet (L), tet (M), tet (O), tet (S) and tet (w)) for identifying the resistance determinants responsible for the tetracycline resistance (Table ). PCR analysis showed that Lb. mucosae NABRII55 carried the tet (S) and neither the genes encoding ribosomal protection proteins tet (M), tet (O), tet (S) or tet (W) nor gene encoding the tetracycline efflux pump tet (L) were detected in Lb. mucosae NABRII53. However, the absence of resistance determinants tet (M), tet (O), tet (S), tet (W), tet (L) and tet (K) may suggest a new mechanism of resistance which can be due either to acquired genes or to the mutation of indigenous genes (EFSA Citation2012). Energy-dependent efflux of tetracycline from the cell, ribosome protection and enzymatic inactivation are three mechanisms of tetracycline resistance in microorganisms (Schaechter and Lederberg Citation2004). Studies have shown that genes conferring resistance to tetracycline are commonly found in the human gut microbiota, both in healthy adults and in breast-fed infants (Gueimonde et al. Citation2006). Similarly, these genes have been found in several lactobacilli isolated from dairy foods and it is vital to avoid their spread to pathogens through the consumption of fermented foods (Muñoz et al. Citation2014). Similar to this study, the absence of the resistance genes tet (M), tet (O), tet (S), tet (W), tet (L) and tet (K) was reported in Lc. Pseudomesenteroides from fermented table olive (Muñoz et al. Citation2014). Overall, lactobacillus resistance to antimicrobials is a relevant scientific topic and one of the crucial properties for identifying safety potential probiotics.
Table 9. Presence of genes associated with tetracycline resistance in Lb. mucosae strains.
PCR detection of genes encoding virulence factors
Investigating the presence of virulence genes is another criterion for assessing the probiotic candidates’ safety with potential applications in food products. The virulence factors are usually associated with competitive advantages of pathogenic strains, and their presence is more common in Enterococcus spp. and other clinical isolates (Semedo et al. Citation2003). The virulence factors usually locate in transferable plasmids. Therefore, due to the concern of transferring the mentioned genetic elements to the intestinal tract’s pathogens, the detection of these genetic elements is inevitable (Eaton and Gasson Citation2001).
The gelE, hyl, asa1, esp, cylA, efaA and ace genes, which encode virulence factors, were screened in the genome of the Lb. mucosae strains (Table ).
Table 10. Presence of genes associated with virulence factors in Lb. mucosae strains.
LAB species, such as Lactococcus spp. can carry different virulence genes, but their presence in the genome is not a definitive indicator of these species’ pathogenesis because of the low capability of expressing these genes, which has been observed in different studies (Casalta and Montel Citation2008; Perin et al. Citation2014).
In this study, the Lb. mucosae NABRII53 and Lb. mucosae NABRII55 strains generated positive PCR results for the ace gene (adhesion of collagen protein). This protein facilitates the binding to collagen and may play a negative role during human infections (Girish and Kemparaju Citation2007); however, on the positive side, it can contribute to better adhesion and colonisation in the GIT (Todorov et al. Citation2017). dos Santos et al. (Citation2015) observed positive results for the ace gene in the studied Lb. rhamnosus and Lb. plantarum strains. Furthermore, two of the investigated lactobacilli strains in this study, including Lb. mucosae NABRII53 and Lb. mucosae NABRII50, similar to the study conducted by de Moraes et al. (Citation2017), carried the cytolysin gene. The cytolysin (cylA) is related to haemolytic activity among enterococci (Jiménez et al. Citation2013). Despite the presence of cytolysin gene, these strains (Lb. mucosae NABRII53 and Lb. mucosae NABRII50) were not able to exhibit haemolytic activity under in vitro assays. The cytolysin is a virulent substance due to its haemolytic potential, but it is also considered as an antibacterial bacteriocin, according to Cotter et al. (Citation2005) classification. The presence of cylA is not enough to activate haemolytic activity by bacteria because the cytolysin expression requires the presence and functionality of eight genes (Perin et al. Citation2014). Additionally, Lb. mucosae NABRII53 showed positive results for the hyl gene. The hyl gene is involved in the production of hyaluronidase enzymes, which break down hyaluronic acid. The ability of bacteria to degrade hyaluronic acid may be a virulence factor and allow hyaluronidase-producing pathogens to penetrate hyaluronic acid-rich tissues (Aubin et al. Citation2017). However, the consequences of hyaluronidase activity among lactobacilli are not clear, as this has not been reported within the context of virulence and pathogenicity yet (Franz et al. Citation2005). The gelE gene, which encodes for the production of Gelatinase, was detected in Lb. mucosae NABRII53. The gelE is commonly found in E. faecalis (Munoz-Atienza et al. Citation2013). However, the presence of gelE gene is not enough for gelatinase activity since the complete fsr operon seems to be essential for its expression (Lopes et al. Citation2006).
Furthermore, the esp and asa1 genes were detected in Lb. mucosae NABRII53 strain. These genes are relevant virulence factor, which contributes to intestinal adhesion through encodes the production of extracellular surface protein (Valenzuela et al. Citation2009; de Moraes et al. Citation2017). According to the results of this study, due to the presence of virulence genes in Lb. mucosae NABRII53, Lb. mucosae NABRII50 and Lb. mucosae NABRII55 these isolates are not recommended for food applications.
PCR detection of genes encoding biogenic amines
Examining the presence of genes encoding biogenic amines is another aspect of assessing the probiotic candidates’ safety because their products could cause health problems. The hdc1, hdc2, tdc and odc genes that are involved in biogenic amine production were searched in the genome of the Lb. mucosae strains (Table ). None of the genes associated with the production of histidine decarboxylase, tyrosine decarboxylase and ornithine decarboxylase was detected in the selected strains. In this study, only hdc1gene was detected in NABRII55.
Table 11. Presence of genes associated with biogenic amines production in Lb. mucosae strains.
Biogenic amines production is an intrinsic property (Franz et al. Citation2005; Lorenzo et al. Citation2010). Lactobacillus strains are usually considered safe organisms in this respect (Arena et al. Citation2002). de Moraes et al. (Citation2017) found none of the genes associated with the production of histamine and cadaverine in the genome of the Lb. mucosae strains. Martín et al. (Citation2005) reported that Lb. gasseri and Lb. fermentum cannot produce biogenic amines. However, the results of the biogenic amines production gene showed that Lb. mucosae NABRII55 isolated from cow carries the hdc1 gene. The formation of biogenic amine from histidine is responsible for allergic reactions. Histamine is formed by the histidine decarboxylation activity of Gram-negative enteric bacteria. Furthermore, it is mainly produced by Gram-positive LAB in some fermented products. dos Santos et al. (Citation2015) detected the hdc1 gene in the genome of the All Lb. rhamnosus strains isolated from Artisanal Coalho cheeses except for Lb. rhamnosus EM1107. Therefore, all examined isolates in this study except for Lb. mucosae NABRII55 are safe in this regard and could be used as starter cultures or other food supplements.
Conclusions
In conclusion, the results of this study indicate that all the Lb. mucosae strains identified from native ruminant intestine of Iran except the Lb. mucosae NABRII50, Lb. mucosae NABRII53 and Lb. mucosae NABRII55 strains were considered as safe for antibiotic resistance, carried virulence factor, and biogenic amine genes. Moreover, they have probiotic properties including ability to survive under simulated gastric conditions, inhibiting bacterial pathogens, harbouring genes related to intestinal adhesion properties under in vitro assessment. Thus, the three Lactobacillus strains could be considered as candidate probiotic strains and should be further studied for their health benefits.
Ethical approval
The protocol followed in this study was approved by the the Ethics Committee recommendations.
Acknowledgements
The authors thank Agricultural Biotechnology Research Institute of Iran (ABRII), for funding of this study.
Disclosure statement
The authors are responsible for the content of this article and declare that there is no conflict of interest associated with the article.
Additional information
Funding
References
- Adetoye A, Pinloche E, Adeniyi BA, Ayeni FA. 2018. Characterization and anti-salmonella activities of lactic acid bacteria isolated from cattle faeces. BMC Microbiol. 18(1):96–106.
- Ahmed T, Kanwal R, Ayub N. 2006. Influence of temperature on growth pattern of Lactococcus lactis, Streptococcus cremoris and Lactobacillus acidophilus isolated from camel milk. Biotechnology. 5(4):481–486.
- Aleksandrzak‐Piekarczyk T, Puzia W, Żylińska J, Cieśla J, Gulewicz KA, Bardowski JK, Górecki RK. 2019. Potential of Lactobacillus plantarum IBB3036 and Lactobacillus salivarius IBB3154 to persistence in chicken after in ovo delivery. Microbiol Open. 8:e620.
- Arena ME, de Nadra MCM, Muñoz R. 2002. The arginine deiminase pathway in the wine lactic acid bacterium Lactobacillus hilgardii X1B: structural and functional study of the arcABC genes. Gene. 301(1–2):61–66.
- Aubin GG, Lavigne JP, Foucher Y, Dellière S, Lepelletier D, Gouin F, Corvec S. 2017. Tropism and virulence of Cutibacterium (formerly Propionibacterium) acnes involved in implant-associated infection. Anaerobe. 47:73–78.
- Bernardeau M, Vernoux JP, Henri-Dubernet S, Gueguen M. 2008. Safety assessment of dairy microorganisms: the Lactobacillus genus. Int J Food Microbiol. 126(3):278–285.
- Bian L, Molan AL, Maddox I, Shu Q. 2011. Antimicrobial activity of Lactobacillus reuteri DPC16 supernatants against selected food borne pathogens. World J Microbiol Biotechnol. 27(4):991–998.
- Buck BL, Altermann E, Svingerud T, Klaenhammer TR. 2005. Functional analysis of putative adhesion factors in Lactobacillus acidophilus NCFM. Appl Environ Microbiol. 71(12):8344–8351.
- Casalta E, Montel MC. 2008. Safety assessment of dairy microorganisms: the Lactococcus genus. Int J Food Microbiol. 126(3):271–273.
- Chatterjee M, Pushkaran AC, Vasudevan AK, Menon KKN, Biswas R, Mohan CG. 2018. Understanding the adhesion mechanism of a mucin binding domain from Lactobacillus fermentum and its role in enteropathogen exclusion. Int J Biol Macromol. 110:598–607.
- Cotter PD, Hill C, Ross RP. 2005. Bacteriocins: developing innate immunity for food. Nat Rev Microbiol. 3(10):777–788.
- de Moraes GMD, de Abreu LR, do Egito AS, Salles HO, da Silva LMF, Nero LA, Todorov SD, Dos Santos KMO. 2017. Functional properties of Lactobacillus mucosae strains isolated from Brazilian goat milk. Probiotics Antimicrob Proteins. 9(3):235–245.
- De Vuyst L, Moreno MF, Revets H. 2003. Screening for enterocins and detection of hemolysin and vancomycin resistance in enterococci of different origins. Int J Food Microbiol. 84(3):299–318.
- Devi SM, Halami PM. 2017. Diversity and evolutionary aspects of mucin binding (MucBP) domain repeats among Lactobacillus plantarum group strains through comparative genetic analysis. Syst Appl Microbiol. 40(4):237–244.
- dos Santos KMO, Vieira ADS, Buriti FCA, do Nascimento JCF, de Melo MES, Bruno LM, de Fátima Borges M, Rocha CRC, de Souza Lopes AC, de Melo Franco BDG, et al. 2015. Artisanal Coalho cheeses as source of beneficial Lactobacillus plantarum and Lactobacillus rhamnosus strains. Dairy Sci Technol. 95(2):209–230.
- Drobná E, Rauova D, Majekova H, Greif G, Mikuš P. 2017. Antifungal activity and aflatoxin binding ability of Lactobacillus species isolated from lamb and goatling stomach mucus. J Food Nutr Res. 56:255–264.
- Dunne C, O’Mahony L, Murphy L, Thornton G, Morrissey D, O’Halloran S, Feeney M, Flynn S, Fitzgerald G, Daly C, et al. 2001. In vitro selection criteria for probiotic bacteria of human origin: correlation with in vivo findings. Am J Clin Nutr. 73:386–392.
- Eaton TJ, Gasson MJ. 2001. Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates. Appl Environ Microbiol. 67(4):1628–1635.
- Ebrahimi SH, Valizadeh R, Heidarian Miri V. 2018. Rumen microbial community of Saanen goats adapted to a high-fiber diet in the Northeast of Iran. Iranian J Appl Anim Sci. 8:271–279.
- EFSA. 2012. Panel on additives and products or substances used in animal feed (FEEDAP), guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 10:2740.
- Ehrmann MA, Kurzak P, Bauer J, Vogel RF. 2002. Characterization of lactobacilli towards their use as probiotic adjuncts in poultry. J Appl Microbiol. 92(5):966–975.
- Etzold S, Kober OI, MacKenzie DA, Tailford LE, Gunning P, Walshaw J, Hemmings AM, Juge N. 2014. Structural basis for adaptation of lactobacilli to gastrointestinal mucus. Environ Microbiol. 16(3):888–903.
- Franz CMAP, Hummel A, Holzapfel WH. 2005. Problems related to the safety assessment of lactic acid bacteria starter cultures and probiotics. Mitteil Geb Lebensm Hyg. 96:39–65.
- Frieri M, Kumar K, Boutin A. 2017. Antibiotic resistance. J Infect Public Heal. 10(4):369–378.
- Gaudana SB, Dhanani AS, Bagchi T. 2010. Probiotic attributes of Lactobacillus strains isolated from food and of human origin. Br J Nutr. 103(11):1620–1628.
- Ge XY, Yuan J, Qin H, Zhang WG. 2011. Improvement of L-lactic acid production by osmotic-tolerant mutant of Lactobacillus casei at high temperature. Appl Microbiol Biotechnol. 89(1):73–78.
- Gentile A, Sconza S, Lorenz I, Otranto G, Rademacher G, Famigli‐Bergamini P, Klee W. 2004. lactic acidosis in calves as a consequence of experimentally induced ruminal acidosis. J Vet Med Series A. 51(2):64–70.
- Girish KS, Kemparaju K. 2007. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 80(21):1921–1943.
- Grispoldi L, Giglietti R, Traina G, Cenci-Goga B. 2020. How to assess in vitro probiotic viability and the correct use of neutralizing agents. Front Microbiol. 11:204–209.
- Gueimonde M, Salminen S, Isolauri E. 2006. Presence of specific antibiotic (tet) resistance genes in infant faecal microbiota. FEMS Immunol Med Microbiol. 48(1):21–25.
- Hall TA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser. 41:95–98.
- Hosseini SV, Arlindo S, Böhme K, Fernández‐No C, Calo‐Mata P, Barros‐Velázquez J. 2009. Molecular and probiotic characterization of bacteriocin-producing Enterococcus faecium strains isolated from nonfermented animal foods. J Appl Microbiol. 107(4):1392–1403.
- Iñiguez-Palomares C, Jimenez-Flores R, Vazquez-Moreno L, Ramos-Clamont-Montfort G, Acedo-Felix E. 2011. Protein-carbohydrate interactions between Lactobacillus salivarius and pig mucins. J Anim Sci. 89(10):3125–3131.
- Iñiguez-Palomares C, Pérez-Morales R, Acedo-Félix E. 2007. Evaluation of probiotic properties in Lactobacillus isolated from small intestine of piglets. Rev Latinoam Microbiol. 49:46–54.
- Jensen H, Grimmer S, Naterstad K, Axelsson L. 2012. In vitro testing of commercial and potential probiotic lactic acid bacteria. Int J Food Microbiol. 153(1–2):216–222.
- Jiménez E, Ladero V, Chico I, Maldonado-Barragán A, López M, Martín V, Fernández L, Fernández M, Álvarez MA, Torres C, et al. 2013. Antibiotic resistance, virulence determinants and production of biogenic amines among enterococci from ovine, feline, canine, porcine and human milk. BMC Microbiol. 13:288–300.
- Kim JA, Bayo J, Cha J, Choi YJ, Jung MY, Kim DH, Kim Y. 2019. Investigating the probiotic characteristics of four microbial strains with potential application in feed industry. PLoS One. 14(6):e0218922.
- Kumar A, Kumar D. 2015. Characterization of Lactobacillus isolated from dairy samples for probiotic properties. Anaerobe. 33:117–123.
- Kuppusamy P, Kim D, Soundharrajan I, Park HS, Jung JS, Yang SH, Choi KC. 2020. Low-carbohydrate tolerant LAB strains identified from rumen fluid: investigation of probiotic activity and legume silage fermentation. Microorganisms. 8(7):1044–1058.
- Leyva-Madrigal KY, Luna-González A, Escobedo-Bonilla CM, Fierro-Coronado JA, Maldonado-Mendoza IE. 2011. Screening for potential probiotic bacteria to reduce prevalence of WSSV and IHHNV in white leg shrimp (Litopenaeus vannamei) under experimental conditions. Aquaculture. 322–323:16–22.
- Li M, Wang Y, Cui H, Li Y, Sun Y, Qiu HJ. 2020. Characterization of lactic acid Bacteria isolated from the gastrointestinal tract of a wild boar as potential probiotics. Front Vet Sci. 7:49.
- London L, Price NPJ, Ryan P, Wang L, Auty MAE, Fitzgerald GF, Stanton C, Ross RP. 2014. Characterization of a bovine isolate Lactobacillus mucosae DPC 6426 which produces an exopolysaccharide composed predominantly of mannose residues. J Appl Microbiol. 117(2):509–517.
- Lopes MDFS, Simões AP, Tenreiro R, Marques JJF, Crespo MTB. 2006. Activity and expression of a virulence factor, gelatinase, in dairy enterococci. Int J Food Microbiol. 112(3):208–214.
- Lorenzo JM, Cachaldora A, Fonseca S, Gomez M, Franco I, Carballo J. 2010. Production of biogenic amines “in vitro” in relation to the growth phase by Enterobactericeae species isolated from traditional sausages. Meat Sci. 86(3):684–691.
- Mahmoudi I, Moussa OB, Khaldi TEM, Kebouchi M, Soligot C, Le Roux Y, Hassouna M. 2016. Functional in vitro screening of Lactobacillus strains isolated from Tunisian camel raw milk toward their selection as probiotic. Small Rumin Res. 137:91–98.
- Maldonado NC, Ficoseco CA, Mansilla FI, Melián C, Hébert EM, Vignolo GM, Nader-Macías MEF. 2018. Identification, characterization and selection of autochthonous lactic acid bacteria as probiotic for feedlot cattle. Livest Sci. 212:99–110.
- Maragkoudakis PA, Papadelli M, Georgalaki M, Panayotopoulou EG, Martinez-Gonzalez B, Mentis AF, Petraki K, Sgouras DN, Tsakalidou E. 2009. In vitro and in vivo safety evaluation of the bacteriocin producer Streptococcus macedonicus ACA-DC 198. Int J Food Microbiol. 133(1–2):141–147.
- Martín R, Olivares M, Marín ML, Fernández L, Xaus J, Rodríguez JM. 2005. Probiotic potential of 3 lactobacilli strains isolated from breast milk. J Hum Lact. 21(1):8–17.
- Mortezaei F, Royan M, Allaf Noveirian H, Babakhani A, Alaie Kordghashlaghi H, Balcázar JL. 2020. In vitro assessment of potential probiotic characteristics of indigenous Lactococcus lactis and Weissella oryzae isolates from rainbow trout (Oncorhynchus mykiss Walbaum). J Appl Microbiol. 129(4):1004–1019.
- Muñoz MDCC, Benomar N, Lerma LL, Gálvez A, Abriouel H. 2014. Antibiotic resistance of Lactobacillus pentosus and Leuconostoc pseudomesenteroides isolated from naturally-fermented Aloreña table olives throughout fermentation process. Int J Food Microbiol. 172:110–118.
- Munoz-Atienza E, Gomez-Sala B, Araujo C, Campanero C, Del Campo R, Hernandez P, Herranz C, Cintas LM. 2013. Antimicrobial activity, antibiotic susceptibility and virulence factors of lactic acid bacteria of aquatic origin intended for use as probiotics in aquaculture. BMC Microbiol. 13:15–27.
- Musikasang H, Tani A, H-Kittikun A, Maneerat S. 2009. Probiotic potential of lactic acid bacteria isolated from chicken gastrointestinal digestive tract. World J Microbiol Biotechnol. 25(8):1337–1345.
- Naeemi Z, Koohsari H, Pordeli H. 2019. Isolation of lactic acid bacteria with probiotic potential from bovine colostrum in livestock farms of Ramian Township in located in the north of Iran. Int J Mol Clin Microbiol. 9:1097–1107.
- Pajarillo EAB, Kim SH, Valeriano VD, Lee JY, Kang DK. 2017. Proteomic view of the crosstalk between Lactobacillus mucosae and intestinal epithelial cells in co-culture revealed by Q EXactive-based quantitative proteomics. Front Microbiol. 8:2459.
- Perin LM, Miranda RO, Todorov SD, Franco BDG, Nero LA. 2014. Virulence, antibiotic resistance and biogenic amines of bacteriocinogenic lactococci and enterococci isolated from goat milk. Int J Food Microbiol. 185:121–126.
- Plessas S, Nouska C, Karapetsas A, Kazakos S, Alexopoulos A, Mantzourani I, Chondrou P, Fournomiti M, Galanis A, Bezirtzoglou E. 2017. Isolation, characterization and evaluation of the probiotic potential of a novel Lactobacillus strain isolated from Feta-type cheese. Food Chem. 226:102–108.
- Repally A, Perumal V, Dasari A, Palanichamy E, Venkatesan A. 2018. Isolation, identification of Lactobacillus mucosae AN1 and its antilisterial peptide purification and characterization. Probiotics Antimicrob Proteins. 10(4):775–786.
- Roos S, Karner F, Axelsson L, Jonsson H. 2000. Lactobacillus mucosae sp. nov., a new species with in vitro mucus-binding activity isolated from pig intestine. Int J Syst Evol Microbiol. 50(1):251–258.
- Sahadeva RPK, Leong SF, Chua KH, Tan CH, Chan HY, Tong EV, Wong SYW, Chan HK. 2011. Survival of commercial probiotic strains to pH and bile. Int Food Res J. 18:1515–1522.
- Salas-Jara M, Ilabaca A, Vega M, García A. 2016. Biofilm forming Lactobacillus: new challenges for the development of probiotics. Microorganisms. 4(3):35.
- Schaechter M, Lederberg J. 2004. Antibiotic resistance in bacteria. In: Davies J, Webb V, editors. The desk encyclopedia of microbiology. 1st ed. San Diego (CA): San Diego State University. p. 35–39.
- Semedo T, Santos MA, Lopes MF, Marques JJF, Crespo MT, Tenreiro R. 2003. Virulence factors in food, clinical and reference enterococci: a common trait in the genus? Syst Appl Microbiol. 26(1):13–22.
- Sharifuzzaman SM, Rahman H, Austin DA, Austin B. 2018. Properties of probiotics Kocuria SM1 and Rhodococcus SM2 isolated from fish guts. Probiotics Antimicrob Proteins. 10(3):534–542.
- Silva JA, Marchesi A, Wiese B, Nader‐Macias MEF. 2019. Technological characterization of vaginal probiotic lactobacilli: resistance to osmotic stress and strains compatibility. J Appl Microbiol. 127(6):1835–1847.
- Smith JL. 2003. The role of gastric acid in preventing foodborne disease and how bacteria overcome acid conditions. J Food Prot. 66(7):1292–1303.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 30(12):2725–2729.
- Timmerman HM, Veldman A, Van den Elsen E, Rombouts FM, Beynen AC. 2006. Mortality and growth performance of broilers given drinking water supplemented with chicken-specific probiotics. Poult Sci. 85(8):1383–1388.
- Todorov SD, Botes M, Guigas C, Schillinger U, Wiid I, Wachsman MB, Holzapfel WH, Dicks LMT. 2008. Boza, a natural source of probiotic lactic acid bacteria. J Appl Microbiol. 104(2):465–477.
- Todorov SD, Perin LM, Carneiro BM, Rahal P, Holzapfel W, Nero LA. 2017. Safety of Lactobacillus plantarum ST8Sh and its bacteriocin. Probiotics Antimicrob Proteins. 9(3):334–344.
- Touré R, Kheadr E, Lacroix C, Moroni O, Fliss I. 2003. Production of antibacterial substances by bifidobacterial isolates from infant stool active against Listeria monocytogenes. J Appl Microbiol. 95(5):1058–1069.
- Valenzuela AS, Ben Omar N, Abriouel H, López RL, Veljovic K, Cañamero MM, Topisirovic MKL, Gálvez A. 2009. Virulence factors, antibiotic resistance, and bacteriocins in enterococci from artisan foods of animal origin. Food Control. 20(4):381–385.
- Wang Z, Wang L, Chen Z, Ma X, Yang X, Zhang J, Jiang Z. 2016. In vitro evaluation of swine-derived Lactobacillus reuteri: Probiotic Properties and Effects on Intestinal Porcine Epithelial Cells Challenged with Enterotoxigenic Escherichia coli K88. J Microbiol Biotechnol. 26(6):1018–1025.
- WMA. 2008. Ferney-Voltaire (Ain): world medical association: declaration of Helsinki. [accessed 2016 Feb 11]. http://www.wma.net/en/30publications/10policies/b3/index.html.
- Yamazaki M, Ohtsu H, Yakabe Y, Kishima M, Abe H. 2012. In vitro screening of lactobacilli isolated from chicken excreta to control Salmonella Enteritidis and Typhimurium. Br Poult Sci. 53(2):183–189.
- Yang E, Fan L, Yan J, Jiang Y, Doucette C, Fillmore S, Walker B. 2018. Influence of culture media, pH and temperature on growth and bacteriocin production of bacteriocinogenic lactic acid bacteria. AMB Expr. 8(1):10–24.
- Zago M, Fornasari ME, Carminati D, Burns P, Suàrez V, Vinderola G, Reinheimer J, Giraffa G. 2011. Characterization and probiotic potential of Lactobacillus plantarum strains isolated from cheeses. Food Microbiol. 28(5):1033–1040.
- Zhang P, Fang F, Chen YP, Shen Y, Zhang W, Yang JX, Li C, Guo JS, Liu SY, Huang Y, et al. 2014. Composition of EPS fractions from suspended sludge and biofilm and their roles in microbial cell aggregation. Chemosphere. 117:59–65.