 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study was aimed to find out the suitable dose of fresh plantain (Plantago lanceolata L.) supplementation for optimum growth, serum antioxidants status, liver health, and meat quality in broilers. A total of 1152-days-old Cobb-500 broilers (average weight: 45 ± 0.7 g) were randomly assigned into four dietary treatments, including (i) control (CON): corn-soya based basal diet, and plantain (PL) supplemented groups (ii) PL40: CON + 40 g fresh PL/kg diet; (iii) PL80: CON + 80 g fresh PL/kg diet; and (iv) PL120: CON + 120 g fresh PL/kg diet. Improved growth efficiency (p < .05) was observed in PL supplemented groups compared to CON, where PL80 and PL120 groups had the highest value. Serum superoxide dismutase and glutathione peroxidase concentrations were comparable in the PL80 and PL120 groups, but higher (p < .05) than other groups. The lowest concentrations of aspartate aminotransferase and alanine aminotransferase were found in the PL80 group, while alkaline phosphatase was the highest in the PL40 group. Furthermore, the PL80 group exhibited the lowest (p = .001) abdominal fat content and the highest (p = .002) breast meat yield. Meat linoleic acid content was nevertheless improved linearly with PL supplement levels, and the highest value was found in the PL120 group. Furthermore, the maximum meat redness (a*) was observed in PL80 and PL120 groups, which was approximately twice that of the CON. Overall, the growth and health responses of both PL80 and PL120 groups were similar, while the latter had improved the meat fatty acid profile.
Supplementation of 80 g plantain/kg diet showed optimum growth performance, health status, and plasma antioxidants level in broilers.
120 g plantain/kg diet might be supplemented with the purpose of producing value-added broiler meat.
HIGHLIGHTS
Introduction
The use of natural herbs and their extracts in broiler diets has been gaining popularity not only to ensure human health concerns but also for safe and value-added livestock products (Zheng et al. Citation2019). The European Union has banned the use of synthetic antibiotic growth promoters in food animals, which provokes scientists to find out suitable alternatives (Selaledi et al. Citation2020). Plantain (PL; Plantago lanceolata L.), a narrow leaf perennial herb belonging to the family Plantaginaceae has been widely used as a human tonic and forage herb for ruminants and poultry (Camy et al. Citation2020; Redoy et al. Citation2020). It contains a rich phytochemical profile, among them acteoside, aucubin and catalpol have a broad aspect of beneficial effects on animal health and productivity (Yap et al. Citation2019).
Bioactive components in PL exert anti-microbial, anti-oxidative, anti-inflammatory, anti-parasitic effects in the broiler (Ferrazzano et al. Citation2015; Boamah et al. Citation2016; Peña-Espinoza et al. Citation2018; Hammami et al. Citation2020). Besides, PL has higher free radical scavenging activity, which might prevent the meat pigments from oxidation leading to improved meat colour and sensory properties (Redoy et al. Citation2020). Moreover, the rich fatty acid profile in PL might enhance broiler meat nutrition (Bajer et al. Citation2016). However, the overdose of PL supplementation in broiler might carry some negative consequences as it also contains tannin, saponin, and other polyphenolic compounds (Ebrahim et al. Citation2015). The higher level of these components leads to reduce feed intake, nutrients absorption, poisoning, bone formation, even death in the broiler (Ebrahim et al. Citation2015). Chacrabati et al. (Citation2013) reported that a 50 g fresh PL/kg diet could be used in the broiler diet as a replacement for a commercial antioxidant to improve the growth performance. In contrast, Mazhari et al. (Citation2016) found improved growth along with better immunity in broiler fed fresh PL at 100 g/kg diet compared to 50 g/kg diet and control diet.
So, there is a dearth of information pertaining to the safe and optimum level of fresh PL supplementation in the broiler to improve production efficiency and meat quality. Therefore, the objective of this research was to find out the optimum dose of fresh PL supplementation in broiler diet concerning growth, plasma lipid profiles, plasma antioxidants, liver health, and meat quality.
Materials and methods
Experimental birds
A total of 1152 one-day-old straight run broiler chicks (Cobb-500) were purchased from a local hatchery and placed in open-sided poultry shed in Manikganj, Dhaka for a period of 35 days. The chicks (initial body weight: 45 ± 0.7 g) were randomly allocated to four dietary treatments with six replications (48 birds/replication). This experiment was conducted using a completely randomised design.
Experimental diets
The experimental diets included (i) CON = corn-soya based basal diet having crude protein = 23.30% and metabolisable energy = 3081 kcal/kg diet, (ii) PL40 = CON + 40 g fresh plantain/kg diet, (iii) PL80 = CON + 80 g fresh plantain/kg diet, (iv) PL120 = CON + 120 g fresh plantain/kg diet. According to the suggested nutrient requirements for commercial Cobb 500 broiler starter feed, the experimental diet (Table ) was formulated and maintained throughout the feeding trial (COBB Citation2018). Plantain herb (PL) cv. Grasslands Lancelot was cultivated nearby a poultry farm and harvested at the pre-flowering stage. After collection, the fresh PL was chopped properly (approximately 0.5‒1.5 cm) by using a locally made manual chopper and mixed thoroughly. This herb was supplemented from the 4th day of the feeding trial at the rate of 5 g, 10 g and 15 g DM/kg of basal diet in PL40, PL80, PL120 groups, respectively.
Table 1. Ingredients and nutritional composition of experimental diets.
Housing and management
Experimental birds were kept in 24-floor pens (4‒5 cm sawdust bedding) having a living space of 1.08 ft2 for each bird. In the first week, the brooding temperature was kept at 34 °C and thereafter weekly decreased 3 °C until it reached 21 °C. The standard lighting program was maintained according to COBB-500 commercial broiler management guidelines (COBB Citation2018). The feed was supplied at 0800 and 1600 h, and birds always had access to clean and fresh water throughout the experimental period. Feeders were cleaned every week, and drinkers were cleaned twice a day. On days 5 and 12, the experimental birds were vaccinated against new castle disease and infectious bursal disease, respectively. Strict bio-security measures were followed during the whole experimental period.
Record keeping and sample collection
Pen live weights were recorded weekly, and live weight gain (LWG) was calculated from the difference between the initial (ILW) and final live weight (FLW). Feed intake (FI) was calculated by subtracting refusal from the supplied feed. Feed conversion ratio (FCR) was determined from the ratio between FI and LWG. Birds were monitored twice a day; dead birds’ weight was used to adjust for feed consumption. The performance efficiency factor (PEF) was calculated using the equation described by Martins et al. (Citation2016). On days 35, 18 birds from each group (three birds/replication) were slaughtered to collect blood and meat samples. Immediate after blood collection, it was centrifuged at 6000 × g for 15 min for plasma separation and stored at 4 °C (Camy et al. Citation2020). Both breast and thigh meat samples were collected for meat colour analysis, but only breast meat was used for proximate and fatty acid profile analysis.
Sample analysis
All the samples were analysed in triplicate. Proximate components of fresh PL, basal feed, and meat samples were analysed according to AOAC (Citation2005). Exactly 1 g of finely ground PL and basal feed samples were digested in 10 mL of a di-acid mixture (HNO3:HClO4 = 2:1) at 180–200 °C. For calcium determination, 1 mL of aliquot was mixed with dibromo-p-methylsulfonazo solution (2.5 mL), hydrochloric acid (1.5 mL), and distilled water (5 mL). For phosphorus determination, sulphomolybdic acid (4 mL) and stannous chloride solution (6 drops) were added to the aliquot (1 mL), followed by adding distilled water to make the volume 100 mL. Finally, the absorbance was taken at 624 nm wavelength for calcium and 600 nm wavelength for phosphorus in a UV spectrophotometer (T60; PG Instruments, UK) as a method followed by Li and Zhai (Citation2020) and De Silva et al. (Citation2015). In order to determine iron content, PL and basal feed samples (1 g) were digested using HNO3 (10 mL) and H2O2 (2 mL) at 120 °C for 2 h. Then, the iron content was determined using a flame atomic absorption spectrophotometer (AA-7000; SHIMADZU, Japan) at 248.3 nm wavelength, where ferric nitrate solution was used as the standard solution (Marinova and Vladimirova Citation2010). For determination of acteoside, aucubin, and catalpol contents in PL, exactly 250 mg of powdered sample was dissolved into 25 mL of methanol (HPLC grade) followed by shaking and filtration. Thereafter, these components were quantified using ultra-high-performance liquid chromatography (UltiMate™ 3000; Thermo Fisher Scientific, USA). In this chromatography, the mobile phase used for acteoside determination was a water–methanol–acetic acid solution (14:6:1, v/v/v), whereas acetonitrile–water (2:98, v/v) solution was used for catalpol and aucubin determination. The flow rate was 0.8 mL/min. Finally, the acteoside was quantified at 330 nm wavelength, and catalpol and aucubin contents were determined at 204 nm wavelength in a diode array detector (DAD-3000; Thermo Fisher Scientific, USA) according to the method described by Al-Mamun et al. (Citation2008). For serum triglyceride and total cholesterol concentration determination, 10 μL serum sample was mixed with 1000 μL triglycerides reagent (Cat. No. 1155010, Linear Chemicals (LC), Spain) and 1000 μL cholesterol aqueous solution (Cat. No. 1118010; LC, Spain), respectively. After that, both the mixtures were incubated at 37 °C for 5 min. Finally, the concentrations of both triglyceride and total cholesterol were measured at 505 nm wavelength in a bio-analyser (Urit-810; URIT Medical Electronic Group Co., Ltd., Guangxi, China). For high-density lipoprotein (HDL) content, 500 μL serum samples were mixed with 1000 μL precipitating reagent (Cat. No. 1133010, LC, Spain) followed by incubating the mixture at room temperature for 10 min, finally, the concentration was measured at 546 nm wavelength in bio-analyser. The low-density lipoprotein (LDL) and very-low-density lipoprotein (VLDL-C) were determined by using the following equations.
(Lee and Siddiqui Citation2021)
(Lee and Siddiqui Citation2021)
For plasma superoxide dismutase (SOD) determination, 20 μL sample was mixed with 200 μL water-soluble tetrazolium salt solution and 20 μL enzyme working solution. The mixture was kept at 37 °C for 20 min and after that took absorbance at 450 nm using a microplate reader (EL 10 A; BIOBASE, China) as instruction provided in Cat. No. 19160, Sigma-Aldrich, Germany. For glutathione peroxidase (GPx) determination specific ELISA kit (Cat. No. 354102, Sigma-Aldrich, Germany) was used, and exactly 20 μL sample was mixed with 50 μL assay buffer, 50 μL co-substrate mixture and, 50 μL NADPH according to manufacturer instruction. Finally, the absorbance was taken at 356 nm wavelength. Similar to SOD and GPx, catalase was determined using an assay kit according to the manufacturer’s protocol (Cat. No. 100, Sigma-Aldrich, Germany). Meat fatty acid profile was analysed using a gas chromatograph (14B; SHIMADZU, Japan) fitted with a flame ionisation detector as method followed by Redoy et al. (Citation2020). Immediately after slaughtering, meat samples were appropriately cleaned by rinsing the running tape water, and meat colour was determined by using a meat calorimeter (CR-410 colorimeter, Minolta, Japan) according to manufacturer guidelines. Meat colour was expressed by lightness (L*), redness (a*), and yellowness (b*).
Statistical analysis
Raw data were organised using the Microsoft Excel program and then analysed using IBM SPSS software (Version 23.0, IBM Crop., USA). All parameters were subjected to one-way analysis of variance (ANOVA), and data were presented as mean ± standard deviation. The orthogonal polynomial contrast test was performed to obtain linear, quadratic and cubic effects of increasing supplementation rate of PL in the basal diet on each tested parameter. Duncan’s multiple range test (DMRT) was conducted to find the difference among the treatment groups, and the differences at p < .05 were considered statistically significant. The following statistical model was considered:
Where Yij was the overall response of broiler; μ was the overall mean; ti was the effect of PL supplementation (treatment effect); eij was the error due to the jth replication of the ith treatments; data were normally distributed with zero mean and constant variance.
Results
Growth performance
Higher LWG (p = .000) and PEF (p = .000), and lower FCR (p = .000) were observed in the PL supplemented groups compared to the CON group (Table ). With the increasing doses of PL supplementation, LWG was improved but better influenced up to the PL80 group (Figure ). Compared to CON, 2.30‒5.75% lower FCR was found in PL supplemented groups where PL80 and PL120 groups exhibited the lowest value.
Figure 1. Effect of different level of fresh plantain herb (PL; Plantago lanceolata L.) supplementation on live weight gain of broilers at 35 days: (a) linear regression; (b) quadratic regression.
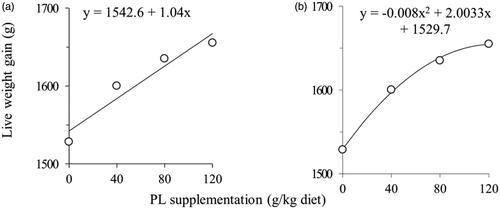
Table 2. Effects of different level of plantain herb (Plantago lanceolata L.) supplementation on performance index of broiler chickens at 35 days.
Plasma lipid profile, antioxidants and liver activity
Based on the result presented in Table , PL had a positive influence (p < .05) on plasma lipid profiles, antioxidants concentration, and liver enzymatic activity. The PL80 group exhibited the lowest (p < .05) triglyceride concentration, whereas HDL and LDL concentrations were similar to the PL120 group but lower (p < .05) than others. Besides, the highest (p < .05) concentrations of SOD and GPx were found in both PL80 and PL120 groups. However, catalase concentration was highest (p < .05) in the PL80 group which was 5% higher than the PL120 group. The AST (p = .002), ALT (p = .000), ALP (p = .005) and AST: ALT (p = .001) were significantly improved in PL supplemented groups and the lowest (p < .05) value was found in both PL80 and PL120 groups.
Table 3. Effect of different level of fresh plantain herb (Plantago lanceolata L.) supplementation on plasma lipid profile, plasma antioxidants and liver enzymatic activity of broilers at 35 days.
Carcase characteristics and meat quality
Compared to CON group, 3‒8% higher dressing percentage, 12‒21% higher breast meat, and 8‒15% lower abdominal fat were found in PL supplemented groups (Table ). Amongst the supplemented groups, both PL80 and PL120 groups exhibited the highest dressing percentage (p = .004) and breast (p = .002) yield, whereas the lowest abdominal fat (p = .001) was recorded in the PL80 group. No difference was found in meat proximate components except ether extract among the treatment groups (p > .05). The lowest meat ether extract was found in both PL80 and PL120 groups compared to PL40 and CON groups. No difference was found in meat saturated and mono-unsaturated fatty acid contents amongst the treatment groups (p > .05). The PL120 group exhibited the highest poly-unsaturated fatty acid, more specifically, higher linoleic acid contents compared to others.
Table 4. Effect of different level of fresh plantain herb (Plantago lanceolata L.) supplementation on carcase characteristics and meat quality of broilers at 35 days.
Meat colour
The addition of PL at different levels in the broiler diet exhibited a positive influence on meat colour (Table ). Antioxidant-rich PL significantly improved the breast and thigh meat colour. In the case of breast meat, both PL80 and PL120 groups exhibited similar L*, a*, and b* values which were better than CON and PL40 groups. The PL supplementation substantially improved (p < .05) the L* and a* values in thigh meat whereas similar (p > .05) L* value was found among the supplemented groups, and the highest (p = .001) a* value was found in both PL80 and PL120 groups compared to others.
Table 5. Effect of different level of fresh plantain herb (Plantago lanceolata L.) supplementation on meat colour of broilers at 35 days.
Discussion
Growth performance
Previous research has demonstrated that supplementation of PL in fresh (Mazhari et al. Citation2016), aqueous extract (Camy et al. Citation2020), or dried powder (Saleheen Citation2020) could improve the growth performance of broiler. In this experiment, fresh PL supplementation improved LWG by 5‒8% compared to the CON group, whereas both the PL80 and PL120 groups exhibited the highest response. Similarly, supplementing broiler diets with Lipia citridora leaves powder (which is high in verbascoside, a compound similar to acteoside in PL) at the rate of 0.5–1.0% maximised growth efficiency, but both the 0.5% and 1.0% doses had a similar response (Mehrparvar et al. Citation2016). On the contrary, Marco et al. (Citation2015) reported that verbascoside (commercial extraction from Lipia citridora) did not influence broiler growth up to 5.0 mg/kg feed. Despite these inconsistent and controversial findings, the improved growth in PL supplemented groups could be attributed to the existence of bioactive components (acteoside, aucubin, and catalpol) that enhanced the broiler’s free radical scavenging activity and thus acted as a natural antioxidant (Chacrabati et al. Citation2013; Boamah et al. Citation2016; Mazhari et al. Citation2016). In addition, flavonoid components in PL induce a lethal chemical interaction with parasite molecules, resulting in a lower parasitic count in the broiler and thus might improve nutrients turnover (Camy et al. Citation2020). However, the dose-related response in growth performance was optimised at the PL80 group (Figure ) and afterward did not carry any significant improvement. One of the reasons that might be responsible for getting optimised growth performance in the PL80 group instead of the PL120 group includes ‒ dietary inclusion of different levels of bioactive components and their interaction. Acteoside in PL is phenylpropanoid glycoside which is effective to alleviate oxidative stress (Marco et al. Citation2015), and aucubin (iridoid glycosides) has a proven effect on immunomodulation by increasing lymphocyte production and interferon-gamma secretion (Venkatalakshmi et al. Citation2016). While the PL120 group obtained the highest concentrations of acteoside and aucubin (4.4 and 2.6 mg/kg DM, respectively), the PL80 group (2.9 and 1.7 mg/kg DM, respectively) also exhibited a similar response in terms of growth efficiency, which might be due to the interaction with other bioactive phytochemicals such as oxalate, tannin, and others. Broilers fed the PL120 diet ingested approximately 1.5-times the amount of oxalate as those fed the PL80 diet (106 vs 71 mg), which could have a detrimental effect on growth efficiency, explaining why the inclusion of higher acteoside and aucubin in the PL120 group failed to improve additional response over the PL80 group (Guil‐Guerrero Citation2001; Zhang et al. Citation2020). Further study is needed to elucidate the antagonistic interaction among the phytochemicals in the broiler.
Plasma metabolites, antioxidants and liver activity
In this experiment, different levels of PL supplementation significantly reduced serum triglycerides in broiler but failed to reduce T-cholesterol content, which was consistent with the previous findings (Mazhari et al. Citation2016; Camy et al. Citation2020). However, some researchers reported that pomegranate pulp (Hosseini-Vashan and Raei-Moghadam Citation2019); garlic powder (Karim et al. Citation2018); turmeric (Mondal et al. Citation2015); thyme and curcumin (Fallah and Mirzaei Citation2016) supplementation in broiler diet reduced plasma T-cholesterol content. On the contrary, Akbari et al. (Citation2016) and Mehrparvar et al. (Citation2016) reported that peppermint and Lipia citridora leaves powder did not affect serum HDL and glucose content in laying hen and broiler, respectively. However, the essential oil extracted from PL successfully exerted the hypocholesteremia effect in mice by reducing the activity of HMG-CoA reductase which is responsible for cholesterol biosynthesis (Najafian et al. Citation2018). But we failed to exert this effect under the current study and the dose of PL supplementation might be a factor. The increased doses of PL oil extract application in mice drastically reduced plasma T-cholesterol levels (Najafian et al. Citation2018), and we found a similar trend but not statistically significant. However, the reduction of triglycerides concentration in PL supplemented groups might be due to the enhanced activity of lipoprotein lipase enzyme which promoted the breakdown of triglycerides into free fatty acids and glycerol (Adiputro et al. Citation2015) and the lowest triglycerides value in the PL80 group might be due to higher serum antioxidant capacity compared to others. As SOD has a negative correlation with triglyceride levels, it aids in the clearance of reactive oxygen species, thus reducing oxidative damage and peroxidation. Triglyceride, a result of lipid peroxidation, decreases as the activity of GPX, SOD and CAT increases (Ma et al. Citation2020). Besides, lower triglycerides concentration in the PL80 group might assist to reduce the VLDL and chylomicron concentration, as both lipoproteins are the transporters of glycerides (Fallah and Mirzaei Citation2016).
The presence of bioactive components in herbs improved serum antioxidants level in the broiler (Cherian et al. Citation2013; Camy et al. Citation2020). Elevated SOD and GPx concentration in both PL80 and PL120 groups might be due to higher consumption of bioactive components, specially acteoside, though it is not clear why the PL120 group exhibited similar results to the PL80 group. Grossly, the acteoside, a polyphenolic component in PL, imparts free radical scavenging activity to the animal body, thereby reducing oxidative stress by increasing serum antioxidant levels (Mazhari et al. Citation2016). Furthermore, PL has a greater capacity for scavenging superoxide anion radicals, which might reduce superoxide anion concentration in broilers, resulting in elevated SOD levels in the supplemented group (Al-Mamun et al. Citation2007). This hypothesis may be supported by the findings of Lien et al. (Citation2008), who reported a similar response in laying hens by dietary flavonoid supplementation. Additionally, the polyphenolic components in ginger powder (Habibi et al. Citation2014), grape pomace (Ebrahimzadeh et al. Citation2018), or Artemisia annua (Wan et al. Citation2016) improved serum antioxidant levels in the broiler. Simultaneously, the uronic acid content of PL is linked to arabinoxylans through an acid group that could be hydrolysed by certain intestinal microbes that possess arabinoxylases, resulting in the formation of arabinoxylan oligosaccharides that act as a prebiotic component (Divani et al. Citation2018). Findings from previous research suggested that prebiotic components exerted antioxidant activity both in vitro and in vivo conditions (Kogan et al. Citation2008; Aluwong et al. Citation2013), supporting the increased serum antioxidant levels in the PL supplemented groups.
Liver enzymatic activity was significantly improved in PL supplemented groups, which was in accordance with Sahoo et al. (Citation2019), who reported similar findings with turmeric and ginger powder in broiler. Previous research illustrated that antioxidant-rich herbs like rosemary leaves (Ahmed et al. Citation2015), neem leaves (Ansari et al. Citation2012), aloe vera (Khan et al. Citation2014) and ajwain seed (Kolbadinejad and Rezaeipour Citation2020) were effective for improving liver enzymatic activity in the broiler. Besides, infusion of PL extract directly to rat blood significantly reduced the serum AST and ALT levels, and the lowest value was found at the infusion rate of 25 mg/kg body weight (Turel et al. Citation2009). In the current study, the lowest ALT and AST concentrations were found in both PL80 and PL120 groups, which indicated that both groups might induce a similar rate of inhibition of the degeneration and necrosis in the liver (Hussan et al. Citation2015). This phenomenon reduces liver enzyme activity by suppressing the reactive oxygen species and cytokine production (Moradi-Ozarlou et al. Citation2020).
Carcase composition and meat quality
Improved dressed and breast meat yield in PL supplemented groups were consistent with the previous findings (Mazhari et al. Citation2016; Camy et al. Citation2020). Besides, supplementation of individual herb or mixture of herbs dry matter at the rate of 0.5‒1.0% in poultry diet significantly reduced abdominal fat and meat fat contents (Narimani-Rad et al. Citation2011; Kusmayadi et al. Citation2019). In broiler, excessive fat is mostly deposited in the abdominal region, and several factors regulate this negative trait, like (i) genetics, (ii) nutrient concentrations in diet, (iii) plasma metabolites level, (iv) dietary inclusion of phytochemicals, etc. Genetics, obviously the prime factor for fat deposition (Fouad and El-Senousey Citation2014) but in this study all the experimental birds were a similar strain, so we can exclude this possibility. Lower energy and protein levels in broiler lead to reduce abdominal fat deposition (Fouad and El-Senousey Citation2014), which contradicts with our findings of reduced abdominal fat contents in PL supplemented groups (Table ). Due to PL supplementation, both energy and protein contents in the diets were increased (<1%), which might not be sufficient to carry to any changes. However, lower plasma LDL and chylomicrons concentrations in PL supplemented groups might reduce the transportation of fatty acid from the liver to adipose tissue (Dubikovskaya et al. Citation2014), which resulted in lower abdominal fat content in the treatment groups. Besides, the lowest plasma triglycerides and LDL contents in the PL80 group might be responsible for getting the lowest abdominal fat content among the treatments. Additionally, polyphenolic compounds have been shown to inhibit dietary fat absorption (Fouad and El-Senousey Citation2014), lipase activity (Kang et al. Citation2012), and/or hepatic lipogenesis (Saha et al. Citation2019) in mouse models, which might be a fact for PL supplemented groups. Along with this, the highest serum antioxidants level in the PL80 group might additionally attribute to get the lowest fat deposition in the abdominal pad.
Fatty acids profiles
Fatty acid composition of broiler meat depends exclusively on dietary manipulation, where (i) fatty acid profile, (ii) antioxidants level, (iii) selenium content of supplied feed contributes significantly. The fresh PL is a good source of poly unsaturated fatty acids (PUFAs), which might help to increase these levels in PL supplemented groups (Mukemre et al. Citation2020). Previous research also illustrated that dietary intervention with PUFAs led to improve these concentrations in broiler meat (Saeed et al. Citation2018; Alagawany et al. Citation2019). The sources of these PUFAs and their dietary levels have a strong influence on lipid metabolism; however, excess inclusion can lead to negative impacts by accelerating lipid oxidation (Wu et al. Citation2019). Besides, phenolic compounds in PL help to exert the anti-oxidative effect, which might stabilise the PUFAs in the intestine and muscle tissues (Brenes et al. Citation2016). Sohaib et al. (Citation2015) reported that PUFAs content in broiler meat changed +4.2% when supplemented by 1% red ginseng (rich in saponin) compared to control. Furthermore, higher serum antioxidant levels improve PUFAs levels in broiler meat either by inhibiting the desaturase enzyme activity or improving the enzyme activity, which converts SFA into PUFA (Chung and Choi Citation2016). Moreover, PL contains selenium, an essential mineral for the anti-oxidative process, ranging from 40 ∼ 50 µg/kg DM, which improved PUFAs content in broiler meat (Puerto et al. Citation2017; Sotek et al. Citation2019). In this experiment, the PL80 group had higher serum antioxidant levels, and the PUFAs content was highest in the PL120 group, which conflicts with the previous argument. The dietary inclusion of PUFAs might be more influential for improving meat PUFAs than other factors (Lee et al. Citation2012). That’s why the PL120 group had a higher value than the PL80 and other groups.
Meat colour
The broiler cannot synthesise meat colour pigments, and the key pigment extracted from the diet sometimes fails to exert the desirable meat colour (Díaz-Gómez et al. Citation2017). Therefore, both synthetic and natural pigments are often used in broiler diets, but natural pigments are mostly safe and eco-friendly (Wang et al. Citation2016). In this experiment, the lower L* value and higher a* and b* values in PL supplemented groups were consistent with the findings of Wang et al. (Citation2016) and Kanani et al. (Citation2017), who reported similar results with marigold extract and cinnamon, respectively. PL contains α-tocopherol and lutein, which might be responsible for increased redness and yellowness of breast and thigh meat in supplemented groups (Elgersma et al. Citation2013). The PL80 and PL120 groups showed similar results, while meat colouration was predicted to improve linearly with the increasing rate of PL supplementation. This phenomenon could be justified in a way that the correlation between the serum antioxidants level and broiler meat colouration (Karadas et al. Citation2016). In the current study, serum antioxidants level was improved consistently up to the PL80 group (Table ) and then eventually did not carry any extra significance for additional supplementation in the PL120 group, which might be reflected in meat colouration. Owing to identical levels of serum antioxidants in both PL80 and PL120 groups, the defense against lipid oxidation, responsible for the formation of brown metmyoglobin from bright red oxygenated myoglobin, might be similar (Salami et al. Citation2015). Besides, elevated serum antioxidant concentrations in these groups might improve mitochondrial stability by (i) reducing the phospholipase A2 activity and/or (ii) by minimising calcium ions leakage, which is directly related to meat discolouration (Pedrão et al. Citation2015; Carvalho et al. Citation2017).
Conclusions
It seems that fresh PL supplementation at the rate of 40 g‒120 g/kg basal diet significantly improved the growth performance, plasma antioxidant concentration, liver enzymatic activity and meat fatty acid profile in the broiler. Both 80 g and 120 g fresh PL supplementation executed similar responses in the aforementioned variables except meat fatty acid profile. The linoleic acid content in meat which is a major interest of consumers was highest at 120 g supplementation. So, it could be concluded that 80 g fresh PL/kg diet could be used for optimum growth performance and health status of broiler provided that poultry entrepreneurs who are interested to produce linoleic acid-enriched meat could supplement up to 120 g PL/kg diet.
Ethical approval
The experimental protocols, bird management and sample collection were reviewed and approved by the Animal Care Committee of Bangladesh Agricultural University Research System, Mymensingh 2202, Bangladesh (BAURES/ESRC/2019/AH/16).
Disclosure statement
The authors declare no conflict of interest. The funding authorities had no role in the experimental design, data collection, interpretation or manuscript writing.
Additional information
Funding
References
- Adiputro DL, Widodo MA, Romdoni R, Sargowo D. 2015. Extract of mangosteen increases high density lipoprotein levels in rats fed high lipid. Universa Med. 32:37–43.
- Ahmed SK, Abdul-Abass MH, Al-Hammed SA. 2015. Testing of the efficacy of dietary natural and synthetic antioxidants on broiler performance: a comparative study. Int J Agri Technol. 11:1075–1088.
- Akbari M, Torki M, Kaviani K. 2016. Single and combined effects of peppermint and thyme essential oils on productive performance, egg quality traits, and blood parameters of laying hens reared under cold stress condition (6.8 ± 3 °C). Int J Biometeorol. 60(3):447–454.
- Alagawany M, Elnesr SS, Farag MR, Abd El-Hack ME, Khafaga AF, Taha AE, Tiwari R, Yatoo MI, Bhatt P, Khurana SK, et al. 2019. Omega-3 and omega-6 fatty acids in poultry nutrition: effect on production performance and health. Animals. 9(8):573–588.
- Al-Mamun M, Abe D, Kofujita H, Tamura Y, Sano H. 2008. Comparison of the bioactive components of the ecotypes and cultivars of plantain (Plantago lanceolata L.) herbs. Anim Sci J. 79(1):83–88.
- Al-Mamun M, Yamaki K, Masumizu T, Nakai Y, Saito K, Sano H, Tamura Y. 2007. Superoxide anion radical scavenging activities of herbs and pastures in northern Japan determined using electron spin resonance spectrometry. Int J Biol Sci. 3(6):349–355.
- Aluwong T, Kawu M, Raji M, Dzenda T, Govwang F, Sinkalu V, Ayo J. 2013. Effect of yeast probiotic on growth, antioxidant enzyme activities and malondialdehyde concentration of broiler chickens. Antioxidants. 2(4):326–339.
- Ansari J, Khan SH, Haq AU, Yousaf M. 2012. Effect of the levels of Azadirachta indica dried leaf meal as phytogenic feed additive on the growth performance and haemato-biochemical parameters in broiler chicks. J Appl Anim Res. 40(4):336–345.
- AOAC. 2005. Official methods of analysis of AOAC International. 18th ed. Arlington (VA): AOAC.
- Bajer T, Janda V, Bajerová P, Kremr D, Eisner A, Ventura K. 2016. Chemical composition of essential oils from Plantago lanceolata L. leaves extracted by hydrodistillation. J Food Sci Technol. 53(3):1576–1584.
- Boamah Ve Agyare C, Odoi H, Dalsgaard A. 2016. Practices and factors influencing the use of antibiotics in selected poultry farms in Ghana. J Antimicrobiol. 2(2):120–128.
- Brenes A, Viveros A, Chamorro S, Arija I. 2016. Use of polyphenol-rich grape by-products in monogastric nutrition: a review. Anim Feed Sci Technol. 211:1–17.
- Camy ML, Redoy MR, Shuvo AA, Ray BC, Rahman MA, Al-Mamun M. 2020. Effect of aqueous herbal extracts on growth, plasma metabolites and meat characteristics of broiler. Bang J Anim Sci. 48(2):108–115.
- Carvalho R, Shimokomaki M, Estévez M. 2017. Poultry meat color and oxidation. In: Petracci M, Berri C, editors. Poultry quality evaluation. 1st ed. Cambridge (UK): Woodhead Publishing; p. 133–157.
- Chacrabati R, Chowdhury R, Yesmin S, Sano H, Al-Mamun M. 2013. Comparison of broiler performance using Plantain (Plantago lanceolata L.), Bio-Sel-E and commercial diet. Bang J Anim Sci. 42(2):123–130.
- Cherian G, Orr A, Burke IC, Pan W. 2013. Feeding Artemisia annua alters digesta pH and muscle lipid oxidation products in broiler chickens. Poult Sci. 92(4):1085–1090.
- Chung T, Choi I. 2016. Growth performance and fatty acid profiles of broilers given diets supplemented with fermented red ginseng marc powder combined with red koji. Rev Bras Cienc Avic. 18(4):733–738.
- COBB. 2018. Broiler management guide. Deer Lodge (TN): Cobb-Vantress Inc.; [accessed 2018 December]. https://www.cobb-vantress.com/assets/5c7576a214/Broiler-guide-R1.pdf.
- De Silva CS, Koralage ISA, Weerasinghe P, Silva NRN. 2015. The determination of available phosphorus in soil: a quick and simple method. OUSL J. 8:1–17.
- Díaz-Gómez J, Moreno JA, Angulo E, Sandmann G, Zhu C, Ramos AJ, Capell T, Christou P, Nogareda C. 2017. High-carotenoid biofortified maize is an alternative to color additives in poultry feed. Anim Feed Sci Technol. 231:38–46.
- Divani F, Kasmani B, Mehri M. 2018. Plantago ovata in broiler chicken nutrition: performance, carcass criteria, intestinal morphology, immunity, and intestinal bacterial population. J Anim Physiol Anim Nutr. 102(1):e353–e363.
- Dubikovskaya E, Chudnovskiy R, Karateev G, Park HM, Stahl A. 2014. Measurement of long-chain fatty acid uptake into adipocytes. Meth Enzymol. 538:107–134.
- Ebrahim R, Liang JB, Jahromi MF, Shokryazdan P, Ebrahimi M, Li Chen W, Goh YM. 2015. Effects of tannic acid on performance and fatty acid composition of breast muscle in broiler chickens under heat stress. Ital J Anim Sci. 14:572–577.
- Ebrahimzadeh SK, Navidshad B, Farhoomand P, Aghjehgheshlagh FM. 2018. Effects of grape pomace and vitamin E on performance, antioxidant status, immune response, gut morphology and histopathological responses in broiler chickens. SA J Sci. 48(2):324–336.
- Elgersma A, Søegaard K, Jensen SK. 2013. Fatty acids, α-tocopherol, β-carotene, and lutein contents in forage legumes, forbs, and a grass-clover mixture. J Agric Food Chem. 61(49):11913–11920.
- Fallah R, Mirzaei E. 2016. Effect of dietary inclusion of turmeric and thyme powders on performance, blood parameters and immune system of broiler chickens. Livest Sci. 7:180–186.
- Ferrazzano GF, Cantile T, Roberto L, Ingenito A, Catania MR, Roscetto E, Palumbo G, Zarrelli A, Pollio A. 2015. Determination of the in vitro and in vivo antimicrobial activity on salivary Streptococci and Lactobacilli and chemical characterisation of the phenolic content of a Plantago lanceolata infusion. Biomed Res Int. 2015:286817.
- Fouad AM, El-Senousey HK. 2014. Nutritional factors affecting abdominal fat deposition in poultry: a review. Asian-Australas J Anim Sci. 27(7):1057–1068.
- Guil‐Guerrero JL. 2001. Nutritional composition of Plantago species (P. Major L., P. Lanceolata L., and P. Media L.). Ecol Food Nutr. 40(5):481–495.
- Habibi R, Sadeghi G, Karimi A. 2014. Effect of different concentrations of ginger root powder and its essential oil on growth performance, serum metabolites and antioxidant status in broiler chicks under heat stress. Br Poult Sci. 55(2):228–237.
- Hammami S, Debbabi H, Jlassi I, Joshi RK, Mokni RE. 2020. Chemical composition and antimicrobial activity of essential oil from the aerial parts of Plantago afra L. (Plantaginaceae) growing wild in Tunisia. S Afr J Bot. 132:410–414.
- Hosseini-Vashan SJ, Raei-Moghadam MS. 2019. Antioxidant and immune system status, plasma lipid, abdominal fat, and growth performance of broilers exposed to heat stress and fed diets supplemented with pomegranate pulp (Punica granatum L.). J Appl Anim Res. 47(1):521–531.
- Hussan F, Mansor AS, Hassan SN, Kamaruddin TN, Tasnim TN, Budin SB, Othman F. 2015. Anti-inflammatory property of Plantago major leaf extract reduces the inflammatory reaction in experimental acetaminophen-induced liver injury. Evidence-Based Complement Altern Med. 2015:1–7.
- Kanani BP, Daneshyar M, Aliakbarlu J, Hamian F. 2017. Effect of dietary turmeric and cinnamon powders on meat quality and lipid peroxidation of broiler chicken under heat stress condition. Vet Res Forum. 8:163–169.
- Kang NH, Lee WK, Yi BR, Park MA, Lee HR, Park SK, Hwang KA, Park HK, Choi KC. 2012. Modulation of lipid metabolism by mixtures of protamine and chitooligosaccharide through pancreatic lipase inhibitory activity in a rat model. Lab Anim Res. 28(1):31–38.
- Karadas F, Erdoğan S, Kor D, Oto G, Uluman M. 2016. The effects of different types of antioxidants (Se, vitamin E and carotenoids) in broiler diets on the growth performance, skin pigmentation and liver and plasma antioxidant concentrations. Rev Bras Cienc Avic. 18(1):101–116.
- Karim MB, Hossain ME, Ali MS, Hossain A. 2018. Effect of garlic powder (Allium sativum) on growth, dressing parameters, serum biochemical contents and profitability of broiler. Bang J Anim Sci. 46(4):215–224.
- Khan MJA, Khan SH, Naz S, Gilani SS, Shafi J, Hassan F, Hassan M, Anwar M. 2014. Effect of dietary supplementation of aloe vera leaves on growth performance and immunity of Fayoumi chicks. Pakistan J Nutrition. 13(4):191–195.
- Kogan G, Pajtinka M, Babincova M, Miadokova E, Rauko P, Slamenova D, Korolenko TA. 2008. Yeast cell wall polysaccharides as antioxidants and antimutagens: can they fight cancer? Neoplasma. 55:387–393.
- Kolbadinejad A, Rezaeipour V. 2020. Efficacy of ajwain (Trachyspermum ammi L.) seed at graded levels of dietary threonine on growth performance, serum metabolites, intestinal morphology and microbial population in broiler chickens. J Anim Physiol Anim Nutr. 104(5):1333–1342.
- Kusmayadi A, Bachtiar KR, Prayitno CH. 2019. The effects of mangosteen peel (Garcinia mangostana L.) and turmeric (Curcuma domestica Val) flour dietary supplementation on the growth performance, lipid profile, and abdominal fat content in Cihateup ducks. Vet World. 12(3):402–408.
- Lee KH, Jung S, Kim HJ, Kim IS, Lee JH, Jo C. 2012. Effect of dietary supplementation of the combination of gallic and linoleic acid in thigh meat of broilers. Asian-Australas J Anim Sci. 25(11):1641–1648.
- Lee Y, Siddiqui WJ. 2021. Cholesterol levels. Treasure Island (FL): StatPearls Publishing; [accessed 2020 Jul 26]. https://www.ncbi.nlm.nih.gov/books/NBK542294/.
- Li XD, Zhai QZ. 2020. Spectrophotometric determination of calcium with dibromo-p-methylsulfonazo. J Chem. 2020:9232385.
- Lien TF, Yeh HS, Su WT. 2008. Effect of adding extracted hesperetin, naringenin and pectin on egg cholesterol, serum traits and antioxidant activity in laying hens. Arch Anim Nutr. 62(1):33–43.
- Ma M, Liu H, Yu J, He S, Li P, Ma C, Zhang H, Xu L, Ping F, Li W, et al. 2020. Triglyceride is independently correlated with insulin resistance and islet beta cell function: a study in population with different glucose and lipid metabolism states. Lipids Health Dis. 19(1):121.
- Marco MD, Salcedo WL, Pastorelli G, Rossi R, Corino C, Bergagna S, Mellia E, Gennero MS, Biasibetti E, Capucchio MT. 2015. Effects of verbascoside supplemented diets on growth performance, blood traits, meat quality, lipid oxidation and histological features in broiler chickens. Ital J Anim Sci. 14:172–178.
- Marinova M, Vladimirova L. 2010. Atomic absorption assessment of mineral iron quantity in ferritin. Spectrosc Lett. 43(3):192–195.
- Martins JM, Carvalho CM, Litz FH, Silveira MM, Moraes CA, Silva MC, Fagundes NS, Fernandes EA. 2016. Productive and economic performance of broiler chickens subjected to different nutritional plans. Rev Bras Cienc Avic. 18(2):209–216.
- Mazhari M, Esmaeilipour O, Mirmahmoudi R, Badakhshan Y. 2016. Comparison of antibiotic, probiotic and great plantain (Plantago major L.) on growth performance, serum metabolites, immune response and ileal microbial population of broilers. Poult Sc J. 4:97–105.
- Mehrparvar M, Mazhari M, Esmaeilipour O, Sami M. 2016. Effect of Lipia citridora leaves powder on growth performance, carcass traits, blood metabolites and meat quality of broilers. Iran J Vet Med. 10:307–317.
- Mondal MA, Yeasmin T, Karim R, Siddiqui MN, Nabi SR, Sayed MA, Siddiky MN. 2015. Effect of dietary supplementation of turmeric (Curcuma longa) powder on the growth performance and carcass traits of broiler chicks. SAARC J Agric. 13(1):188–199.
- Moradi-Ozarlou M, Javanmardi S, Tayefi H, Ashrafizadeh M. 2020. Hepato-and reno-protective impacts of Plantago major in rats: an experimental model of testicular ischemia/reperfusion. Iran J Vet Surg. 15:78–84.
- Mukemre M, Konczak I, Uzun Y, Dalar A. 2020. Phytochemical profile and biological activities of Anatolian plantain (Plantago anatolica). Food Biosci. 36:100658.
- Najafian Y, Hamedi SS, Farshchi MK, Feyzabadi Z. 2018. Plantago major in traditional Persian medicine and modern phytotherapy: a narrative review. Electron Physician. 10(2):6390–6399.
- Narimani-Rad M, Nobakht A, Shahryar HA, Kamani J, Lotfi A. 2011. Influence of dietary supplemented medicinal plants mixture (Ziziphora, Oregano and Peppermint) on performance and carcass characterization of broiler chickens. J Med Plant Res. 5:5626–5629.
- Pedrão MR, Kato T, Soares AL, Ida EI, Coró FAG, Grespan M, Paião F, Shimokomaki M. 2015. Influence of cooling on the glycolysis rate and development of PSE (pale, soft, exudative) meat. Braz Arch Biol Technol. 58(2):272–277.
- Peña-Espinoza M, Valente AH, Thamsborg SM, Simonsen HT, Boas U, Enemark HL, López-Muñoz R, Williams AR. 2018. Antiparasitic activity of chicory (Cichorium intybus) and its natural bioactive compounds in livestock: a review. Parasit Vectors. 11(1):475–489.
- Puerto DM, Cabrera MC, Saadoun A. 2017. A note on fatty acids profile of meat from broiler chickens supplemented with inorganic or organic selenium. Int J Food Sci. 2017:7613068–7613069.
- Redoy MR, Shuvo AA, Cheng L, Al-Mamun M. 2020. Effect of herbal supplementation on growth, immunity, rumen histology, serum antioxidants and meat quality of sheep. Animal. 14(11):2433–2441.
- Saeed M, Yatao X, Hassan FU, Arain MA, El-Hack ME, Noreldin AE, Sun C. 2018. Influence of graded levels of L-theanine dietary supplementation on growth performance, carcass traits, meat quality, organs histomorphometry, blood chemistry and immune response of broiler chickens. Int J Mol Sci. 19(2):462–478.
- Saha P, Talukdar AD, Nath R, Sarker SD, Nahar L, Sahu J, Choudhury MD. 2019. Role of natural phenolics in hepatoprotection: a mechanistic review and analysis of regulatory network of associated genes. Front Pharmacol. 10:509–534.
- Sahoo N, Mishra S, Swain R, Acharya A, Pattnaik S, Sethy K, Sahoo L. 2019. Effect of turmeric and ginger supplementation on immunity, antioxidant, liver enzyme activity, gut bacterial load and histopathology of broilers. Indian J Anim Sci. 9:774–779.
- Salami SA, Majoka MA, Saha S, Garber A, Gabarrou F. 2015. Efficacy of dietary antioxidants on broiler oxidative stress, performance and meat quality: science and market. Avian Biol Res. 8(2):65–78.
- Saleheen E. 2020. Efficacy of different drying methods for Plantain herb (Plantago lanceolata L.) preservation: a way forward to produce year-round safe additives for broiler [MS thesis]. Mymensingh (Bangladesh): Bangladesh Agricultural University.
- Selaledi AL, Hassan MZ, Manyelo TG, Mabelebele M. 2020. The current status of the alternative use to antibiotics in poultry production: an African perspective. Antibiotics. 9(9):594–612.
- Sohaib M, Butt MS, Shabbir MA, Shahid M. 2015. Lipid stability, antioxidant potential and fatty acid composition of broilers breast meat as influenced by quercetin in combination with α-tocopherol enriched diets. Lipids Health Dis. 14:61–76.
- Sotek Z, Białecka B, Pilarczyk B, Drozd R, Pilarczyk R, Tomza-Marciniak A, Kruzhel B, Lysak H, Bąkowska M, Vovk S. 2019. Antioxidant activity and selenium and polyphenols content from selected medicinal plants natives from various areas abundant in selenium (Poland, Lithuania, and Western Ukraine). Processes. 7(12):878–891.
- Turel I, Özbek H, Erten R, Oner OC, Cengiz N, Yilmaz O. 2009. Hepatoprotective and anti-inflammatory activities of Plantago major L. Indian J Pharmacol. 41(3):120–124.
- Venkatalakshmi P, Vadivel V, Brindha P. 2016. Role of phytochemicals as immunomodulatory agents: a review. Int J Green Pharm. 10:1–18.
- Wan XL, Niu Y, Zheng XC, Huang Q, Su WP, Zhang JF, Zhang LL, Wang T. 2016. Antioxidant capacities of Artemisia annua L. leaves and enzymatically treated Artemisia annua L. in vitro and in broilers. Anim Feed Sci Technol. 221:27–34.
- Wang S, Zhang L, Li J, Cong J, Gao F, Zhou G. 2016. Effects of dietary marigold extract supplementation on growth performance, pigmentation, antioxidant capacity and meat quality in broiler chickens. Asian-Australas J Anim Sci. 30(1):71–77.
- Wu YB, Li L, Wen ZG, Yan HJ, Yang PL, Tang J, Xie M, Hou SS. 2019. Dual functions of eicosapentaenoic acid-rich microalgae: enrichment of yolk with n-3 polyunsaturated fatty acids and partial replacement for soybean meal in diet of laying hens. Poult Sci. 98(1):350–357.
- Yap KH, Yee G, Candasamy M, Md S, Majeed A, Bhattamisra S. 2019. Effect of catalpol on liver glucose homeostasis in high fat diet/low dose streptozotocin-induced type 2 diabetes mellitus. Br J Pharmacol. 176:3051–3052.
- Zhang LH, He TF, Hu JX, Li M, Piao XS. 2020. Effects of normal and low calcium and phosphorus levels and 25-hydroxycholecalciferol supplementation on performance, serum antioxidant status, meat quality, and bone properties of broilers. Poultry Sci. 99(11):5663–5672.
- Zheng M, Mao P, Tian X, Meng L. 2019. Growth performance, carcass characteristics, meat and egg quality, and intestinal microbiota in Beijing-you chicken on diets with inclusion of fresh chicory forage. Ital J Anim Sci. 18(1):1310–1320.
