Abstract
This study evaluated the effects of dietary coated sodium butyrate (CSB) on lipopolysaccharide (LPS)-induced inflammatory response in weaned lambs. A basal diet was supplemented with CSB at 0, 2 or 3 g/kg feed for 28 days and/or intraperitoneally injected with 100 μg/kg BW Escherichia coli LPS (0 g/kg CSB, 0 g/kg CSB and LPS, 2 g/kg CSB and LPS, 3 g/kg CSB and LPS). The results showed that CSB supplementation conferred significant protective effects against LPS-induced inflammatory response by reducing in the levels of D-lactate (DLA), diamine oxidase (DAO), inflammatory cytokines (TNF-α, IL-1β and IL-8) in the serum and upregulated expression of tight junction proteins (claudin-3, ZO-1 and occludin) in the ileum. Meanwhile, CSB pre-treatment significantly suppressed LPS-induced TLR4/NF-κB signalling pathway mRNA expression (MyD88, TLR4, NF-κB, IL-6 and IL-1β) and down-regulated TLR4/NF-κB signalling pathway protein expression (MyD88, TLR4, NF-κB p65 and p-NF-κB p65). Furthermore, dietary CSB supplementation improved intestinal morphology. Lastly, bacterial 16S rRNA gene sequencing indicated that CSB could alter intestinal microbiota composition at genus levels. Taken together, our results indicate CSB could maintain intestinal health, improve intestinal morphology, modulate the microbial community and decrease LPS-induced inflammatory response in lambs.
Graphical Abstract
The addition of CSB improves the permeability of lamb’s intestinal tract
The addition of CSB enhances lamb’s intestinal barrier function
CSB alter intestinal microbiota composition at genus levels
CSB alleviates LPS-induced intestinal injury in weaned lambs through TLR4/NF-κB signalling pathway
HIGHLIGHTS
Introduction
In recent years, China’s sheep industry has gradually moved from extensive to modern intensive production. In modern sheep production, lambs are vulnerable to pathogens such as bacteria, viruses and non-pathogens such as lipopolysaccharides that stimulate immune stress, resulting in the destruction of intestinal mucosal barrier function and leading to digestive dysfunction, growth retardation, diarrhoea and other problems (Shyer et al. Citation2015; Rigby et al. Citation2016).
The healthy development of the intestine is key to ensuring animal growth and potential production performance. In addition, intestinal microbes play significant roles in nutrient digestion and intestinal health. Butyric acid is one of the main short-chain fatty acids (SCFAs) in intestinal microbial metabolites. Sodium butyrate (SB) is hydrolysed in the animal gastrointestinal tract to form butyrate. It is not only the first choice for energy metabolism of intestinal epithelial cells and the major regulator of cell proliferation and differentiation but is also considered as a potential therapeutic drug for the clinical treatment of enteritis (Chen and Vitetta Citation2018; Perego et al. Citation2018). According to reports, adding SB to the diet can improve growth performance as well as improve rumen epithelial inflammation induced by high-concentrate feed, thereby protecting the goat rumen epithelial integrity and barrier function (Dai et al. Citation2017; Zhang et al. Citation2018). Previous reports have shown that adding SB to the diet can reduce the content of lipopolysaccharide (LPS) and pro-inflammatory cytokines in ruminants and attenuate the activation of apoptosis pathways to maintain mammary health (Chang et al. Citation2018). Górka et al. found that dietary supplementation of exogenous SB had a beneficial effect on the development of the small intestine of sheep (Górka et al. Citation2018).
The anti-inflammatory effect of CSB has been largely confirmed in monogastric animals and humans (Segain et al. Citation2000; Zhang et al. Citation2011; Xu et al. Citation2016; Feng et al. Citation2018). In spite of the excellent aforementioned biological functions, information is extremely scarce regarding dietary CSB supplementation on ruminants under immune stress. CSB may aid in gut health during periods of intestinal imbalance induced by LPS. Hence, the purpose of this research was to explore the effect of CSB on the intestinal function of LPS-stressed lambs from the combined perspectives of nutrition and immunity.
Materials and methods
Animals and diets
The program for the animal trial was allowed by the Institutional Animal Care Committee of the Heilongjiang Bayi Agricultural University (Daqing, China). The animal feeding experiment was completed in the animal experimental centre of the College of Animal Science and Veterinary Medicine. Basal diet without antibiotics was formulated in accordance with the Chinese feeding standards for meat-producing sheep (NY/T 816-2004) (Gao et al. Citation2019) to conform to the nutritional requirements of weaned lambs, and the composition and nutrient contents of the basal diet are shown in Table . Coated sodium butyrate was purchased from Herunde Biological Bosen Import and Export Co., Ltd (CAT: 20190321, Shandong Province, China).
Table 1. The composition and nutrient contents of basal diet (air-dry basis) (%).
Experimental design
A total of 24 weaned male lambs (German Merino × Dorper sheep) at 42 days of age with average body weight (11.79 ± 0.54 kg) were randomised into four groups with six replicates in each group: basal diet without CSB (the CON group); basal diet without CSB challenged by LPS (the LPS group); 2 g/kg CSB added to the basal diet and challenged by LPS (the CSB2L group); 3 g/kg CSB added to the basal diet and challenged by LPS (the CSB3L group). In this experiment, the pre-feeding period was seven days, and the experimental period was 28 days. The weaned lambs were fed an equal amount of food twice a day in the morning and at 07:00 and 17:00 in the evening and were given access to food and water ad libitum. Sterile saline solution was injected intraperitoneally in the CON group, and the other groups were injected intraperitoneally with 100 μg/kg BW Escherichia coli LPS serotype O55:B5 (L2880 Sigma-Aldrich, St. Louis, MO, USA), three hours before slaughter on the 28th day of the experiment.
Blood and intestine sample collection
On the 28th day of the formal test period, blood samples were collected from the jugular vein of the weaned lamb. Then centrifuged at 3000 × g for 10 min to obtain serum after 30 min at room temperature and stored at −20 °C until the serum index was tested.
The abdominal cavity of the weaned lamb was opened quickly along the midline of the abdomen, and the junction between the small intestine and large intestine was ligated with cotton thread. The tissues of the small intestine of lambs were taken midway and washed with cold phosphate buffer three times straightway. The intestinal segment samples were divided into two portions. A portion of the intestinal tissue sample was scraped with a sterile slide, and then the scrapings were immediately transferred to liquid nitrogen and stored at −80 °C to extract DNA and RNA. Another intestinal tissue sample of weaned lambs was deposited in 4% paraformaldehyde (Solarbio, Shanghai, China) for analysis of intestinal morphology. Additionally, the colonic digesta sample (approximately 20 g) stored at −80 °C was employed for microflora analysis.
Serum DLA, DAO and cytokines
Serum D-lactate (DLA) and diamine oxidase (DAO) were analysed using a commercial ELISA kit (Alisa Biotechnology Co., Ltd., Shanghai, China) according to the instructions provided by the manufacturers. The concentrations of the inflammatory cytokines in serum were determined separately by commercially available ELISA kits (Alisa Biotechnology Co., Ltd., Shanghai, China), and the absorbance was monitored at 450 nm using an iMark microplate reader (Bio-Rad). Each sample was measured three times while the serum indicators were being tested.
Intestinal morphology analysis
To examine the morphology of the small intestine, the tissue samples were embedded in paraffin to prepare sections. After staining with haematoxylin and eosin, photomicrographs were obtained using an optical microscopy system (Olympus Corporation, Tokyo, Japan). Image Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA) was utilised to measure the intestine villus height (the apex of the villus to the junction of the villus and the crypt) and crypt depth (the vertical distance from the junction of intestinal gland villi to the base of the intestinal gland) of nine complete villi for each weaned lamb, with average villus height and crypt depth ratio, then calculated per weaned lamb.
Bacterial Illumina MiSeq sequencing and bioinformatic analysis
Colon contents samples were thawed at room temperature. Microbial genomic DNA was extracted from the contents using the E.Z.N.A® soil DNA Kit (Omega Bio-tek, Norcross, GA, USA) according to the requirements of the instructions. The purity and content of the extracted DNA were determined spectrophotometrically by measuring the NanoDrop 2000 (Thermo Scientific, Wilmington, USA), and DNA isolation was confirmed by electrophoresis using 1% agarose gels. Subsequently, the 16S rRNA gene hypervariable V3 to V4 of the bacteria was amplified using specific primers 338 F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806 R (5′-GGACTACHVGGGTWTCTAAT-3′) provided by Meiji Biomedical Technology Co., Ltd. (Shanghai, China).
After PCR amplification and purification, the purified amplicons were combined in equimolar amounts, and paired-end sequencing was performed using the Illumina MiSeq platform (Illumina, San Diego, USA). UPARSE (7.0) was used to cluster operational taxonomic units (OTUs) of each sample at the 97% similarity level. The classification of each 16SrRNA gene sequence was analysed using the ribosomal database program (RDP) classification algorithm and the Silva132 16SrRNA database (using a 70% confidence threshold).
Quantitative real-time PCR
Total RNA extraction from frozen intestinal mucosa using the TRIzol reagent (Invitrogen, ThermoFisher Scientific, Inc., Waltham, Massachusetts, USA). A NanoDrop2000 was used to assess the content and quality of total RNA. Subsequently, cDNA was synthesised using a Prime Script RTreagent kit with gDNA Eraser (TaKaRa, Tokyo, Japan). All the primers were commercially synthesised from Sangon Biotechnology (Shanghai, China) Co., Ltd. And the sequences of primers are presented in Table . The CFX96TM real-time PCR detection system (Bio-Rad, Hercules, California, USA) was chosen by us to perform real-time fluorescence quantitative PCR. The target gene and β-actin expression levels were detected using the TB Green® Premix Ex TaqTM II Kit (TaKaRa, Tokyo, Japan). The reaction was performed with a total reaction volume of 25 µL, including 12.5 µL of TB Green® Premix Ex TaqTM II, 0.5 µL each of the forward and reverse primers (10 µM), 2 µL of the cDNA template and 9.5 µL of enzyme-free water. The PCR procedure consisted of 1 cycle (pre denaturation at 95 °C for 30 s), 40 cycles (denaturation at 95 °C for 5 s, annealing extension at 60 °C for 30 s). To analyse relative gene expression, we used the 2−ΔΔCt method.
Table 2. List of primer sequences for quantitative real-time PCR.
Western blotting
The ileal mucosal tissue sample was broken with a tissue grinder. After treatment, the sample was harvested, lysed and the total protein was extracted for western blotting using the commercial kit (P0013, Beyotime Biotechnology, Shanghai, China). Protein concentration was determined using the BCA Protein Assay Kit (23227, Pierce, Rockford, IL, USA). Protein samples were separated by SDS-PAGE and then transferred to a PVDF membrane (Millipore, Massachusetts, USA) by electrophoresis. After blocking with 5% skimmed milk powder (BD, New Jersey, USA), the membrane sections were incubated with indicated primary antibodies incubation overnight at 4 °C. The following primary antibodies were used: rabbit anti-claudin-3 (AF0129), rabbit anti-ZO-1 (AF5145), rabbit anti-occludin (DF7504), rabbit anti-MyD88 (AF5195), rabbit anti-TLR4 (AF7017), rabbit anti-p-NF-κB p65 (AF2006), rabbit anti-NF-κB p65 (AF5006) and mouse anti-β-actin (T0022) (Affinity Bioscience, USA). Perform western blot analysis as before (Jin et al. Citation2019). After the primary antibody incubation, the membranes were washed and then incubated with appropriate secondary antibodies. Subsequently, the GelDocTM XR Plus system (Bio-Rad, USA) was used to perform the chemiluminescence detection.
Statistical analysis
Experimental data were subjected to ANOVA, using the general linear model procedure of SAS 9.2 software (SAS Institute, Cary, NC, USA) followed by Duncan’s multiple range tests. All data were expressed as treatment means with their pooled SEM, and significance was determined as p < .05.
Results
Inflammatory cytokines on serum
As shown in Table , increased IL-1β, IL-8 and TNF-α levels in the serum of weaned lambs were observed challenged with LPS (p < .05). In comparison to the LPS group, serum IL-1β and TNF-α levels were remarkably lower in the CSB2L and CSB3L groups (p < .05). TNF-α level was significantly increased in the CSB2L and CSB3L groups than in the CON group (p < .05). In addition, compared with the LPS stimulated lambs, the content of serum IL-8 was down-regulated in the lambs supplemented with 3 g/kg CSB (p < .05). However, the content of serum IL-8 in the CSB2L group was not found significant differences between the groups (p > .05). Despite no obvious difference in the serum IL-10 concentration in the LPS group in comparison with the CON group (p > .05), the IL-10 concentration in the CSB2L and CSB3L groups was significantly higher than that in the LPS group (p < .05). We also observed no difference in the concentrations of inflammatory factors between the CSB2L and CSB3L groups (p > .05).
Table 3. Effects of coated sodium butyrate (CSB) on serum inflammatory cytokines of weaned lambs challenged with LPS.
Intestinal barrier function
The serum contents of DLA and DAO are considered to be important indicators of intestinal leakiness. When the intestinal barrier is damaged, the concentrations of serum DLA and DAO will increase. The levels of serum DLA and DAO were significantly increased after LPS infusion (p < .05) in weaned lambs (Table ). Supplementation of 2 g/kg CSB and 3 g/kg CSB diets reduced DLA and DAO levels of weaned lambs after LPS infusion. There were no significant differences between the CSB2L and CSB3L groups in the DLA or DAO concentrations of weaned lambs (p > .05).
Table 4. Effects of coated sodium butyrate (CSB) on serum D-lactate (DLA) and diamine oxidase (DAO) of weaned lambs challenged with LPS.
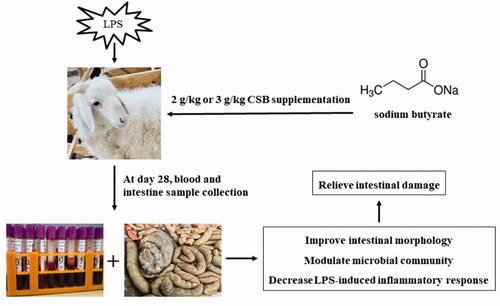
Significantly decreased claudin-3, ZO-1 and occludin proteins expression levels of the ileum were observed in the LPS group (p < .05) than in the CON group (Figure ). Nevertheless, dietary CSB supplementation to weaned lambs led to greater protein expression of claudin-3, occluding and ZO-1 in ileum compared to the LPS group (p < .05).
Figure 1. Effects of coated sodium butyrate (CSB) on protein levels of claudin-3, ZO-1 and Occludin in the ileal mucosa of weaned lambs challenged with LPS. Data are shown as means ± SE. (A) The representative western blot images. (B) Ratio of claudin-3 to β-actin. (C) Ratio of ZO-1 to β-actin. (D) Ratio of Occludin to β-actin. CON: basal diets without supplement; LPS: control diet with lipopolysaccharide; CSB2L: LPS treatment group supplemented with 2 g/kg CSB. CSB3L: LPS treatment group supplemented with 3 g/kg CSB. a, b, c Mean within a row with different superscripts are different at p < .05.
Intestinal morphology analysis
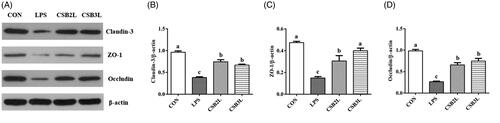
LPS administration to lambs resulted in disordered ileal villi arrangement and shedding of tip epithelial cells following intraperitoneal injection (Figure ). LPS stimulation significantly decreased the villus height and villus height/crypt depth of the jejunum and ileum (p < .05) (Figure ). The decline of villus height and villus height/crypt depth in the jejunum and ileum was alleviated after dietary CSB was added (p < .05). The heights of duodenal villi in the CSB2L and CSB3L groups were significantly greater than in the CON and LPS groups (p < .05). Significantly rising ileal villus height and villus height/crypt depth in the CSB3L group compared within the CON group (p < .05).
Figure 2. Effects of coated sodium butyrate (CSB) on intestinal morphology of weaned lambs with LPS. (A) Ileum stained with H&E (bars, 200 μm). Villus height, crypt depth and the ratio of villous height to crypt depth in the Duodenum, Jejunum and Ileum (B). CON: basal diets without supplement; LPS: control diet with lipopolysaccharide; CSB2L: LPS treatment group supplemented with 2 g/kg CSB. CSB3L: LPS treatment group supplemented with 3 g/kg CSB. a, b, c Mean within a row with different superscripts are different at p < .05.
Diversity and structure of the colonic microbiota composition
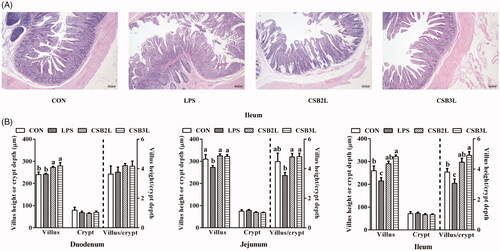
Good’s coverage was at least 97% for each sample, and the obtained sequences were bacterial. In total, 229,656 high-quality sequences were obtained from the 12 samples of the gut bacteria. The Venn diagram showed that the numbers of OTUs were 1382, 1120, 1177 and 1351 for the CON, LPS, CSB2L and CSB3L groups, respectively. The number of shared OTUs was 655, and the respective numbers of unique OTUs were 156, 140, 105 and 157 (Figure ).
Figure 3. Venn diagram demonstrating the unique and shared OTUs in the colonic digesta in different treatments. CON: basal diets without supplement; LPS: control diet with lipopolysaccharide; CSB2L: LPS treatment group supplemented with 2 g/kg CSB. CSB3L: LPS treatment group supplemented with 3 g/kg CSB.
In this study, in terms of colonic richness and diversity of weaned lambs with LPS (Table ), the LPS group had decreased community richness (ACE and Chao1 index) compared with the CON, CSB2L and CSB3L groups (p < .05). However, no significant differences were observed in community diversity (Shannon and Simpson indices and Good’s coverage) between groups.
Table 5. Effects of coated sodium butyrate (CSB) on colonic richness and diversity of weaned lambs with LPS.
Effect of CSB supplementation on the relative abundance of colonic bacterial communities
We observed the composition of the microbiota of the colon at the phylum level (Table ). The most abundant phylum in the CON group was Firmicutes (57.13%) followed by Bacteroidetes (38.41%), Spirochaetes (1.81%), Tenericutes (1.00%), Verrucomicrobia (0.59%), Epsilonbacteraeota (0.35%) and Proteobacteria (0.26%). Compared with the CON group, the LPS group had decreased (p < .05) relative abundance of Tenericutes, whereas compared with the LPS group, the CSB2L and CSB3L groups contained increased proportions of Tenericutes. There were no significant differences in the relative abundances of Firmicutes, Bacteroidetes, Spirochaetes, Verrucomicrobia, Epsilonbacteraeota, or Proteobacteria in the treatment groups (p > .05).
Table 6. Effects of coated sodium butyrate (CSB) on relative abundance in colon at phylum level of weaned lambs with LPS (%).
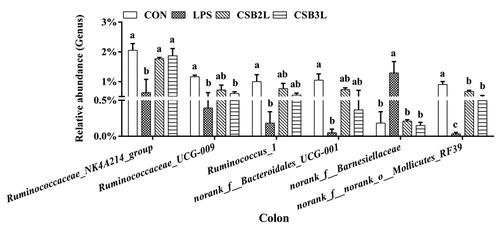
Of the 38 genera observed with over 1% relative abundance, six differed significantly among the groups (Figure ). Compared with the CON group, the abundances of Ruminococcaceae_NK4A214_group, Ruminococcaceae_UCG-009, Ruminococcus_1, norank_f__Bacteroidales_UCG-001, norank_f__norank_o__Mollicutes_RF39 were significantly decreased (p < .05), and the relative abundance of norank_f__Barnesiellaceae was significantly increased in the LPS group (p < .05). However, supplemental CSB suppressed the reduction of Ruminococcaceae_NK4A214_group, norank_f__Barnesiellaceae and norank_f__norank_o__Mollicutes_RF39 compared with the LPS group (p < .05).
Figure 4. Effects of coated sodium butyrate (CSB) on relative abundances of bacterial genera (accounted for ≥1% in the samples that affected) in colon of weaned lambs with LPS (%). CON: basal diets without supplement; LPS: control diet with lipopolysaccharide; CSB2L: LPS treatment group supplemented with 2 g/kg CSB. CSB3L: LPS treatment group supplemented with 3 g/kg CSB. a, b, c Mean within a row with different superscripts are different at p < .05.
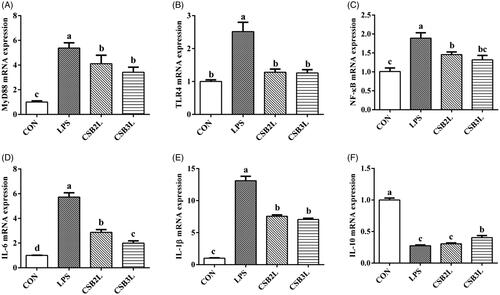
Gene expression in the ileal mucosa
We found that LPS stimulation induced mRNA expression of MyD88, TLR4 and NF-κB as well as inflammatory cytokines (p < .05). CSB-treated weaned lambs showed lower expression levels of these genes in the ileum compared to the LPS-treated lambs (p < .05) (Figure ). Compared with the LPS group, the expression level of IL-10 was increased in the CSB3L group (p < .05). Interestingly, the expression levels of IL-10 and IL-6 in the CSB2L and CSB3L groups showed significant differences (p < .05).
Figure 5. Effects of CSB supplementation on the mRNA expression of genes in the ileal mucosa of weaned lambs challenged with LPS. (A) MyD88 mRNA expression level. (B) TLR4 mRNA expression level. (C) NF-κB mRNA expression level. (D) IL-6 mRNA expression level. (E) IL-1β mRNA expression level. (F) IL-10 mRNA expression level. CON: basal diets without supplement; LPS: control diet with lipopolysaccharide; CSB2L: LPS treatment group supplemented with 2 g/kg CSB. CSB3L: LPS treatment group supplemented with 3 g/kg CSB. a, b, c Mean within a row with different superscripts are different at p < .05.
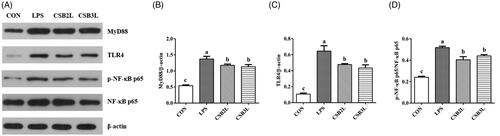
Protein expression in the ileal mucosa
LPS improved the protein expression levels of TLR4, MyD88 and the phosphorylation of NF-κB p65 signalling pathways in the ileum (p < .05) (Figure ). CSB treatment significantly inhibited the expression of TLR4, MyD88 and the phosphorylation of NF-κB p65 (p < .05).
Figure 6. Effects of coated sodium butyrate (CSB) on Protein levels of MyD88, TLR4, pNF-κB and NF-κB P65 in the ileal mucosa of weaned lambs challenged with LPS. Data are shown as means ± SE. (A) The representative western blot images. (B) Ratio of MyD88 to β-actin. (C) Ratio of TLR4 to β-actin. (D) Ratio of phosphorylated NF-κB P65 to total NF-κB P65. CON: basal diets without supplement; LPS: control diet with lipopolysaccharide; CSB2L: LPS treatment group supplemented with 2 g/kg CSB. CSB3L: LPS treatment group supplemented with 3 g/kg CSB. a, b, c Mean within a row with different superscripts are different at p < .05.
Discussion
In this experiment, weaned lambs showed depression, unstable standing and intra-abdominal ascites after LPS injection. LPS is the main component of the cell wall of Gram-negative bacteria, which can directly or indirectly induce apoptosis and regulate the immune response (Bruewer et al. Citation2003). When the body is confronted by microbial antigens such as LPS, macrophages, monocytes and endothelial cells will release a series of cytokines, oxygen-free radicals, histamines and other inflammatory mediators to cause an inflammatory reaction and reduce barrier function (Tazuke et al. Citation2003). SB has been shown to alleviate intestinal inflammation (Guo et al. Citation2019; Liu et al. Citation2019; Zou et al. Citation2019). LPS stress could significantly increase the level of serum proinflammatory cytokines in broilers, and dietary supplementation of SB inhibited the increases of serum IL-6 and TNF-α (Zhang et al. Citation2011). In the current study, LPS stimulation increased the levels of proinflammatory cytokines TNF-α, IL-1 β and IL-8 in the serum of weaned lambs. The CSB2L and CSB3L groups showed inhibition of TNF-α, IL-1β and IL-8 levels and increased levels of IL-10. These discoveries are steady with previous reports suggesting that SB can alleviate the inflammatory response induced by LPS in weaned lambs. The reason might be that butyric acid hydrolysed by SB can alleviate inflammation induced by LPS via inhibiting the activity of the NF-κB pathway, and then inhibiting the release of proinflammatory cytokines.
The integrity of intestinal mucosa is necessary to ensure its normal barrier function (Blikslager et al. Citation2007; Wijtten et al. Citation2011). The number of intestinal epithelial cells is decreased and intestinal permeability is increased in injury to the intestinal mucosal barrier. DLA is a metabolic and lytic product of intestinal bacteria such as Lactobacillus and Escherichia coli that exist in the intestinal tract (Venn et al. Citation2020). DAO, as a sign of relative stability of intestinal mucosal maturity and integrity, is a highly active intracellular enzyme that can deaminate histamine (Zhao et al. Citation2011). DLA and DAO enter the blood and lymph and show increased activity in the blood when the intestinal mucosal barrier is damaged (Aschenbach et al. Citation2007). Increased concentrations of DLA and DAO have been reported after LPS stimulation (Zhang et al. Citation2018; Luo et al. Citation2019). An appropriate concentration of butyrate has been demonstrated to have positive effects on the function of the intestinal barrier (Dengler et al. Citation2015; Zou et al. Citation2019). However, whether this effect would be present in lambs challenged with LPS remained unknown. In this study, the levels of DLA and DAO in the serum of weaned lambs were increased by LPS stimulation, in line with the above results. The results showed that LPS stimulation damaged the intestinal barrier. However, dietary CSB could alleviate this increase in intestinal permeability. This result may be attributed to the fact that CSB can inhibit the expression of inflammatory cytokines by promoting the expression of tight junction proteins, thus reducing the injury to intestinal mucosal barrier. The role of SB in maintaining the intestinal mucosal barrier can also be attributed to the activation of the signal transduction pathway that regulates the immune response through the binding of SCFAs to G-protein-coupled receptors on the cell surface (Li et al. Citation2018). There is evidence that butyrate enhances the intestinal defense barrier via decreasing small intestinal permeability and increasing the expression of tight junction protein (Feng et al. Citation2018).
The villus height, crypt depth and villus height/crypt depth values of the small intestine are important indicators of intestinal development and function. The increased crypt depth, lower villus height and villus height/crypt depth values in the small intestines of the LPS-stimulated group compared to the CON group were in accordance with recent researches (Chen et al. Citation2018; Chen et al. Citation2018; Luo et al. Citation2019). Moreover, previous research has demonstrated that feeding SB increased the jejunal villus height and villus height/crypt depth (Wang et al. Citation2018). In this study, examination of the intestinal morphology indicated that dietary inclusion of CSB could inhibit the decreases in villus height and villus height/crypt depth induced by LPS stimulation. The data suggest that CSB treatment may improve the intestinal mucosal barrier function and may help to reduce the occurrence of inflammatory reactions and maintain immune balance. On the one hand, this may be due to the hydrolysis of CSB to butyric acid, which can not only provide energy for intestinal epithelial cells to promote their proliferation and differentiation but also promote the expression of growth factors of intestinal epithelial tissue and the maturation rate of the small intestine and the absorption of nutrients by the intestinal epithelium. On the other hand, studies have shown that an increase in the content of SCFAs in the intestine can cause the downregulation of gene expression of insulin-binding protein 3 and can increase insulin-like growth factor 1 expression in the digestive tract epithelium, thereby promoting the growth of intestinal epithelial cells (Van Landeghem et al. Citation2015). In addition, increased release of inflammatory cytokines leads to the loss of small intestinal villi and the impairment of the barrier function (Zhu et al. Citation2013). Therefore, the expression of inflammatory factors was determined.
The intestinal microbiota has a critical role in the host’s intestinal function and health. Intestinal microbiota can not only provide energy for the host (Den Besten et al. Citation2013) and protect against pathogens (Baumler and Sperandio Citation2016) but also regulate the host’s immunity under normal circumstances (Gensollen et al. Citation2016). However, this beneficial relationship may be disrupted by an altered microbial composition. The current study found that dietary CSB could modify intestinal inflammation by decreasing the concentration of inflammatory cytokines and by lowering intestinal permeability. To understand the underlying mechanism of CSB in alleviating LPS-induced intestinal injury, 16S rRNA gene sequencing was applied to explore the reaction of intestinal microorganisms to CSB supplementation in weaned lambs treated with LPS. Firmicutes and Bacteroidetes have been shown to be two major phyla of sheep colonic bacteria (Zhang et al. Citation2018). We also demonstrated that the structure of the intestinal bacteria at the phylum level was in agreement with those found in previous studies. Firmicutes play a significant role in the degradation of fibre and cellulose by ruminants (Thoetkiattikul et al. Citation2013). The previous study has reported that Bacteroides could increase the utilisation of complex carbohydrates and promote digestion (Spence et al. Citation2006). Interestingly, Tenericutes was the sole taxon that differed among treatment groups at the phylum level. Tenericutes is a frequent component of the rumen microbiome in ruminants (Creevey et al. Citation2014; Myer et al. Citation2015). Consistent with the present study, previous research has indicated that SB supplement ameliorated intestinal microbial community and diversity on broilers (Wu et al. Citation2018). In addition, LPS or CSB also altered bacterial composition at the genus level. Our data showed that CSB alleviated the decrease of Ruminococcaceae_NK4A214_group, norank_f__norank_o__Mollicutes_RF39 and the increase of norank_f__Barnesiellaceae in the colon caused by LPS. Thus, these three bacterial genera may be associated with the microbial metabolism of volatile fatty acids. Ruminococcus has been proven to be able to ferment xylose, glucose and certain indigestible dietary fibres, and produce acetate from pyruvate via acetyl-CoA through the Wood-Ljungdahl pathway. Notably, Ruminococcus has been proven to be able to ferment xylose, glucose and certain indigestible dietary fibres, which produce acetate from pyruvate under the action of acety1-CoA via the Wood-Ljungdahl pathway (Crost et al. Citation2013). In addition, it was also reported that the abundance of SCFAs was highly correlated with Ruminococcaceae_NK4A214 (Wang B et al. Citation2019; Calderón-Pérez et al. Citation2020). RF39 is a novel Tenericutes lineage of Bacilli reported in the guts of humans and domestic animals (Zhang et al. Citation2015; Nayfach et al. Citation2019). A survey using Illumina MiSeq next-generation sequencing to analyse the intestinal microflora of college students with different levels of fibre intake showed that Barnesiellaceae was higher in those with low fibre intake (Whisner et al. Citation2018). Therefore, we consider that modifying dysbiosis and enriching biodiversity might be important mechanisms in CSB amelioration of intestinal injury in weaned lambs challenged with LPS.
It is obvious that intestinal barrier function is important for maintaining homeostasis (Ghosh et al. Citation2020). LPS is widely understood to promote the expression of pro-inflammatory cytokines (Hou et al. Citation2013; Zhu et al. Citation2013), which may be an important cause of intestinal damage. Previous studies have noted the sufficiency of inducing intestinal inflammation challenged with an intraperitoneal injection of LPS (Mercer et al. Citation1996). It is particularly noteworthy that TNF-α has been proved to regulate intestinal epithelial cell apoptosis, survival, as well as mediate gastrointestinal injury (Stuber et al. Citation1999; Marini et al. Citation2003). According to several reports, the levels of pro-inflammatory cytokines and the expression of TLR4-NF-κB signalling were upregulated in the small intestine under the stimulation of LPS (Hou et al. Citation2013; Gong et al. Citation2019). Furthermore, studies have indicated that intraperitoneal injection of LPS-TLR4 signalling inhibitor TAK-242 reduces the adverse effects of intestinal barrier disruption (Horioka et al. Citation2020). Similarly, LPS stimulation increased the mRNA expression of MyD88, TLR4, NF-κB, IL-6, IL-1β and protein expression of MyD88, TLR4, p-NF-κB p65/NF-κB p65 in the ileum. Host TLR4 be directly activated by LPS to transmit immune-related signals to the nucleus through NF-κB transcription factors (Ke et al. Citation2013), resulting in the production of a variety of pro-inflammatory cytokines (Lu et al. Citation2008). Therefore, the down-regulation of TLR-4 mRNA expression may partly explain the inhibitory effect of butyrate on LPS. On the basis of recent evidence, SB revealed to protect diarrhoea caused by weaning stress or enterohemorrhagic Escherichia coli (Biagi et al. Citation2007; Takao et al. Citation2014). SB was also revealed to reduce the expression of intestinal proinflammatory cytokines (Segain et al. Citation2000; Xu et al. Citation2016; Feng et al. Citation2018). CSB may have relieved inflammatory damage via inhibiting the TLR4/MyD88/NF-κB signalling pathway. Therefore, the attenuation of cytokine expression by butyrate may be part of its gut protection mechanism, as it may enhance epithelial immune defences rather than impairing epithelial integrity.
Conclusions
Relief of intestinal damage in response to CSB supplementation in lambs challenged with LPS may be explained because of the enhanced intestinal function. CSB led to improved barrier function in the intestine and had positive effects on the microbial community in LPS-challenged lambs. Our study found that CSB supplement induced anti-inflammatory actions by blocking TLR4/NF-κB signalling. These findings may provide a novel nutritional strategy of adding CSB to the feed used in modern intensive lamb husbandry to maintain intestinal health, but this proposal is worth further research.
Ethical approval
All experimental procedures were conducted according to the international procedures and approved by the Institutional Animal Care Committee of the Heilongjiang Bayi Agricultural University
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Aschenbach JR, Ahrens F, Schwelberger HG, Furll B, Roesler U, Hensel A, Gabel G. 2007. Functional characteristics of the porcine colonic epithelium following transportation stress and Salmonella infection. Scand J Gastroenterol. 42(6):708–716.
- Baumler AJ, Sperandio V. 2016. Interactions between the microbiota and pathogenic bacteria in the gut. Nature. 535(7610):85–93.
- Biagi G, Piva A, Moschini M, Vezzali E, Roth FX. 2007. Performance, intestinal microflora, and wall morphology of weanling pigs fed sodium butyrate. J Anim Sci. 85(5):1184–1191.
- Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. 2007. Restoration of barrier function in injured intestinal mucosa. Physiol Rev. 87(2):545–564.
- Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. 2003. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 171(11):6164–6172.
- Calderón-Pérez L, Gosalbes M, Yuste S, Valls R, Pedret A, Llauradó E, Jimenez-Hernandez N, Artacho A, Pla-Pagà L, Companys J, et al. 2020. Gut metagenomic and short chain fatty acids signature in hypertension: a cross-sectional study. Sci Rep. 10(1):6436.
- Chang G, Yan J, Ma N, Liu X, Dai H, Bilal MS, Shen X. 2018. Dietary sodium butyrate supplementation reduces high-concentrate diet feeding-induced apoptosis in mammary cells in dairy goats. J Agric Food Chem. 66(9):2101–2107.
- Chen J, Vitetta L. 2018. Inflammation-modulating effect of butyrate in the prevention of colon cancer by dietary fiber. Clin Colorectal Canc. 17:541–544.
- Chen L, Li S, Zheng J, Li W, Jiang X, Zhao X, Li J, Che L, Lin Y, Xu S. 2018. Effects of dietary Clostridium butyricum supplementation on growth performance, intestinal development, and immune response of weaned piglets challenged with lipopolysaccharide. J Anim Sci Biotechnol. 9(1):1–14.
- Chen Y, Zhang H, Cheng Y, Li Y, Wen C, Zhou Y. 2018. Dietary l-threonine supplementation attenuates lipopolysaccharide-induced inflammatory responses and intestinal barrier damage of broiler chickens at an early age. Br J Nutr. 119(11):1254–1262.
- Creevey CJ, Kelly WJ, Henderson G, Leahy SC. 2014. Determining the culturability of the rumen bacterial microbiome. Microb Biotechnol. 7(5):467–479.
- Crost EH, Tailford LE, Gall GL, Fons M, Henrissat B, Juge N. 2013. Utilisation of mucin glycans by the human gut symbiont Ruminococcus gnavus is strain-dependent. PLoS One. 8(10):e76341.
- Den Besten G, Van Eunen K, Groen AK, Venema K, Reijngoud D, Bakker BM. 2013. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 54(9):2325–2340.
- Dengler F, Rackwitz R, Benesch F, Pfannkuche H, Gabel G. 2015. Both butyrate incubation and hypoxia upregulate genes involved in the ruminal transport of SCFA and their metabolites. J Anim Physiol Anim Nutr. 99(2):379–390.
- Feng W, Wu Y, Chen G, Fu S, Li B, Huang B, Wang D, Wang W, Liu J. 2018. Sodium butyrate attenuates diarrhea in weaned piglets and promotes tight junction protein expression in colon in a GPR109A-dependent manner. Cell Physiol Biochem. 47(4):1617–1629.
- Gao Q, Zhu H, Dong L, Shi W, Chen R, Song Z, Huang C, Li J, Dong X, Zhou Y, et al. 2019. Integrated proteogenomic characterization of HBV-related hepatocellular carcinoma. Cell. 179(5):1240.
- Gensollen T, Iyer SS, Kasper DL, Blumberg RS. 2016. How colonization by microbiota in early life shapes the immune system. Science. 352(6285):539–544.
- Ghosh SS, Wang J, Yannie PJ, Ghosh S. 2020. Intestinal barrier dysfunction, LPS translocation, and disease development. J Endocr SoC. 4(2):1–15.
- Gong Q, He L, Wang M, Zuo S, Gao H, Feng Y, Du L, Luo Y, Li J. 2019. Comparison of the TLR4/NFκB and NLRP3 signalling pathways in major organs of the mouse after intravenous injection of lipopolysaccharide. Pharm Biol. 57(1):555–563.
- Górka P, Śliwiński B, Flaga J, Olszewski J, Nawrocka P, Sobkowiak K, Miltko R, Godlewski MM, Zabielski R, Kowalski ZM. 2018. Effect of exogenous butyrate on the gastrointestinal tract of sheep. II. Hydrolytic activity in the rumen and structure and function of the small intestine. J Anim Sci. 96(12):5325–5335.
- Guo J, Wang Y, Jiang P, Yao H, Zhao C, Hu X, Cao Y, Zhang N, Fu Y, Shen H. 2019. Sodium butyrate alleviates lipopolysaccharide-induced endometritis in mice through inhibiting inflammatory response. Microb Pathog. 137:103792.
- Dai H, Liu X, Yan J, Aabdin ZU, Bilal MS, Shen X. 2017. Sodium butyrate ameliorates high-concentrate diet-induced inflammation in the rumen epithelium of dairy goats. J Agric Food Chem. 65(3):596–604.
- Horioka K, Tanaka H, Isozaki S, Konishi H, Fujiya M, Okuda K, Asari M, Shiono H, Ogawa K, Shimizu K. 2020. Acute colchicine poisoning causes endotoxemia via the destruction of intestinal barrier function: the curative effect of endotoxin prevention in a murine model. Dig Dis Sci. 65(1):132–140.
- Hou Y, Wang L, Yi D, Ding B, Yang Z, Li J, Chen X, Qiu Y, Wu G. 2013. N-acetylcysteine reduces inflammation in the small intestine by regulating redox, EGF and TLR4 signaling. Amino Acids. 45(3):513–522.
- Jin X, Zhang M, Yang Y. 2019. Saccharomyces cerevisiae β-glucan-induced SBD-1 expression in ovine ruminal epithelial cells is mediated through the TLR-2-MyD88-NF-κB/MAPK pathway. Vet Res Commun. 43(2):77–89.
- Ke X, Chen J, Zhang X, Fang W, Yang C, Peng J, Chen Y, Sferra TJ. 2013. Qing Hua Chang Yin attenuates lipopolysaccharide-induced inflammatory response in human intestinal cells by inhibiting NF-κB activation. Exp Ther Med. 6(1):189–193.
- Li M, Van Esch BCAM, Henricks PAJ, Folkerts G, Garssen J. 2018. The anti-inflammatory effects of short chain fatty acids on lipopolysaccharide- or tumor necrosis factor α-stimulated endothelial cells via activation of GPR41/43 and inhibition of HDACs. Front Pharmacol. 9:533–545.
- Liu J, Chang G, Huang J, Wang Y, Ma N, Roy A, Shen X. 2019. Sodium butyrate inhibits the inflammation of lipopolysaccharide-induced acute lung injury in mice by regulating the toll-like receptor 4/nuclear factor κB signaling pathway. J Agric Food Chem. 67(6):1674–1682.
- Lu Y, Yeh W, Ohashi PS. 2008. LPS/TLR4 signal transduction pathway. Cytokine. 42(2):145–151.
- Luo J, Chen D, Mao X, He J, Yu B, Cheng L, Zeng D. 2019. Purified β-glucans of different molecular weights enhance growth performance of LPS-challenged piglets via improved gut barrier function and microbiota. Animal. 9(9):602.
- Marini M, Bamias G, Rivera-Nieves J, Moskaluk C, Hoang S, Ross W, Pizarro T, Cominelli F. 2003. TNF-alpha neutralization ameliorates the severity of murine Crohn’s-like ileitis by abrogation of intestinal epithelial cell apoptosis. PNAS. 100(14):8366–8371.
- Mercer DW, Smith GS, Cross JM, Russell DH, Chang L, Cacioppo J. 1996. Effects of lipopolysaccharide on intestinal injury; potential role of nitric oxide and lipid peroxidation. J Surg Res. 63(1):185–192.
- Myer PR, Smith TPL, Wells JE, Kuehn LA, Freetly HC. 2015. Rumen microbiome from steers differing in feed efficiency. PLoS One. 10(6):e0129174.
- Nayfach S, Shi ZJ, Seshadri R, Pollard KS, Kyrpides NC. 2019. New insights from uncultivated genomes of the global human gut microbiome. Nature. 568(7753):505–510.
- Perego S, Sansoni V, Banfi G, Lombardi G. 2018. Sodium butyrate has anti-proliferative, pro-differentiating, and immunomodulatory effects in osteosarcoma cells and counteracts the TNFα-induced low-grade inflammation. Int J Immunopathol Pharmacol. 32:394632017752240.
- Rigby RJ, Carr J, Orgel K, King SL, Lund PK, Dekaney CM. 2016. Intestinal bacteria are necessary for doxorubicin-induced intestinal damage but not for doxorubicin-induced apoptosis. Gut Microbes. 7(5):414–423.
- Segain J, Raingeard de la Blétière D, Bourreille A, Leray V, Gervois N, Rosales C, Ferrier L, Bonnet C, Blottière H, Galmiche J. 2000. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut. 47(3):397–403.
- Shyer AE, Huycke TR, Lee CH, Mahadevan L, Tabin CJ. 2015. Bending gradients: how the intestinal stem cell gets its home. Cell. 161(3):569–580.
- Spence C, Wells WG, Smith CJ. 2006. Characterization of the primary starch utilization operon in the obligate anaerobe bacteroides fragilis: regulation by carbon source and oxygen. J Bacteriol. 188(13):4663–4672.
- Stuber E, Buschenfeld A, Von FA, Arendt T, Folsch UR. 1999. Intestinal crypt cell apoptosis in murine acute graft versus host disease is mediated by tumour necrosis factor alpha and not by the FasL-Fas interaction: effect of pentoxifylline on the development of mucosal atrophy. Gut. 45(2):229–235.
- Takao M, Yen H, Tobe T. 2014. LeuO enhances butyrate-induced virulence expression through a positive regulatory loop in enterohaemorrhagic Escherichia coli. Mol Microbiol. 93(6):1302–1313.
- Tazuke Y, Drongowski RA, Teitelbaum DH, Coran AG. 2003. Interleukin-6 changes tight junction permeability and intracellular phospholipid content in a human enterocyte cell culture model. Pediatr Surg Int. 19(5):321–325.
- Thoetkiattikul H, Mhuantong W, Laothanachareon T, Tangphatsornruang S, Pattarajinda V, Eurwilaichitr L, Champreda V. 2013. Comparative analysis of microbial profiles in cow rumen fed with different dietary fiber by tagged 16S rRNA gene pyrosequencing. Curr Microbiol. 67(2):130–137.
- Van Landeghem L, Santoro MA, Mah AT, Krebs AE, Dehmer JJ, Mcnaughton KK, Helmrath MA, Magness ST, Lund PK. 2015. IGF1 stimulates crypt expansion via differential activation of 2 intestinal stem cell populations. FASEB J. 29(7):2828–2842.
- Venn EC, Barnes AJ, Hansen RJ, Boscan P, Twedt DC, Sullivan LA. 2020. Serum D-lactate concentrations in dogs with parvoviral enteritis. J Vet Intern Med. 34(2):691–699.
- Wang B, Ma MP, Diao QY, Tu Y. 2019. Saponin-induced shifts in the rumen microbiome and metabolome of young cattle. Front Microbiol. 10:356–370.
- Wang CC, Wu H, Lin FH, Gong R, Xie F, Peng Y, Feng J, Hu C. 2018. Sodium butyrate enhances intestinal integrity, inhibits mast cell activation, inflammatory mediator production and JNK signaling pathway in weaned pigs. Innate Immun. 24(1):40–46.
- Whisner CM, Maldonado J, Dente B, Krajmalnik-Brown R, Bruening M. 2018. Diet, physical activity and screen time but not body mass index are associated with the gut microbiome of a diverse cohort of college students living in university housing: a cross-sectional study. BMC Microbiol. 18(1):210–221.
- Wijtten PJA, Der Meulen JV, Verstegen MWA. 2011. Intestinal barrier function and absorption in pigs after weaning: a review. Br J Nutr. 105(7):967–981.
- Wu W, Xiao Z, An W, Dong Y, Zhang B. 2018. Dietary sodium butyrate improves intestinal development and function by modulating the microbial community in broilers. PLoS One. 13(5):e0197762.
- Xu J, Chen X, Yu S, Su Y, Zhu W. 2016. Effects of early intervention with sodium butyrate on gut microbiota and the expression of inflammatory cytokines in neonatal piglets. PLoS One. 11(9):e0162461.
- Zhang H, Shao M, Huang H, Wang S, Ma L, Wang H, Hu L, Wei K, Zhu R. 2018. The dynamic distribution of small-tail han sheep microbiota across different intestinal segments. Front Microbiol. 9:32–42.
- Zhang K, Meng M, Gao L, Tu Y, Bai Y. 2018. Sodium butyrate improves high-concentrate-diet-induced impairment of ruminal epithelium barrier function in goats. J Agric Food Chem. 66(33):8729–8736.
- Zhang L, Huang X, Xue B, Peng Q, Wang Z, Yan T, Wang L. 2015. Immunization against rumen methanogenesis by vaccination with a new recombinant protein. PLoS One. 10(10):e0140086.
- Zhang WH, Jiang Y, Zhu QF, Gao F, Dai SF, Chen J, Zhou GH. 2011. Sodium butyrate maintains growth performance by regulating the immune response in broiler chickens. Br Poult Sci. 52(3):292–301.
- Zhang X, Chen X, Zhang H, Guan S, Wen S, Huang W, Liu Z. 2018. Propofol does not reduce pyroptosis of enterocytes and intestinal epithelial injury after lipopolysaccharide challenge. Dig Dis Sci. 63(1):81–91.
- Zhao Y, Qin G, Sun Z, Che D, Bao N, Zhang X. 2011. Effects of soybean agglutinin on intestinal barrier permeability and tight junction protein expression in weaned piglets. Int J Mol Sci. 12(12):8502–8512.
- Zhu H, Liu Y, Xie XL, Huang JJ, Hou Y. 2013. Effect of L-arginine on intestinal mucosal immune barrier function in weaned pigs after Escherichia coli LPS challenge. Innate Immun. 19(3):242–252.
- Zou X, Ji J, Qu HX, Wang J, Shu DM, Wang Y, Liu TF, Li Y, Luo C. 2019. Effects of sodium butyrate on intestinal health and gut microbiota composition during intestinal inflammation progression in broilers. Poult Sci. 98(10):4449–4456.
