Abstract
This study evaluated the impacts of inclusion of Moringa Oleifera leaves extract (MOLE) in semen extender on rams cryopreserved semen quality and fertilization potential. Forty ejaculates were collected from eight fertile Barki rams, pooled and divided into five groups. The semen extender of the control group was without additives. The semen extender of the second and third groups was supplemented with MOLE at doses of 300 and 600 µg/mL, respectively. The semen extender of the fourth and fifth groups was supplemented with a combination of vitamin E and selenium at doses of 2.5 and 5 µg/mL, respectively. One hundred ten multiparous Barki ewes were artificially inseminated with the semen supplemented without or with MOLE or vitamin E and selenium combination. Inclusion of MOLE in semen extender significantly elevated the motility, viability index, membrane integrity and fertilization capacity of the post-thawed spermatozoa, as well as the activities of semen catalase, total antioxidant capacity, superoxide dismutase, glutathione peroxidase, and glutathione reductase. However, it significantly decreased acrosomal defects and DNA fragmentation of spermatozoa, the activities of semen alkaline and acid phosphatase and the concentration of malondialdehyde compared with the other groups. Similarly, vitamin E and selenium significantly improved the above-mentioned parameters compared to those of the control group. This study indicated that inclusion of MOLE to semen extender improved the quality and fertility of the post-thawed rams’ semen through enhancing the activities of the antioxidant enzyme system and decreasing the spermatozoa DNA fragmentation and lipid peroxidation.
Moringa Oleifera leaves extract (MOLE) protected spermatozoa against cryopreservation induced oxidative stress.
MOLE enhanced cryopreserved semen quality.
MOLE enhanced post-thawed spermatozoa fertilization capacity.
Highlights
Introduction
Cryopreservation is a process aimed to preserve functional spermatozoa to produce viable embryos (Agarwal et al. Citation2003). It also facilitates the distribution of highly fertile males (Demianowicz and Strzezek Citation1995). Cryopreservation is a non-physiological method involving a series of processes such as dilution, incubation, cooling, freezing and thawing that alter the spermatozoal ultrastructure, biochemistry and functions (Watson Citation2000; Öztürk et al. Citation2019). Subsequently, cryopreservation results in irreversible damage to sperms leading to loss of their motility (Medeiros et al. Citation2002). Ram spermatozoa plasma membranes are more susceptible to lipid peroxidation (LPO) than other ruminant because they are rich in polyunsaturated fatty acids (Van Tran et al. Citation2017). Thus, reactive oxygen species (ROS) generated during the cryopreservation is considered the principal cause for rams’ spermatozoal deterioration through the development of oxidative stress and impairment of antioxidant defines system that induce the spermatozoa membranes LPO and damage (Mahanta et al. Citation2012). In order to protect spermatozoa during the cryopreservation process, the semen extender should include some essential nutrients and antioxidants (Bucak et al. Citation2009; Coyan et al. Citation2011; Bucak et al. Citation2012).
Artificial insemination is very important procedure in farm animals’ production because it improves genetic characters and allows the entrance of new foreign breeds through the frozen semen (Casali et al. Citation2017). In ewes, unlike cows, it is difficult to insert the insemination pipette and deposit the semen intrauterine through the cervix because of its small, narrow, rigid and tortuous structure (Kershaw et al. Citation2005; Bathgate et al. Citation2013). So the cervical insemination in sheep is usually by deposition of the semen at the entrance of the cervix through the vaginal canal (Casali et al. Citation2017).
Moringa Oleifera leaves are rich in many nutrients and active compounds such as polyphenols, β-carotene, vitamins A, C and E, protein, calcium, amino acids and several natural anti-oxidiant agents hence; it is widely used in the developing country (Luqman et al. Citation2012). In addition, it possesses many phytochemicals, including crude fibres, reducing sugars, resins, alkaloids, flavonoids, organic acids, sterols, tannins, saponins, proteins, polyphenols and other antioxidants (Mishra et al. Citation2011) such as kaempferol, quercetin, ellagic acid, and apigenin glucoside (Mousa et al. Citation2019). Moringa Oleifera leaves extract (MOLE) enhances the male reproductive potentials by lowering the free radical concentration in sperm cytoplasm (D'cruz and Mathur Citation2005). Also, addition of MOLE to the Friesian bull semen extender as an antioxidant (El-Nagar Citation2017) or antimicrobial agent (Hammad et al. Citation2019) improves sperm quality.
It has been reported that the combination of vitamin E and selenium (Se) exerts a powerful antioxidant activity (Milad et al. Citation2001). Bilodeau et al. (Citation2001) showed that supplementing the semen extender with vitamin E and selenium prevents the loss of sperm motility by inhibiting the formation of ROS that induces LPO in frozen-thawed bull and goat semen. Therefore, the current study aimed to evaluate the impact of including of Moringa Oleifera leaves extract in rams’ semen extender on the post-thawed spermatozoa quality and fertilization capacity compared with a combination of vitamin E and selenium.
Material and methods
Plant materials and extraction procedure
Moringa Oleifera leaves were purchased from a local farm in Sadat City, Menoufia, Egypt. Leaves were identified and authenticated at the department of Biochemistry and Chemistry of Nutrition, Faculty of Veterinary Medicine, University of Sadat City. The alcoholic extract of Moringa Oleifera leaves was prepared following the method described by Mousa et al. (Citation2019) with slight modification. In brief, fresh Moringa Oleifera leaves were washed with distilled water, dried in shaded area at room temperature then grinded and sieved. The powder was submerged in ethyl alcohol 70% (ethanol: water, 70: 30 v/v) at room temperature for 48 h with intermittent shaking then filtered through Whatmann filter paper (size No. 1), and air dried at room temperature. In our previous study (Mousa et al. Citation2019) this extract was analysed phytochemically to identify its constituents by using Liquid chromatography-mass spectrometry (LC-MS), Shimadzu LC-10 HPLC with a Grace Vydac Everest Narrowbore C18 column (100 mm × 2.1 mm i.d., 5 μm, 300 Å) connected to an LCQ electrospray ion trap MS (Thermo Finnigan, San Jose, CA).
Preparation of vitamin E and selenium combination
Vitamin E acetate (sigma- Aldrich-Japan- T1157) and selenium sulphide (sigma- Aldrich-Japan-S4882) were purchased from sigma-Aldrich, Japan. Vitamin E acetate was prepared to a final concentration of 100 µM of vitamin E according to the manufacture’s procedure. Briefly, one mg of bovine serum albumin was added to keep vitamin E in an aqueous form. Stock solution of selenium sulphide was prepared by adding of 100 µM of selenium powder to 100 mL of distilled water. Then one volume of vitamin E solution was added to one volume of selenium solution to make a combination of them.
Semen processing
Eight ejaculates were collected during breeding season (October and November) from eight fertile Barki rams (one per ram) aged 2–3 years old and weighted 50–70 Kg weekly for five weeks (total forty ejaculates) by using pre-warmed (42 °C) artificial vagina (Neustadt/Aisch, Müller, Nürnberg, Germany). The ejaculates were incubated at 37 °C for evaluation. All rams were of good general health conditions and clinically free from external and internal parasites with the sound history of fertility in the herd. Rams were housed free in well ventilated semi open shed stalls and exposed to the natural light. This study was carried out at the Animal Reproduction Research Institute (ARRI), Agriculture Research Centre (ARC), Giza, Egypt (Latitude 30° 00′ 60.00" N; Longitude: 31° 12′ 60.00" E). The climatic data of Giza as summarised in Ministry of Lands and Survey Atlas of Giza district was as follows: the mean annual rainfall was 2500 mm; the temperature range was 16.5–27.5 °C and the humidity range was 50–70%.
Preparation of semen extender
The commercial Tris-based extender (BullXcell) was prepared by mixing one volume of stock diluent to 3 volumes of pure distilled water and 1 volume of egg yolk according to manufacturer’s instructions (IMV technologies, L'Aigle, France) with slight modification.
Semen dilution
Semen samples with motility rate ≥70% and spermatozoal concentration ≥ 2 × 109 sperm/ mL were pooled and diluted with the extender without additives (BullXcell) for the control group or supplemented either with MOLE or a combination of vitamin E and Se to reach 150 × 106 sperms per straw. Then the straws were cryopreserved.
Determination of the optimum doses of Moringa oleifera leaves extract and vitamin E and selenium
To determine the optimum dose of MOLE to be added to the semen extender, twenty four ejaculates were collected from eight rams (one ejaculate per ram) for three successive weeks and cryopreserved in semen extender supplemented with MOLE at doses of 60, 150, 180, 300, 600, 900 or 1200 µg/ mL. Then, the motility percentage and viability index of the post-thawed semen were determined. Addition of MOLE to semen extender at doses of 300 and 600 µg/ mL resulted in the highest post-thawed sperms motility percentage and viability index compared to other doses. Thus, MOLE at doses of 300 and 600 µg/ mL was used in the rest of the experiment (Table ).
Table 1. Post-thawed Barki ram’s spermatozoa motility percentage and viability index after addition of Moringa Oleifera leaves extract to semen extender.
Similarly, Twenty four ejaculates were collected from eight rams (one ejaculate per ram) for three successive weeks and cryopreserved in semen extender supplemented with vitamin E and Se combination at doses of 0.5, 1, 2.5, 5, 8.5 and 10 µg/mL. Addition of vitamin E and Se combination to semen extender at doses of 2.5 and 5 µg/mL resulted in the highest post-thawed sperms motility percentage and viability index compared with other doses. Thus, vitamin E and Se combination at doses of 2.5 and 5 µg/ mL was used in the rest of the experiment (Table ).
Table 2. Post-thawed Barki ram’s spermatozoa motility percentage and viability index after addition of vitamin E and Se to semen extender.
Experimental design
Forty ejaculates were collected from 8 Barki rams (one ejaculate per ram) over 5 weeks, pooled and divided into five groups according to the additives into:
Control group: The semen extender was without additives.
Second group (MOLE 300): MOLE was added to the semen extender at a dose of 300 µg/ mL.
Third group: (MOLE 600): MOLE was added to the semen extender at a dose of 600 µg/ mL.
Fourth group (Vit 2.5): vitamin E and selenium combination was added to the semen extender at a dose of 2.5 µg/ mL.
Fifth group (Vit. 5): vitamin E and selenium combination was added to the semen extender at a dose of 5 µg/ mL.
Cryopreservation procedures
Semen was diluted with BullXcell extender gradually at 37 °C. The diluted semen specimens were cooled to 5 °C for 2 h (IMV technologies, L'Aigle, France). The French straws (0.25 Ml) (IVM Technologies, L'Aigle, France) were filled with the diluted semen samples, arranged horizontally inside a foam box and suspended in liquid nitrogen tank 5 cm above the liquid nitrogen (−120 °C) for 15 min then frozen in liquid nitrogen (Najafi et al. Citation2014).
Evaluation of post thawing spermatozoa characters
After one-month, frozen rams semen was thawed by collecting the straws from the liquid nitrogen and incubating them in water bath at 37 °C for 30 s. The thawed semen was then transferred into pre-warmed sterile glass test tubes and incubated in a water bath at 37 °C for 3 h for assessment of the viability index (Milovanov Citation1962).
Determination of post-thawed sperm motility
A drop of post-thawed semen was placed on a pre-warmed glass slide, covered with a coverslip and examined by a phase contrast microscope equipped with warm stage (38 °C, Nikon Corporation, Tokyo, Japan). The average of the motility percentages was recorded in four different microscopic fields and used as the final sperm motility score (Hafez and Hafez Citation2000).
Determination of post-thawed viability index
The evaluation of motility was done hourly for 3 successive hours. The viability index equal ½ post-thawing motility + the post-thawing motility of 1st + 2nd + 3rd hours after thawing (Milovanov Citation1962).
Evaluation of post-thawed sperm abnormalities:
The smears were prepared by placing a drop from a semen sample and one to two drops of pre-warmed (37 °C) eosin-nigrosine stain on a clean glass slide. The smears were allowed to dry in the air and then examined using high power (100×), oil immersion objective, (Olympus, Tokyo, Japan). A total of 200 sperms were examined for each sample and the numbers of sperm cells with morphological abnormalities were recorded (3 slides were examined per each sample) (Bathgate et al. Citation2013).
Assessment of post-thawed sperm plasma membrane integrity
The integrity of the sperm plasma membrane was evaluated by hypo-osmotic swelling test (HOST) as described by Revell and Mrode (Citation1994). The HOS solution composed of (sodium citrate 0.73 g and fructose 1.35 g, dissolved in 100 mL distilled water with osmotic pressure ∼190 mOsmol kg−1) (Gonotec Osmomate 030). This assay was performed by mixing 50 μl of the semen sample to 500 μl of HOS solution and incubating them at 37 °C for 30 min. After incubation, a drop of semen sample was examined under phase contrast microscope (×400; Olympus BX40, Japan). Two hundred spermatozoa were counted in at least five different microscopic fields. The percentage of spermatozoa bearing swollen and curved tails was recorded as positive to the test (functional membranes) (Buckett et al. Citation1997).
Assessment of post-thawed sperm acrosomal membrane integrity
The integrity of the acrosomal membranes of spermatozoa was evaluated by using silver nitrate stain (Elshamy et al. Citation2019). The spermatozoal suspension was spread over slides and dried at room temperature. The preparations were fixed by ethyl alcohol 70% for 2 min and ethyl alcohol 95% for another 2 min. The preparations were stained with the silver nitrate solution for 2 h in an incubator at 65 °C and complete humidity. After the preparations turned into gold colour, the chemical reaction was stopped and the slides were rinsed with distilled water several times. The preparations were then dried at room temperature and analysed for acrosomal integrity using a light microscope (Olympus BX50) with a 100× magnification. The percentage of the acrosome-intact spermatozoa was counted and 200 sperm cells were checked per slide (3 slides per sample were examined) (Chinoy et al. Citation1992).
Analysis of post-thawed seminal plasma lipid peroxidation and antioxidant activity biomarkers
Semen samples of five straws for each treatment were thawed at 37 °C, pooled and centrifuged at 1500 rpm for 15 min. Then the supernatant was collected as the crude extract of enzyme in the semen.
The activities of the seminal plasma acid phosphatase (ACP), alkaline phosphatase (ALP), superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), glutathione reductase (GR) and total antioxidant capacity (TAC) and the seminal plasma levels of ascorbic acid and malondialdehyde (MDA) were analysed calorimetrically by using diagnostic kits purchased from Bio Diagnostic, Company, Cairo, Egypt with the catalogue numbers of (Bio Diagnostic AC 10 10 for ACP, Bio Diagnostic AP 10 20 for ALP, Cat. No. Bio Diagnostic SD 25 21 for SOD, Bio Diagnostic GPx 25 24 for GPx, Bio Diagnostic GR 25 23 for GR, Bio Diagnostic CA 25 17 for catalase, Bio Diagnostic AS 25 15 for ascorbic acid, Bio Diagnostic TA 25 13 for TAC and Bio Diagnostic MD 25 29 for NDA).
Assessment of DNA integrity of post thawed spermatozoa
Semen samples of five straws for each treatment were thawed at 37 °C, pooled and centrifuged at 1500 rpm for 15 min and the pellets were collected and used for assessment of DNA integrity. The comet assay was used to assess sperm DNA integrity according to (Boe-Hansen et al. Citation2005). Two hundred sperms were examined by using fluorescence microscope (Olympus, Japan). DNA damage was calculated from DNA fragmentation percentage, tail length and tail moment using an image analysis system (TriTek Comet-Score program freeware version 1.5) (Fraser Citation2004). Classification of images was carried out according to the fluorescence intensity in the comet tail. The damaged DNA was detected by a tail of fragmented DNA that migrated from the sperm head, inducing a ‘comet’ pattern whereas whole sperm heads without a comet were not considered damaged (Boe-Hansen et al. Citation2005).
Assessment of pregnancy rate
One hundred and ten multiparous Barki ewes (body condition score of 3.0 ± 0.1; scale 0e5) (Russel et al. Citation1969) were synchronised in two consecutive days to receive fixed time artificial insemination (FTAI) after a short-term progesterone protocol described by Menchaca and Rubianes (Citation2004). The treatment for FTAI involved the insertion of an intravaginal silicone device (20 mg flugestone acetate, Chronogest Sponges®, MSD, France), and one dose of eCG (300 IU, Choriomon 5000, IBSA, Switzerland) on day 0 and prostaglandin F2alpha analog (125 mg sodium cloprostenol, Estrumate, MSD, France) was given intramuscularly in the morning of day 6 (Casali et al. Citation2017). The ewes were allocated into three groups according to the type of semen used (MOLE, Vit E and Se or control) and assigned to cervical insemination. The FTAI was performed for each group in the morning of the day 8 (52–56 h after device removal) using 150 × 106 sperms per dose in a 0.25 mL French straw (IVM Technologies, l'Aigle, France). The insemination was done by using a vaginoscope with light source and a multi-dose insemination gun (Walmur, Montevideo, Germany) that allow semen deposition at the end of the vagina in the external Os of the cervix (Casali et al. Citation2017). The pregnancy rate was determined by trans-rectal ultrasonography using a 7.5 MHz transducer (EXAPad mini, IMV, France) 35 days after insemination.
Statistical analysis
The data were presented as mean ± standard error. The statistical analysis was done by one-way of variance (ANOVA) followed by Bonferroni Multiple comparison test to determine the significance of the differences among groups at p value < .05. All tests were carried out using SPSS analysis program (IBM SPSS, Version 25).
Results
Determination of the effective dose of MOLE and the combination of vitamin E and selenium
To select the effective dose of MOLE to be included in semen extender, different doses of MOLE starting from 60 µg/ mL to 1200 µg/mL were added to semen extender. The motility percentage and viability index of the post-thawed sperms were determined. The motility percentage and viability index were gradually increased till reach its peak at doses 300 and 600 µg/mL then declined again. Thus, the effective doses of MOLE that used in the rest of the experiment were 300 and 600 µg/mL (Table ).
A combination of vitamin E and Se at doses beginning from 0.5 µg/ mL to 10 µg/ mL was added to semen extender. There was no significant difference in the post-thawing motility of spermatozoa supplemented with the combination of vitamin E and Se at doses of 1, 2.5 and 5 µg/ mL. Also, there was no significant difference in the post-thawing motility of spermatozoa supplemented with the combination of vitamin E and Se at doses of 0.5, 5, 8.5, and 10 µg/ mL compared with the control group (Table ). However, the viability index of spermatozoa supplemented with the combination of vitamin E and Se at doses 2.5 and 5 µg/ mL was significantly increased compared to those of the control group and those supplemented with the combination of vitamin E and Se at other doses. Thus, the effective doses of the combination of vitamin E and Se that used in the rest of the experiment were 2.5 and 5 µg/mL (Table ).
Effect of semen additives on post-thawed sperms motility, viability index, membrane integrity and acrosomal defect
Supplementation of semen extender with MOLE at doses of 300 and 600 µg/ mL significantly (p < .05) elevated the motility, viability index and membrane integrity of the post-thawed spermatozoa compared with those of the control groups. In addition, acrosome integrity was better preserved in MOLE 300 and 600 µg/mL groups than the other groups. However, MOLE at doses of 300 and 600 µg/mL significantly (p < .05) decreased the acrosomal defect of post-thawed spermatozoa compared to the other groups (Table ).
Table 3. Post-thawed Barki ram’s spermatozoa characters after addition of Moringa Oleifera leaves extract and vitamin E & Se to semen extender.
Supplementation of semen extender with the combination of vitamin E and Se at a dose of 2.5 µg/ mL significantly (p < .05) increased the spermatozoa motility, viability index and membrane integrity compared to the post-thawed spermatozoa of the control group. While it significantly (p < .05) decreased the acrosomal defect of post-thawed spermatozoa compared to the control group (Table ).
Supplementation of semen extender with the combination of vitamin E and Se at a dose of 5 µg/mL numerically increased the spermatozoa motility and significantly increased the viability index and membrane integrity compared to the spermatozoa of the control group. While it significantly (p < .05) decreased the acrosomal defect compared to the control group (Table ).
On the other hand, supplementation of semen extender with MOLE or the combination of vitamin E and Se did not have significant effects on sperm abnormalities compared to those of the control group (Table ).
Effect of semen additives on antioxidant defense system and MDA concentration
Supplementation of semen extender with MOLE at doses of 300 and 600 µg/ mL and the combination of vitamin E and Se at doses of 2.5 and 5 µg/ mL had no significant effects (p > .05) on ascorbic acid levels of the post-thawed seminal plasma compared to the control group (Figure ).
Figure 1. Effect of addition of different of MOLE and vitamin E & Se to semen extender on post-thawed Barki ram’s semen antioxidant enzyme system activity and LPO. Eight ejaculates from eight fertile Barki rams were collected weekly for five weeks (total 40 ejaculates). The ejaculates were pooled and divided into five groups according to additives into control group, in which semen extender was without additives, MOLE 300 group in which MOLE was added to semen extender at a dose of 300 µg/ mL, MOLE 600group in which MOLE was added to semen extender at a dose of 600 µg/ mL, Vit. E & Se 2.5 in which vitamin E and selenium combination was added to semen extender at a dose of 2.5 µg/ mL and Vit. & Se 5 group in which vitamin E and selenium combination was added to semen extender at a dose of 5 µg/ mL. The concentrations of ascorbic acid and malondialdehyde and the activities of alkaline phosphatase, acid phosphatase, total antioxidant capacity, Catalase, superoxide dismutase, glutathione reductase and glutathione peroxidase of the post-thawed seminal plasma were analysed. Values expressed as means ± SEM. Columns carrying different superscripts in the same row are significantly different at (p < .05).
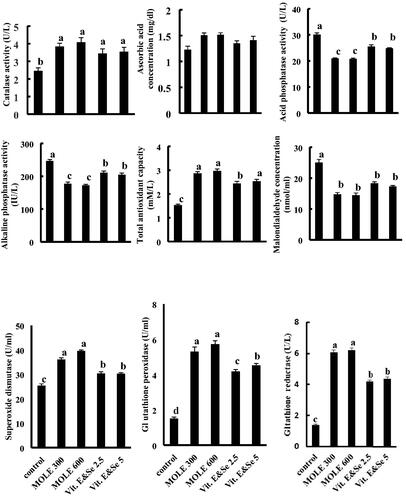
Supplementation of semen extender with MOLE at doses of 300 and 600 µg/ mL and the combination of vitamin E and Se at doses of 2.5 and 5 µg/ mL significantly (p < .05) increased the activities of catalase, SOD, GPx, GR and TAC of the post-thawed seminal plasma compared to the control group (Figure ).
Supplementation of semen extender with MOLE at doses of 300 and 600 µg/ mL and the combination of vitamin E and Se at doses of 2.5 and 5 µg/mL significantly (p < .05) decreased the activities of ACP and ALP and the concentration of MDA in post-thawed semen compared to those of the control group.
Addition of MOLE at doses of 600 µg/mL to semen extender exert highest values concerning the activities of catalase, SOD, GPx, GR and TAC while it exerted the lowest values for the activities of ACP and ALP and the concentration of MDA (Figure ).
Effect of semen additives on spermatozoa DNA fragmentation
Addition of MOLE at doses of 300 and 600 µg/mL and the combination of vitamin E and Se at a doses of 2.5 and 5 µg /mL to semen extender significantly (p < .05) decreased the post-thawed spermatozoa DNA fragmentation, tail length and tail moment compared to the control group (Table and Figure ). In addition, MOLE at doses of 300 and 600 µg/ mL significantly decreased the post-thawed spermatozoa tail moment compared to the combination of vitamin E and Se at doses of 2.5 and 5 µg /mL (Table and Figure ).
Figure 2. Comet assay for evaluation of post thawed sperms DNA fragmentation control (A), MOLE at dose 300 µg/mL (B), MOLE at dose 600 µg/mL (C), vitamin E and selenium at 2.5 µg/mL (D) and vitamin E and selenium at 5 µg/mL (E) White arrows show fragmented DNA.
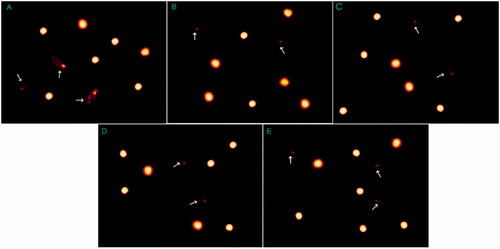
Table 4. Post-thawed Barki ram’s spermatozoa DNA fragmentation, tail length and tail moment after addition of Moringa Oleifera leaves extract and vitamin E and Se to semen extender.
Effect of semen additives on pregnancy rate
Insemination of ewes with post-thawed semen supplemented with MOLE at a dose of 600 µg/ mL resulted in 52.4% (22/42) pregnancy rate and insemination of ewes with post-thawed semen supplemented with vitamin E and Se combination at a dose of 2.5 µg/mL resulted in 39.6% (13/33) pregnancy rate while insemination of ewes with pot-thawed semen without additive resulted in 28.6% (10/35) pregnancy rate. Thus, addition of MOLE at a dose of 600 µg/ mL to semen extender increased the fertilization capacity of the post-thawed sperms by 83.21% compared to the fertilization capacity the post-thawed semen of the control group. In addition, inclusion of vitamin E and Se combination at a dose of 2.5 µg/mL in semen extender increased the fertilization capacity of the post-thawed sperms by 38.46% compared to the fertilization capacity of the post-thawed semen of the control group.
Discussion
Mammalian sperms are more sensitive to LPO by free radicals including, hydrogen peroxide, hydroxyl and superoxide radicals (Alvarez and Storey Citation1989) because they are rich in polyunsaturated fatty acids that are more prone to oxidation by ROS decreasing its fertilising capacity (Lenzi et al. Citation2002). Cryopreservation of semen inversely influences spermatozoa motility; viability, morphology and DNA (Asturiano et al. Citation2007) because lowering sperm's environmental temperature to zero stops production of ATP and increased the generation of ROS that hinder sperms activities (Meryman Citation2007). Thus, inclusion of antioxidants to semen extender is required to improve the post-thawed spermatozoa quantity and quality (Topraggaleh et al. Citation2014). The results of the current study revealed that the addition of MOLE to semen extender had a positive impact on post-thawed spermatozoa motility, viability index and membranes integrity while it decreased acrosomal defect and DNA damage. These findings were in accordance with that of Doidar et al. (Citation2018) who reported that addition of MOLE to buffalo bull semen extenders improved semen parameters. Also, supplementation of extender for rabbit and bull semen with MOLE increases sperm motility (Ghodiah Citation2016). In addition, our previous study indicated that administration of MOLE to Barki rams enhanced their cryopreserved semen characters (Shokry et al. Citation2020). These enhancement effects of inclusion of MOLE in semen extender on post-thawed sperms characters can be attributed to its powerful antioxidant activity as it increased the activities of TAC, catalase, SOD, GPx and GR while it reduced LPO of the post-thawed semen (Figure ) because the generation of ROS during cryopreservation process induces spermatozoal membranes LPO and cellular damage through impairment of the antioxidant defense system in seminal plasma (Mahanta et al. Citation2012). Freezing and thawing of semen decrease sperms motility, normal morphology, viability and increases spermatozoa DNA damage (Asturiano et al. Citation2007; Öztürk et al. Citation2019) due to decreasing of the temperature of sperms to zero during cryopreservation process generates ROS, which stops the production of ATP leading to sperm cells either hibernate or die (Meryman Citation2007).
This finding was in line with our previous study that indicated that supplementation of Barki rams with MOLE reduced LPO while enhanced the activities of the antioxidant enzyme system of their cryopreserved semen (Shokry et al. Citation2020). In addition, previous studies indicated that addition of some essential nutrients and substances such as trehalose (Uysal and Bucak Citation2009), glycerol and trehalose or taxifoline hydrate to ram semen ectender (Bucak et al. Citation2020; Öztürk et al. Citation2020), cysteine to Saanan goat semen extender (Bucak and Uysal Citation2008) or glycerol and cysteine to bull semen extender (Büyükleblebici et al. Citation2014) produced more cryoprotective activity and improve the post-thawed spermatozoa quality.
Seminal plasma contains its own antioxidant defense system including reduced glutathione, SOD, CAT, GPx, GR, vitamin C, vitamin E, and Carnitine (Agarwal et al. Citation2005), which prevent LPO (Mahanta et al. Citation2012). These improvement effects of MOLE on post-thawed semen antioxidant enzymes system activity can be attributed to its contents of potent antioxidants such as Kaempferol, Ellagic acid, Quercetin and Apigenin glucoside (Mousa et al. Citation2019). The aforementioned antioxidant constituents of MOLE have been shown to have powerful antioxidant activities through preventing LPO, increasing antioxidant enzymes activities and the concentration of the antioxidant molecule, reduced glutathione (Abd Eldaim et al. Citation2017; Mousa et al. Citation2019). Furthermore, MOLE contains phenolics such as catechin, epicatechin, ferulic acid, ellagic acid and myricetin, essential oil, monoterpenes, carotinoids, glycosides and flavonoids (Sinha et al. Citation2012).
Moreover, supplementation of ram’s semen extender with MOLE decreased cryopreservation induced DNA damage in post-thawed spermatozoa (Table ) as it has been shown that cryopreservation of rams semen induces generation of ROS that promotes spermatozoa DNA damage (Peris et al. Citation2004, Citation2007). In addition, incubation of human spermatozoa with exogenous ROS for long time increases the sperm DNA fragmentation, while addition of antioxidants to semen decreases it (Lopes et al. Citation1998). However, addition of antioxidants to Barki rams semen extender such as trehalose and raffinose (Elshamy et al. Citation2019; Keskin et al. Citation2020), GPx and SOD to dog semen extender (Chatterjee and Gagnon Citation2001), glutathione to Boer buck semen (Rawash et al. Citation2018) and selenium to water buffalo semen (Dorostkar et al. Citation2012) improves DNA integrity of spermatozoa and reduces cryopreservation induces DNA damage. Taken together it is became clear that addition of MOLE to rams semen extender in our study protected spermatozoa DNA against cryopreservation induced spermatozoa DNA damage via its antioxidant activity.
Furthermore, our results indicated that addition of vitamin E and Se to the cryopreserved semen extender improved the quality of frozen semen of ram, which were in agreement with other in vitro studies in ram (Seremak et al. Citation1999), cattle (Siegel et al. Citation1980) and buffalo (Dorostkar et al. Citation2012). Further, addition of vitamin E at a dose of 60 µg/mL and selenium at a dose 2 µg/mL to tris extender for rams’ semen storage in liquid state improves semen quality (Mohammadi et al. Citation2017). The improvement effect of post-thawed semen supplemted with vitamin E and Se combination can be explained by the antioxidant activity of vitamin E as it has been indicated that the addition of vitamin E and glutathione to cool stored goat semen enhances sperm motility and viability and increases semen GPx and SOD activity while reduces sperm abnormality and seminal MDA concentration (Sarangi et al. Citation2017).
In addition, inclusion of both MOLE and the combination of vitamin E and Se in semen extender decreased the activities of both ACP and ALP in pot-thawed semen. This finding may be related the protective effects of these additives on cryopreserved spermatozoa as it has been indicated that ALP and AC leak from the spermatozoa during the cryopreservation process (Upreti et al. Citation1996) and there is a negative correlation between the activities of the post-thawed semen ALP and ACP and the quality of post-thawed spermatozoa of stallion and ram of (Bucci et al. Citation2016 and Salamon and Maxwell Citation1995).
Finally, the addition of MOLE or the combination of vitamin E and Se to the semen extender enhanced the fertilising capacity of the post-thawed sperm that represented by increasing of pregnancy rate compared to the cryopreserved semen without additives. This finding possibly due to the protective effects of MOLE or the combination of vitamin E and Se on the cryopreserved spermatozoa as Aisen et al. (Citation2002) and Jafaroghli et al. (Citation2011) indicated that the addition of trehalose to semen extenders improved post-thawed semen fertility, also addition of ethylene glycol to bull semen extender improves the conception rats of cows (Büyükleblebici et al. Citation2014). In addition, addition of fish oil to egg yolk-based extender enhanced the post-thawed semen after artificial insemination in ewes (Abdi-Benemar et al. Citation2015). Furthermore, inclusion of reduced glutathione in the ram semen extender increased the pregnancy rates in ewes that artificially inseminated with this semen represented by increasing pregnancy rate (Kubovičová et al. Citation2010).
Conclusion
Supplementation of ram’s semen extender with Moringa oleifera leaves extract at doses of 300 and 600 µg/ mL enhanced the post-thawed sperm quality and fertilization capacity through enhancing the post-thawed semen antioxidant defense system activity and reducing the cryopreservation induced oxidative stress. Moringa oleifera leaves extract had more potent effects compared with the combination of vitamin E and Se. This study suggested that Moringa oleifera leaves extract can be used as a good substitute for the antioxidant component for conventional semen extender.
Ethical approval
All the experimental procedures were approved by the Research Ethics Committee of the Faculty of Veterinary Medicine, University of Sadat City, Egypt under the approval code Vusc-016-1-19 that follows the Guide for the Care and Use of Laboratory Animals 8th edition Washington (DC): National Academies Press (US); 2011.
Software and data repository resources
Authors Declared That There Were No Data Or Models Were Deposited From Any Sources In This Manuscript.
Acknowledgments
The authors thank Taif University for the financial support of Taif University Researchers Supporting Project Project number (TURSP-2020/76), Taif University, Taif, Saudi Arabia.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the paper.
Additional information
Funding
References
- Abd Eldaim MA, Shaban Abd Elrasoul A, Abd Elaziz SA. 2017. An aqueous extract from Moringa oleifera leaves ameliorates hepatotoxicity in alloxan-induced diabetic rats. Biochem Cell Biol. 95(4):524–530.
- Abdi-Benemar H, Jafaroghli M, Khalili B, Zamiri MJ, Ezazi H, Shadparvar AA. 2015. Effects of DHA supplementation of the extender containing egg yolk and α-tocopherol on the freezability and post-thawing fertility of ram semen. Small Rumin Res. 130:166–170.
- Agarwal A, Saleh RA, Bedaiwy MA. 2003. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril. 79:829–843.
- Agarwal A, Gupta S, Sharma RK. 2005. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 3:28.
- Aisen EG, Medina VH, Venturino A. 2002. Cryopreservation and post-thawed fertility of ram semen frozen in different trehalose concentrations. Theriogenology. 57 (7):1801–1808.
- Alvarez JG, Storey BT. 1989. Role of glutathione peroxidase in protecting mammalian spermatozoa from loss of motility caused by spontaneous lipid peroxidation. Gamete Res. 23(1):77–90.
- Asturiano JF, Marco-Jiménez F, Peñaranda DS, Garzón DL, Pérez L, Vicente JS, Jover M. 2007. Effect of sperm cryopreservation on the European eel sperm viability and spermatozoa morphology. Reprod Domest Anim. 42(2):162–166.
- Bathgate R, Mace N, Heasman K, Evans G, Maxwell WM, de Graaf SP. 2013. Birth of kids after artificial insemination with sex-sorted, frozen-thawed goat spermatozoa. Reprod Domest Anim. 48(6):893–898.
- Bilodeau JF, Blanchette S, Gagnon C, Sirard MA. 2001. Thiols prevent H2O2-mediated loss of sperm motility in cryopreserved bull semen. Theriogenology. 56(2):275–286.
- Boe-Hansen GB, Morris ID, Ersbøll AK, Greve T, Christensen P. 2005. DNA integrity in sexed bull sperm assessed by neutral Comet assay and sperm chromatin structure assay. Theriogenology. 63(6):1789–1802.
- Bucak MN, Çoyan K, Öztürk C, Güngör Ş, Ömür AG. 2012. Methionine supplementation improves ram sperm parameters during liquid storage at 5 °C. Cryobiology. 65(3):335–337.
- Bucak MN, Keskin N, İli P, Bodu M, Akalın PP, Öztürk AE, Özkan H, Topraggaleh TR, Sarı F, Başpinar N, et al. 2020. Decreasing glycerol content by co-supplementation of trehalose and taxifolin hydrate in ram semen extender: Microscopic, oxidative stress, and gene expression analyses. Cryobiology. 96:19–29.
- Bucak MN, Tuncer PB, Sarıözkan S, Ulutaş PA. 2009. Comparison of the effects of glutamine and an amino acid solution on post-thawed ram sperm parameters, LPO and anti-oxidant activities. Small Rumin Res. 81(1):13–17.
- Bucak MN, Uysal O. 2008. The role of antioxidants in freezing of saanen goat semen. Indian Vet J. 85:148–150.
- Bucci D, Giaretta E, Spinaci M, Rizzato G, Isani G, Mislei B, Mari G, Tamanini C, Galeati G. 2016. Characterization of alkaline phosphatase activity in seminal plasma and in fresh and frozen-thawed stallion spermatozoa. Theriogenology. 85(2):288–295.
- Buckett WM, Luckas MJ, Aird IA, Farquharson RG, Kingsland CR, Lewis-Jones DI. 1997. The hypo-osmotic swelling test in recurrent miscarriage. Fertil Steril. 68(3):506–509.
- Büyükleblebici S, Tuncer PB, Bucak MN, Eken A, Sarıözkan S, Taşdemir U, Endirlik BU. 2014. Cryopreservation of bull sperm: effects of extender supplemented with different cryoprotectants and antioxidants on sperm motility, antioxidant capacity and fertility results. Anim Reprod Sci. 150(3–4):77–83.
- Casali R, Pinczak A, Cuadro F, Guillen-Muñoz JM, Mezzalira A, Menchaca A. 2017. Semen deposition by cervical, transcervical and intrauterine route for fixed-time artificial insemination (FTAI) in the ewe. Theriogenology. 103:30–35.
- Chatterjee S, Gagnon C. 2001. Production of reactive oxygen species by spermatozoa undergoing cooling, freezing, and thawing. Mol Reprod Dev. 59(4):451–458.
- Chinoy NJ, Ranga GM, Highland HN, D'Souza KJ, Sequeira E. 1992. A modified method for the differential staining of spermatozoa using alcoholic acidic silver nitrate. Int J Fertil. 37(4):232–236.
- Coyan K, Başpınar N, Bucak MN, Akalın PP. 2011. Effects of cysteine and ergothioneine on post-thawed Merino ram sperm and biochemical parameters. Cryobiology. 63(1):1–6.
- D'cruz S, Mathur P. 2005. Effect of piperine on the epididymis of adult male rats. Asian J Androl. 7(4):363–368.
- Demianowicz W, Strzezek J. 1995. The effect of lipoprotein fraction from egg yolk on some of the biological properties of boar spermatozoa during storage of the semen in liquid state. Reprod Domest Anim. 31(1):279–280.
- Dorostkar K, Alavi-Shoushtari SM, Mokarizadeh A. 2012. Effects of in vitro selenium addition to the semen extender on the spermatozoa characteristics before and after freezing in water buffaloes (Bubalus bubalis). Vet Res Forum. 3(4):263–268.
- Doidar Y, El-Nagar H, Elrefy A, Mousbah A. 2018. Cryopreservation and quality assessment of buffalo bull (Bubalus bubalis) semen using new Moringa extender and antioxidant Co-Q10. J Anim Poultry Prod Mans Univ. 9(9):375–381.
- El-Nagar H. 2017. Effect of some semen extenders as a natural source of antioxidants on quality of frozen Friesian semen. J Anim Poultry Prod. 8(9):391–397.
- Elshamy A, Wehaish E-BE-SF, Shaker HM, Elseady Y, Abd El-Fatah E. 2019. Effect of different sugars concentrations on the quality of post- thawed Barki ram semen quality and DNA integrity. Mansoura Vet Med. SVII:81–95.
- Fraser L. 2004. Structural damage to nuclear DNA in mammalian spermatozoa: its evaluation techniques and relationship with male infertility. Pol J Vet Sci. 7(4):311–321.
- Ghodiah AEB. 2016. Physiological studies on some factors affecting semen quality and preservation of rabbit bucks fed moringa., in Faculty of Agriculture (PhD)n Mansoura University, Egypt.
- Hafez ESE, Hafez B. 2000. Reproduction in farm animals. 7th ed. Baltimore (MD): Lippincott Williams & Wilkins.
- Hammad M, Wafa W, Gabr A, Elkishky A. 2019. Different types and levels of Moringa oleifera leaf extract as a source of antibiotics in Friesian bull semen extender. J Anim Poultry Prod Mansoura Univ. 10(3):67–71.
- Jafaroghli M, Khalili B, Farshad A, Zamiri MJ. 2011. The effect of supplementation of cryopreservation diluents with sugars on the post-thawing fertility of ram semen. Small Rumin Res. 96(1):58–63.
- Kershaw CM, Khalid M, McGowan MR, Ingram K, Leethongdee S, Wax G, Scaramuzzi RJ. 2005. The anatomy of the sheep cervix and its influence on the transcervical passage of an inseminating pipette into the uterine lumen. Theriogenology. 64(5):1225–1235.
- Keskin N, Erdogan C, Bucak MN, Ozturk AE, Bodu M, Ili P, Baspinar N, Dursun S. 2020. Cryopreservation effects on ram sperm ultrastructure. Biopreserv Biobank. 18(5):441–448.
- Kubovičová E, Ríha Ľ, Makarevich AV, Apolen D, Pivko J. 2010. Effect of different semen extenders and additives to insemination doses on ewes pregnancy rate. Slovak J Anim Sci. 43:118–122.
- Lenzi A, Gandini L, Lombardo F, Picardo M, Maresca V, Panfili E, Tramer F, Boitani C, Dondero F. 2002. Polyunsaturated fatty acids of germ cell membranes, glutathione and blutathione-dependent enzyme-PHGPx: from basic to clinic. Contraception. 65(4):301–304.
- Lopes S, Jurisicova A, Sun JG, Casper RF. 1998. Reactive oxygen species: potential cause for DNA fragmentation in human spermatozoa. Hum Reprod. 13(4):896–900.
- Luqman S, Srivastava S, Kumar R, Maurya AK, Chanda D. 2012. Experimental assessment of Moringa oleifera leaf and fruit for its antistress, antioxidant, and scavenging potential using in vitro and in vivo assays. Evid Based Complement Alternat Med. 2012:519084.
- Mahanta R, Gogoi A, Chaudhury PN, Roy S, Bhattacharyya IK, Sharma P. 2012. Association of oxidative stress biomarkers and antioxidant enzymatic activity in male infertility of north-East India. J Obstet Gynecol India. 62(5):546–550.
- Medeiros CM, Forell F, Oliveira AT, Rodrigues JL. 2002. Current status of sperm cryopreservation: why isn’t it better? Theriogenology. 57(1):327–344.
- Menchaca A, Rubianes E. 2004. New treatments associated with timed artificial insemination in small ruminants. Reprod Fertil Dev. 16(4):403–413.
- Meryman HT. 2007. Cryopreservation of living cells: principles and practice. Transfusion. 47(5):935–945.
- Milad K, Kovac G, Rácz O, Sipulova A, Bajová V. 2001. Effect of vitamin E and selenium on blood glutathione peroxidase activity and some immunological parameters in sheep. Veterinarni Med. 46(1):1–5.
- Milovanov VK. 1962. The biology of reproduction and the artificial insemination of animals. Moscow: Seljhozizdat.
- Mishra G, Singh P, Verma R, Kumar S, Srivastav S, Jha K, Khosa R. 2011. Traditional uses, phytochemistry and pharmacological properties of Moringa oleifera plant: an overview. Der Pharm Lettre. 3:141–164.
- Mohammadi P, Tabatabaei VS, Mamouei M. 2017. The interaction effects of vitamin E and selenium on Arabian ram spermatozoa quality parameters during semen storage in liquid condition. Anim Sci J. 30:43–56.
- Mousa AA, El-Gansh HAI, Eldaim MAA, Mohamed MAE, Morsi AH, El Sabagh HS. 2019. Protective effect of Moringa oleifera leaves ethanolic extract against thioacetamide-induced hepatotoxicity in rats via modulation of cellular antioxidant, apoptotic and inflammatory markers. Environ Sci Pollut Res Int. 26(31):32488–32504.
- Najafi A, Najafi MH, Zanganeh Z, Sharafi M, Martinez-Pastor F, Adeldust H. 2014. Cryopreservation of ram semen in extenders containing soybean lecithin as cryoprotectant and hyaluronic acid as antioxidant. Reprod Domest Anim. 49(6):934–940.
- Öztürk AE, Bodu M, Bucak MN, Ağır V, Özcan A, Keskin N, Ili P, Topraggaleh TR, Sidal H, Başpinar N, et al. 2020. The synergistic effect of trehalose and low concentrations of cryoprotectants can improve post-thaw ram sperm parameters. Cryobiology. 95:157–163.
- Öztürk AE, Bucak MN, Bodu M, Başpinar Çelik, Shu Z, Keskin N, Gao D. 2019. Cryobiology and cryopreservation of sperm. In: Quain M, editor. Cryopreservation: current advances and evaluations. IntechOpen; pp. 75–116.
- Peris SI, Bilodeau JF, Dufour M, Bailey JL. 2007. Impact of cryopreservation and reactive oxygen species on DNA integrity, lipid peroxidation, and functional parameters in ram sperm. Mol Reprod Dev. 74(7):878–892.
- Peris SI, Morrier A, Dufour M, Bailey JL. 2004. Cryopreservation of ram semen facilitates sperm DNA damage: Relationship between sperm andrological parameters and the sperm chromatin structure assay. J Androl. 25(2):224–233.
- Rawash Z, Ibrahim EA, El-Raey M. 2018. Effects of reduced glutathione on Boer goat semen freezability. Asian Pac J Reprod. 7(1):33.
- Revell S, Mrode R. 1994. An osmotic resistance test for bovine semen. Anim Reprod Sci. 36(1–2):77–86.
- Russel A,J, Doney J, Gunn R. 1969. Subjective assessment of body fat in live sheep. J Agric Sci. 72(3):451–454.
- Salamon S, Maxwell WMC. 1995. Frozen storage of ram semen II. Causes of Low fertility after cervical insemination and methods of improvement. Anim Reprod Sci. 38(1–2):1–36.
- Sarangi A, Singh P, Virmani M, Yadav AS, Sahu S, Ajithakumar HM, Kumari A, Rath AP. 2017. Effect of antioxidants supplementation on the quality of Beetal buck semen stored at 4 °C. Vet World. 10(10):1184–1188.
- Seremak B, Udala J, Lasota B. 1999. Influence of selenium additive on ram semen freezing quality. Electron J Pol Agric Univ Anim Husbandry Ser. 2:1.
- Shokry DM, Badr MR, Orabi SH, Khalifa HK, El-Seedi HR, Abd Eldaim MA. 2020. Moringa oleifera leaves extract enhances fresh and cryopreserved semen characters of Barki rams. Theriogenology. 153:133–142.
- Siegel RB, Murray FA, Julien W, Moxon A, Conrad H. 1980. Effect of in vitro selenium supplementation on bovine sperm motility. Theriogenology. 13(5):357–367.
- Sinha M, Das DK, Datta S, Ghosh S, Dey S. 2012. Amelioration of ionizing radiation induced lipid peroxidation in mouse liver by Moringa oleifera Lam. leaf extract. Indian J Exp Biol. 50 (3):209–215.
- Topraggaleh TR, Shahverdi A, Rastegarnia A, Ebrahimi B, Shafiepour V, Sharbatoghli M, Esmaeili V, Janzamin E. 2014. Effect of cysteine and glutamine added to extender on post-thaw sperm functional parameters of buffalo bull. Andrologia. 46 (7):777–783.
- Upreti G, Payne S, Duganzich D, Oliver J, Smith J. 1996. Enzyme leakage during cryopreservation of ram spermatozoa. Anim Reprod Sci. 41(1):27–36.
- Uysal O, Bucak MN. 2009. The role of different trehalose concentrations and cooling rates in freezing of ram semen. Ankara Üniv Vet Fak Derg. 56:99–103.
- Van Tran L, Malla BA, Kumar S, Tyagi AK. 2017. Polyunsaturated fatty acids in male ruminant reproduction - a review. Asian-Australas J Anim Sci. 30 (5):622–637.
- Watson P. 2000. The causes of reduced fertility with cryopreserved semen. Animal Rep Sci. 60–61:481–492.
