Abstract
In sows, the synthesis of the colostrum begins several days before farrowing and the different amino acid composition of colostrum compared to milk can justify the hypothesis of different dietary requirements to produce it. The objective of the study was to test the enrichment with threonine (Thr), tryptophan (Trp) and valine in the peripartum diet of sows on the quality of colostrum, health and growth of piglets, and the variations of backfat and lean thickness and the faecal microbiome of sows. Ninety-nine sows were divided into two groups, balanced by parity number and fed: (1) the standard formula used on late gestation to early lactation (CON; 50 sows; (2) CON added with 0.074% L-Thr, 0.034% L-Trp and 0.077% L-valine (TRT; 49 sows). The number of pigs born alive and their average live weight, the number of weaned pigs, their growth and the mortality on d1 did not change with the diet, but the percentage of total dead piglets was reduced when sows were fed TRT (−3.5%, p = .049). Backfat loss of sows in lactation was marginally reduced (p = .097). The IgA content of colostrum increased with TRT, but not protein, fat, lactose or urea. Bacterial richness (Chao1) decreased in faeces of sows at the end of lactation with TRT, but not bacterial diversity. Selenomonas and Bacillus were more abundant in CON and TRT, respectively. Adjusting the amino acid profile of the feed provided in the transition phase between final gestation and early lactation to the colostrum profile could improve the survival of suckled piglets.
The amino acid profile of colostrum differs from that of milk.
The pre-weaning mortality can be decreased by feeding sows a diet with an amino acid profile closer to that of colostrum.
Raising the dietary ratio of threonine (Thr), tryptophan (Trp) and valine to lysine (Lys) in the peripartum diet of sows improves the IgA content of colostrum.
Highlights
Introduction
A reduction in the average birth weight of piglets and an increase in its variability is observed with the genetic selection for high-prolific sows (Yuan et al. Citation2015), with unfavourable repercussions on piglet survival and subsequent growth performance. Furthermore, the increase in the number of newborn per litter is not followed by a concomitant increase in the synthesis of milk solids (Auldist et al. Citation1998), while the dilution of the secreted milk protein with increasing prolificacy (Vadmand et al. Citation2015) can lead to a lower growth rate of piglets. On the other hand, high quality and quantity of colostrum are essential to support newborn piglets in the first days post-farrowing to ensure their health and growth (Quesnel et al. Citation2012; Trevisi et al. Citation2020). It is known that the synthesis of colostrum components begins about a week before the farrowing (Theil Citation2015), so the feeding practices of the sows in the days before farrowing must be carefully evaluated.
The different composition of colostrum compared to milk can justify the hypothesis of different nutrient needs to produce it. According to widespread feeding practices, in the very last days of gestation the intake of feed is lowered (e.g. 2.5 kg or less, from −3 d to farrowing) (Tabeling et al. Citation2003; Harper et al. Citation2021), to avoid constipation and overfeeding. It has been calculated that, in this phase, the feeding the sows according to current standards leads to a negative balance for nitrogen, but not for lysine (Lys), which usually is the first limiting amino acid to which other essential amino acids (EAAs) and energy are compared with (Theil Citation2015). This may indicate that the dietary intake is adequate for Lys, but not for other EAAs. It is not known whether this can affect the quantitative and qualitative production of colostrum.
The EAA requirements are closely related to the composition of the proteins that will be produced. The amino acid composition of the sow’s colostrum differs from milk and almost all EAAs are more represented in colostrum than in milk, when their values are calculated as a ratio to the Lys content in each of these products (Elliott et al. Citation1971; Wu and Knabe Citation1994; Csapo et al. Citation1996; Hurley Citation2015), reflecting the higher presence of certain proteins. For instance, immunoglobulin G (IgG), is rich in valine and proline (Eguchi-Ogawa et al. Citation2012), heavy chain of polymeric IgA and IgM, in threonine (Thr) (www.ensembl.org/pig), beta-lactoglobulin, in leucine (Rezaei et al. Citation2016), alpha-lactalbumin, in tryptophan (Trp) (Heine et al. Citation1996). All of these proteins are proportionally lower in Lys, than in mature milk proteins. This therefore leads to the hypothesis that in relative terms the need for these EAAs, expressed as a ratio to Lys, is proportionally greater for the production of colostrum than for milk.
It can therefore be hypothesised that the ideal protein requirements for the pre-parturition week and the first post-farrowing days differ substantially from the needs for milk production, even without considering the final needs for the foetuses, so much so that Theil (Citation2015) concludes that ideally the nutritional requirements of the sow should be expressed as daily requirement of each gestation or lactation day and not expressed on a standard feed basis.
Feeding requirements for sows in gestation and lactation are routinely updated by leading research organisations, including NRC (Citation2012). These are usually based on the EAAs composition of the milk, with adjustment for estimations of extraction efficiency and are validated with dose-response tests for each amino acid. However, there are no specific requirements listed for the transition phase which begins about a week before farrowing and ends when the production of colostrum changes to mature milk production (Theil Citation2015). To define EAA requirements for transition sows, a first preliminary step could be to compare the values of EAAs composition of colostrum protein with the published EAA requirements for gestation and lactation (NRC Citation2012), always taking the values of Lys as a reference unit. Thus, the EAA feeding guidelines could be adjusted for the transition sow. The evaluation of optimal feeding at this phase requires a consideration of the appropriate response variables, among which above all the growth and health of the litter. There is also interest to consider the gut microbial profile of the sow, considering the findings that supplementary free amino acids can change the porcine gut microbial community (Dai et al. Citation2010; Luise et al. Citation2020; Spring et al. Citation2020), with an impact on the piglet microenvironment. Furthermore, amino acid supplementation in late pregnancy affected the intestinal microbiota and the health of their offspring (Azad et al. Citation2020).
Starting from the differential composition of colostrum with respect to milk, this study focuses on the effect of supplementing the sows with the EAAs Thr, Trp and Val that are proportionally more concentrated in colostrum than milk. It was hypothesised that the integration of these EAAs in the diets of sows in transition (between gestation and lactation) has potential beneficial effects. Thus, the aim of this study was to evaluate the effects of a colostrum-oriented amino acid dietary supply during the transition period on the colostrum composition, productive performance up to weaning and on the health of the sows. Interest in including this measure was stimulated by the findings that supplementary free amino acids can change the porcine gut microbial community (Dai et al. Citation2010; Luise et al. Citation2020; Spring et al. Citation2020), with an impact on the piglet microenvironment. Furthermore, amino acid supplementation in late pregnancy affected the intestinal microbiota and the health of their offspring (Azad et al. Citation2020).
Materials and methods
Definition of the levels of inclusion of amino acids in the diet
In a preliminary study, the level of inclusion of amino acids in the experimental diet was defined using the composition of the colostrum of the sows present in the farm under test. Specifically, four pools were defined using the parity number as a reference (1; 2–3; 4–5; 6–10) (Table ). The number of samples per pool reflected the different parity of the sows in the farm in which the study was conducted. Colostrum samples were collected at the time of farrowing, but before the end of this, across all teats. For each pool the samples included were mixed homogeneously. The pools were then analysed by HPLC method to determine the amino acid profile outsourced to an external laboratory certified to ISO17025, NEOTRON laboratory (Modena, Italy). The analyses evidenced a clear homogeneity of amino acid content between the colostra from sow of the different parity classes (Figure ). Then, the average values of the individual amino acids in the colostrum were calculated, and these values were compared to those typical of milk, used as a reference (NRC Citation2012). From the comparison, carried out with the same Lys content, it was seen that colostrum contains more Thr (+40%), Trp (+54%) and valine (Val) (+19%) than mature milk (Figure ). This led to hypothesise that the transition diet should contain a greater amount of these AAs compared to Lys and compared to the corresponding values used in lactation feed, to allow adequate secretion of colostrum proteins. Using this ratio with respect to the NRC lactation requirement, expressed in standardised ileal digestibility values, for the colostral phase, the ratio to Lys was calculated as 88.2, 29.3, and 101.3%, respectively, for Thr, Trp, and Val.
Figure 1. Lysine content and ratio of the other essential amino acids to lysine in the pools of colostra collected from sows of the different parity class in the farm.
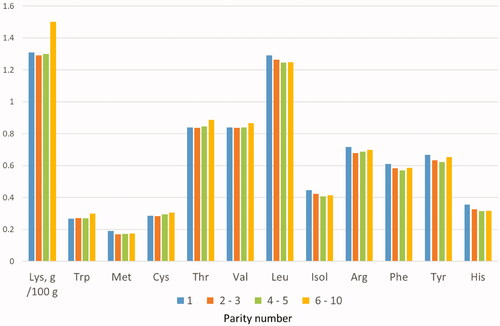
Figure 2. Comparison between the ratio of amino acids to lysine (=100) in colostra obtained in the farm (general mean of pooled samples) and sow mature milk (NRC Citation2012).
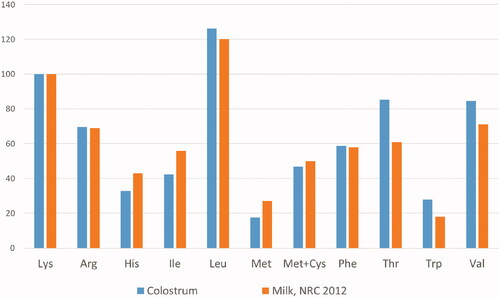
Table 1. Composition of colostrum pools used in the preliminary test for the assessment of colostrum composition.
In vivo trial
The trial was designed to assess the supplementation with Thr, Trp and Val of a feed mixture normally designed for the last phase of gestation to the initial lactation phase. The in vivo trial was carried out in a commercial multiplication unit located in the Italian Po valley and piglets were produced for the production of PDO Parma ham. The animals involved in this study were sows and piglets subjected to conventional farm rearing condition in Europe (EU) by the Dir. 120/2008 EC. The Committee for Animal Welfare of the University of Bologna stated that there was no procedure requiring additional approvals for the experimental condition on the animals.
The trial involved a total of 100 sows (PIC hybrid line), but one sow was excluded because of a leg failure before the farrowing day. Following the EU rules to guarantee pig welfare, from the fourth-week post insemination, sows were kept in groups of twenty on average and fed twice a day the same liquid diet normally used in the farm for this phase. Upon entering the delivery room, 5 d before farrowing, sows were housed in single farrowing crates of 4.5 m2 and were divided into two groups, balanced by parity number (): (i) Control group (CON; 50 sows) received the diet consisting of the formula normally used on the farm in the late gestation till the third day of lactation (Table ); (ii) Treated group (TRT; 49 sows), received the same diet as the CON group in which, however, the ratio of Thr, Trp and Val to Lys was increased, providing 0.074% L-Thr, 0.034% L-Trp and 0.077% L-Val in addition in the feed. As presented in , the inclusion of the three amino acids was calculated starting from the values in the control feed until reaching the ratios with Lys as defined with respect to the ideal value for colostrum (Figure ).
Table 2. Ingredient composition and calculated nutrient contents of the control diet and of the supplemented transition diet.
The different diets were provided to the two experimental groups at the time of entering the farrowing room and were administered with an automatic system, according to the feeding plan normally used in the farm, for 8 d during the peripartum period, with an average of 5 d before delivery and 3 d post-partum (including the day of delivery): 2.5 kg feed per day, 1 kg on the day of farrowing, 1.5 kg feed on the first day of lactation and 2.5 kg on the second and then ad libitum.
After eight experimental days, all sows were given the same standard lactating diet, therefore, without variation of amino acid supply.
Surveys and samplings
On the day of the differentiation of the diets and three days before weaning, the thickness of the dorsal back fat and muscle in the P2 position (65 mm laterally from the middle line of backbone, corresponding to the 12th rib) was measured by an ultrasound probe in all sows to determine starting body condition and subsequent tissue loss during lactation. At farrowing, the total number of piglets, the total number of alive piglets, the number of stillbirth piglets were recorded for each sow. Cross-fostering of piglets occurred among litters of the same treatment within the d1 post-farrowing, as a practice to balance the number of suckled piglets per sow. Cross-fostering of piglets in the litters was registered, to obtain the numerosity of suckled pigs per each sow after d1 post-weaning. After the cross-fostering, the dead pigs in each litter were never replaced by the additional cross-fostering pigs.
A colostrum sample was collected after the first piglet was born. This was done manually milking each sow from at least four mammary glands and involving anterior, middle and posterior glands, directly into test tubes which were immediately frozen and preserved at −20 °C. The farrowing was not induced and the sows were not treated with oxytocin before the sampling. A saliva sample was taken from the same sows approximately 2 h after the first-morning meal after farrowing, with a Salivette©-like swab, positioned for a few minutes inside the corner of the sows’ mouth.
The individual body weight (BW) of the piglets at birth and weaning (23–25 d of age) was recorded. The individual average daily gain was calculated for each piglet in each litter, including those that were added with the cross-fostering.
At weaning, a rectal disposable sterile swab was obtained by careful insertion into the anal orifice and then immediately returned to a sterile tube and frozen, from a subgroup of sows (34 sows per each group, balanced for parity number) to determine the faecal microbial profile. Interest in including this measure was stimulated by the findings that supplementary free amino acids can change the porcine gut microbial community (Dai et al. Citation2010; Luise et al. Citation2020; Spring et al. Citation2020), with an impact on the piglet microenvironment. Furthermore, amino acid supplementation in late pregnancy affected the intestinal microbiota and the health of their offspring (Azad et al. Citation2020).
Colostrum and saliva analysis
The composition of sow colostrum was analysed in triplicate for protein, fat, lactose and urea contents with infrared spectroscopy using a Milkoscan FT2 (FOSS A/S, Padova, Italia).
The content of immunoglobulins (namely IgA, IgG and IgM), in colostrum and saliva was analysed through pig immunoglobulin enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s recommendations (Bethyl Laboratories, Montgomery, TX). Briefly, Nunc Maxisorp plates (Thermo Fisher Scientific, Roskilde, Denmark) were incubated overnight at room temperature (RT) with 100 mL of purified goat antibodies with anti-pig IgA/IgG/IgM-affinity diluted to 1:100 in 0.05 M carbonate-bicarbonate coating buffer, pH 9.6. Each well was washed five times with Tris-buffered saline solution (TBS) pH 8.0, containing 0.05% Tween 20 (TBS-Tw) and then blocked at RT for 30 min with TBS blocking buffer containing 1% BSA; 100 µL of the diluted samples and the Pig Immunoglobulin Reference Serum (BETHYL, Laboratories, Montgomery, TX) which was used as standard, were added in each well in duplicate and incubated at RT for 2 h. The colostrum samples were analysed at dilutions of 1:50,000, 1:500,000 and 1:10,000 for the IgA, IgG and IgM, respectively, while the saliva samples were diluted 1:4000 for the IgA and 1:2500 for IgG analysis. The wells were then washed and reacted with 100 µL per well of the goat anti-pig sIgA/slgG/slgM-HRP conjugate (BETHYL Laboratories, Montgomery, TX) at RT for 2 h. After three washes, the ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid)) substrate (Thermo Fisher Scientific, Rockford, IL) was added to the plates, and the colour reaction was developed at RT for 15 min. The reaction was quantified spectrophotometrically at an absorbance of 405 nm by a microplate reader (Multiskan™ FC Microplate Photometer – Thermo Fisher Scientific). Concentration values, expressed in mg/mL, were calculated using a four-point parametric curve. Of note, the intra- and inter-assay CVs for these ELISA assay were ≤3 and 25%, respectively.
Analysis of faecal microbiota
From rectal swabs, total bacterial DNA was extracted using FastDNATMSpin Kit for Soil (MP Biomedicals, Santa Ana, CA, USA). The microbiological profile was characterised by sequencing the V3–V4 regions of the 16S rRNA gene. Briefly, the DNA was amplified for the V3–V4 hypervariable regions of the 16S rRNA gene amplicons were produced using the primersPro341F: 50-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNBGCASCAG-30 and Pro805R:50GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACNVGGGTATCTAATCC-30 using the PlatinumTM Taq DNA Polymerase High Fidelity (Thermo Fisher Scientific, Milan, Italy). The libraries were prepared using the standard protocol for MiSeq Reagent Kit v3 and sequenced on MiSeq platform (Illumina Inc., San Diego, CA, USA). Microbiota analysis was performed by using DADA2 pipeline (Callahan et al. Citation2016) and taxonomic categories were assigned by using Silva Database (release 132) as reference (Quast et al. Citation2013). The datasets on microbiota data are available in the PiGutNet area of the European Nucleotide Database (ENA) repository with the number PRJEB43707.
Statistical analysis
Data on the number of pigs in litters at birth and subsequently, of colostrum and saliva composition, body measures on sows were analysed using the GLM procedure of SAS version 9.3 (SAS Inst. Inc., Cary, NC) through a linear model including, diet and class of parity (1; 2; 3; 4–5; >5) as factors. The interaction between diet and parity class was tested and when it was not significant, it was removed from the model. Depending on the parameters several covariates were used and those included in the final models are reported in the tables. The individual daily gain from birth to weaning was analysed by a linear model including, diet and parity class as factors, using sows as a random effect for testing these factors.
The GENMOD procedure of SAS version 9.3 was used with a binomial distribution and Logit function to analyse mortality parameters and data on litter size ratio expressed in percentage, considering the diet, the class of parity and their interaction as factors.
Alpha and beta diversity and the abundance of taxonomic categories were analysed with R software version 3.6, by using the PhyloSeq (McMurdie and Holmes Citation2013), Vegan (Dixon Citation2003) and lme4 (Bates et al. Citation2015) packages. Alpha diversity indices were analysed with an ANOVA model, considering experimental treatment and parity class as factors. Beta diversity was analysed with a PERMANOVA model (‘Adonis’ procedure) including treatment and class of parity as factors. The effect of the diet and the parity class on Bray Curtis distance were visualised using a non-metric multidimensional scaling (NMDS) approach. The differences in taxonomic abundances between the two experimental groups were analysed with DESeq2 package (Love et al. Citation2014), based on negative binomial generalised linear models and applying the Benjamini–Hochberg method for multiple testing correction (Love et al. Citation2014).
Results
Reproductive and productive performance of sows
The effect of the supplementation of amino acids on the diet of transition sows on the litter characteristics and survival is shown in Table .
Table 3. Influence of amino acid supplementation to the diet of transition sow on litter characteristics and survival.
The diet did not influence the number of born alive (14.45 average across the two diets), of stillborn and total born pigs. Average live weight at birth (mean and SD 1328 ± 213 g, average across the two diets) and after cross-fostering, the number of totals weaned pigs and the percentages of stillborn to total born, of dead piglets first 24 h to pigs born alive, of dead piglets after 24 h to total pigs post-adoption were not affected by treatment. The supplementation of amino acids on the transition diet reduced the percentage of total dead piglets on total suckled pigs (−3.5%, p = .049), while the average daily gain of piglets until weaning was not affected by the treatments. The class of parity of sows was statistically significant for several parameters (average live weight at birth and after cross-fostering, percentage of stillborn pigs, average daily gain to weaning) and thus the least squares estimated values for the two diets were equalised for this factor.
The supplementation of amino acids on the diet of transition sow did not influence the content of protein, fat, lactose and urea of colostrum (Table ). Conversely, fat and lactose content differed according to the parity class. Particularly the fat content of colostrum for gilts was significantly higher than that of sows of all the other parity classes (p=or <.004, data not shown).
Table 4. Influence of amino acid supplementation to the diet of transition sow on the composition of colostrum.
Body condition and health of sows
The effect of the amino acid supplementation of the diet on sow metabolic effort was tested on back fat and muscle depth at 5 d before farrowing and at weaning in the two groups of sows (Table ). The absolute values for both tissues were not changed at both times, however, the supplementation of the amino acid marginally reduced (p = .097) the loss of back fat during lactation.
Table 5. Back fat and muscle depth in the two groups of sows at the start of the trial and at weaning.
The effect of the tested diets of transition sows on the concentration of Igs in colostrum and saliva is presented in Table . The supplemented diet showed an increased concentration of IgA in colostrum by 29% (p = .002), while the concentrations of IgG and IgM in colostrum and of IgA and IgG in the saliva of sows were not influenced by the diet.
Table 6. Influence of amino acid supplementation to the diet of transition sow on the concentration of immunoglobulins (Ig) of colostrum and saliva.
Faecal microbiota of sows
All the faecal samples subjected to the sequencing of the V3–V4 region of 16S rRNA microbial gene produced on average a good number of sequences (43,400 on average).
The rarefaction curves, considered a qualitative control of the sequencing method, shown in represent the number of taxonomic units (amplicon sequence variant [ASV]) identified in relation to the depth of sequencing.
Overall, all samples reached the plateau point indicating that the sequencing procedure was able to identify all the taxonomic variability present in that specific ecosystem, thus demonstrating that sequencing was optimal. The DADA2 algorithm identified a total of 8591 different ASVs. A total of 21 different phyla (mainly represented by: Firmicutes 54.8 ± 0.4% and Bacteroidetes 29.4 ± 0.3%), 66 families (mainly represented by: Ruminococcaceae 30.2 ± 0.3%, Prevotellaceae 11.4 ± 0.2% and Rikenellaceae 9.7 ± 0.5%) and 224 genera (mainly represented by: Rikenellaceae_RC9_gut_group 8.9 ± 0.5%, Treponema_2 7.9 ± 1.1% and Ruminococcaceae_UCG-002 6.0 ± 0.6%) were identified. shows the relative abundance of the most representative phyla, families and genera.
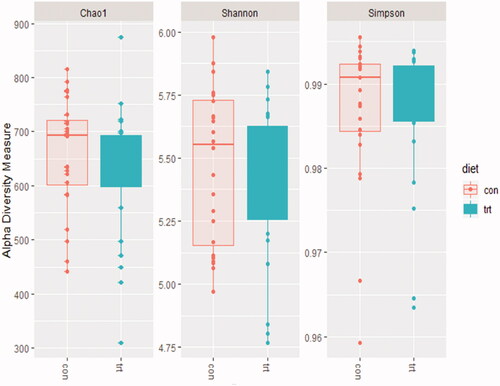
The values of Alpha diversity for the index of Chao1, Shannon and Simpson are shown in Figure . The group fed with the supplementation of the amino acids had a significantly lower value of Chao1 than the control group (p = .031).
Figure 3. Alpha diversity for the index of Chao1, Shannon and Simpson in the two dietary groups of sows. Chao1 index differed for p = .031.
Regarding the Beta diversity, no significant difference was observed between the two groups (Table ).
Table 7. Results for Adonis test for the diet and parity classTable Footnotea effect on faecal microbiota of sows.
The NMDS graph shown in Figure confirms what was observed with the statistical test as it is not possible to identify net clusters between the two diets.
Figure 4. NMDS plot for the effect of diet and parity class using the Bray–Curtis matrix of distance.
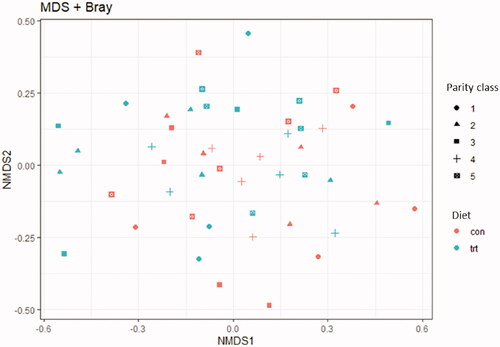
Significant differences in the beta diversity were found between sows of different parity classes (R2 0.121 p < .01) (Table ); most of the differences were related to the primiparous sows (1 vs. 5, R2 0.10 p adj <.01; 1 vs. 4, R2 0.10 p adj=.07; 1 vs. 2, R2=0.10 p adj=.05, 2 vs. 5 R2=0.07 padj =.06).
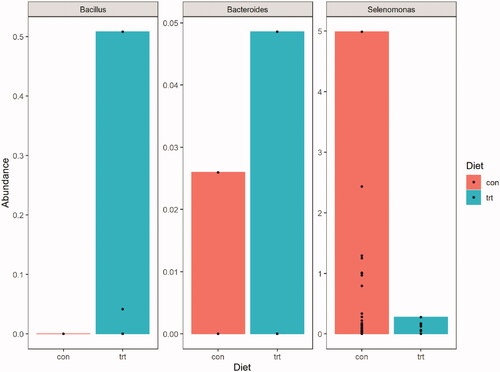
As regards the taxonomic composition, the algorithm used by DEseq2 did not show significant differences between the two groups at phylum and family taxonomical levels. Aggregating the data at the genus level, bacteria from genus Selenomonas (log2FC: −8.95 FDR <0.01) were more abundant in the control group than in the TRT group. Instead, bacteria from genus Bacillus were more represented in TRT group (log2FC:17.95, FDR: <0.01). Relative abundance of taxa differently expressed between diets is reported in Figure . At the taxonomic level of ASVs, a single ASV (ASV_475; log2FC: −10.43; FDR: <0.001) assigned to bacteria belonging to the order of Bacteroidetes that was significantly higher in the CON group compared with the TRT group.
Discussion
The concept of dedicated feeding for the gestating to lactating transition phase in sows is scientifically sounded due to the rapidly changing metabolic demands (Theil Citation2015). However, while attention was in general paid to protein, Lys, fibre concentration and total feed supply (Feyera et al. Citation2017; Feyera and Theil Citation2017; Gourley, Swanson, DeRouchey, et al. Citation2020; Gourley, Swanson, Royall, et al. Citation2020; Krogh et al. Citation2020; Feyera et al. Citation2021), the changing needs of the other EAAs received scarce attention and this is probably due to the prevalent focus on Lys demands of body tissues, foetuses and for the mature milk synthesis. In this study, we did not fully try to model the overall EAAs requirements of transition sows, but only to focus on some amino acids that are more represented in the colostrum proteins, than in milk, based on their relative ratio to Lys.
It is acknowledged that the control diet had some limitation with respect to very specific requirements suggested by recent research for the transition phase, for instance, those proposed by Feyera and Theil (Citation2017) or studied by Gourley, Swanson, DeRouchey, et al. (2020). However, this study was performed in a commercial farm. Notwithstanding the study may have this limitation, it can also have the advantage of representing a typical productive condition. Furthermore, there is a diffuse presumption by the breeders that giving gestation diets from late gestation to the first one to two days can preserve the mammary glands reducing the mammary pressure when the suckling ability of piglets is still limited (Theil Citation2015).
The main results were the reduction of the overall mortality of piglets on the total number of suckled litter and the increase of the concentration of IgA in colostrum. Among several factors that could have contributed to the improvement of piglet survival, after the piglet weight at birth, colostrum affects litter performance (Quesnel Citation2011; Declerck et al. Citation2016), due to both nutrient supply and transfer of passive immunity (Rooke and Bland Citation2002). The colostrum intake by piglets was not quantified, but it could be hypothesised that the colostrum quality could have contributed to a improved resistance of offspring of treated sows. A positive correlation between IgG in colostrum and IgG in the blood of piglets was seen (Cabrera et al. Citation2012). In this study, the concertation of IgG in colostrum was in the range reported in the literature (Quesnel Citation2011; Cabrera et al. Citation2012; Hurley Citation2015), but the TRT group of sows did not differ from CON. Increased Lys and energy provision increased the IgG colostrum content in Gourley, Swanson, DeRouchey, et al. (2020), while in an older trial, the reduction of the supply of conventional sow diet during the last 14 d of gestation from 3.4 to 1 kg did not affect the IgG content in the colostrum (Göransson Citation1990). Conversely in this latter study, the concentration of IgA in the colostrum was negatively affected by the lower feeding plan. A different origin of the different classes of Igs may explain the differentiated effects. In fact, the study of the transfer of immunoglobulins IgG, IgA and IgM from serum to colostrum and milk in the sow evidenced that all colostral IgG and a high proportion of IgM are derived from serum, but 60% of IgA are produced at the mammary level (Bourne and Curtis Citation1973). Thus, while IgG and IgM may be already in the bloodstream, it could be that the formation of IgA was more related to the mammary gland final maturation and, therefore, more depending on the nutrient provision in the last pre-farrowing days. This hypothesis is also supported by the observation that conversely no effect of the diet was seen for salivary IgA in this study.
It is possible also that an increased ratio of Thr, Trp and Val to Lys in the diet improved the sow health, resulting in a beneficial effect on the piglets’ health. Partially in agreement with this study, a previous study suggested that Trp:Lys ratio during lactation can influence piglet mortality (Fan et al. Citation2016). It is known that Trp has an important metabolite and that its requirement is associated with a response to stress. In fact, under production of cortisol and inflammatory cytokines Trp enters the kynurenine pathway and serum level decreases (Le Floc’h et al. Citation2018). Sows exhibiting a behaviour less susceptible to human presence (less immobility and more activity) in mid-gestation, had faster farrowing per piglet born and also a lower plasmatic content of Trp during the first week of lactation (Mosnier et al. Citation2009). This could indicate that Trp catabolism varies depending on individual sows and that the welfare of transition sows could be affected by Trp dietary content. Interestingly lactating sows supplemented with Trp responded with an increased serotonin level in plasma (Paulicks et al. Citation2004). Supplementary Thr in the transition phase when feed supply was restricted could have helped maintain the IgA levels at the mammary and intestinal level in the first part of lactation, and thus protect sows or piglets against local infections. In fact, dietary Thr helped piglets to produce more IgG ( 10–25 kg BW, Wang et al. Citation2006; intrauterine growth-retarded weanling piglets, Zhang et al. Citation2019) and, particularly, more IgA for piglets susceptible to enterotoxigenic Escherichia coli challenged with this microorganism (Trevisi et al. Citation2015). Finally, Val was seen to activate mTOR signalling pathway in mammary epithelial cells and thus promoted the production of protein in the colostrum of gilts (Che et al. Citation2019). Supplementing Val during late gestation improved the average daily gain of piglets and litters (Gao et al. Citation2019). However, in this study, no changes were seen for protein content in colostrum with the addition of the mix of the three AAs, nor were piglet or litter growth to weaning affected. This could indicate that the overall milk production was not changed and there was no specific effect on mammary gland tissue. This agrees with the absence of effect of increased Val to Lys ratios in lactation diet (in the range of 0.84–0.96 to 100) on the sow performance (Carter et al. Citation2000; Strathe et al. Citation2016). Conversely, the Val supplementation up to 93% Lys during late gestation in gilts linearly improved the content of DNA, RNA, and total protein in the mammary tissues at day 1 of lactation, with a positive effect on colostrum composition and litter growth (Che et al. Citation2020).
No changes were also seen for the other main components of colostrum. Dietary variations have often scarce effect on the lactose and fat content of colostrum (Hurley Citation2015). Likewise the measures of variation of body composition of the sows (back fat and muscle thickness from late gestation to weaning) were not affected, except for a marginal reduction of the loss of backfat thickness at the end of the lactation. These observations indicate that supplementing with the three EAAs only marginally changed the overall energetic state with no effect on the protein state in the body. This could also be due to the fact that the experimental treatment was not covering all the lactation. The feed in the period of supplementation was provided in fixed amounts while during the rest of lactation it was ad libitum, but it was not quantified. However, it cannot be excluded that there was a residual effect of amino acid supplementation after the end of the treatment. Actually, a positive effect of Trp addition over NRC was seen on feed intake of sows, but when the treatment was extended to the full lactation (Libal et al. Citation1997 ; Fan et al. Citation2016; Miao et al. Citation2019). Conversely, high ratios of Val (Craig et al. Citation2016) or Thr (Greiner et al. Citation2019) to Lys for the full lactation did not increase feed intake of sows). Thus, we cannot fully conclude on a possible residual effect of the additions on feed intake and/or on energetic efficiency. Nevertheless, the absence of effect on nitrogen balance agrees also with the unchanged urea content in colostrum.
Microbiological results show that the supplementation of amino acid decreased faecal bacterial richness (Chao1) but did not affect bacterial diversity (Shannon and InvSimpson) in sows. A modification in the faecal bacterial richness could be related to the higher availability of free amino acids in the gut that could have favoured the proliferation of bacteria that can use it as a growth substrate (Dai et al. Citation2010) and therefore reduced the colonisation of the gut by other bacteria. The lack of effect on bacterial diversity (Shannon and InvSimpson) was also observed in a previous study with free amino acid supplementation (Arg; Luise et al. Citation2020). Not many dissimilarities in taxonomic composition were observed, the only differences were related to the Selenomonas and Bacillus genus. The Selenomonas genus was more abundant in the control group and is known to be involved in the fibre utilisation; even though it does not have a direct fibrolytic activity, it can activate fibrolytic bacteria through an interaction known as ‘cross-feeding’ (Sawanon et al. Citation2011). The Bacillus genus was more abundant in the treated group and that could be explained by the fact that Bacilli, belonging to the so-called Clostridium clusters (Bacillus-Lactobacillus-Streptococcus), are among the most abundant amino acid fermenting bacteria (Dai et al. Citation2010). This evidence also supports the idea that the amino acid surplus favoured the proliferation of amino acid fermenting bacteria, and this modification persisted over time until the end of the lactation period. In fact, the sampling time was chosen in order to have a vision of the eventual long-term effect of the transition feed, but this may not consider of mild changes occurring in the early lactation. Furthermore, the maternal faecal microbiota represents one of the main drivers for the initial microbial colonisation of the piglet’s gastro-intestinal tract (Trevisi et al. Citation2021). Thus, proper colonisation in the early stages is fundamental in the development of the piglet’s intestinal mucosa and immune system (Chen et al. Citation2018). The modification of the sow intestinal microbiota profile induced by the treatment could have had favoured a different colonisation of the piglets’ gut, possibly affecting the immune response and reducing their mortality. This was seen also after supplementing a balanced mixture of methionine and cysteine from the end of gestation to lactation of gilts, with effect on their offspring’s blood metabolites and gut microbiome (Azad et al. Citation2020). The supplementation of the amino acids could also have made available more Thr, Trp and/or Val for other processes after the colostrum synthesis. It is well known that the amino acids are involved in supporting and maintain the gut health and the local interplay with the gut microbiota (Le Floc’h et al. Citation2018; Chalvon-Demersay et al. Citation2021). For instance, this could be the case of Thr, for the production of Igs, as already previously discussed, but also for mucins (Wang et al. Citation2010), that are important factors of the variation of the microbiota (Tailford et al. Citation2015). Finally, the effect of the parity class on the beta diversity of the faecal microbiota agrees with what seen by Berry et al. (Citation2020), who observed that the remodelling of the gut microbiome induced during gestation acts differentially in sows of first parity than those of higher parity number.
Conclusions
The trial provided a proof of concept that more attention should be paid to the specific amino acid requirements for colostrum production in sows. Considering a specific amino acid content of the diet for sows of good prolificacy during the transition phase between the end of gestation and early lactation could improve the survival of suckling piglets. In particular, this was observed for the combined supplementation with Thr, Trp and valine that increased the survival of suckling piglets and the immune content of the colostrum. Further studies are required to confirm if better piglets’ survival is directly associated with an improved transfer of immunity from the colostrum to the offspring.
Ethical approval
Procedures approved by the Italian Ministry of Health (N.2975 − 6/6/2018).
tjas_a_1960210_sm2186.docx
Download MS Word (1,012.4 KB)Acknowledgements
The authors acknowledge the support of Agricola Tre Valli Soc.Coop. and Veronesi Group, and thank Dr. Maurizio Veneri of the farm Tenuta Pasina for the help in the trial conduction.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets on microbiota data are available in the PiGutNet area of the European Nucleotide Database (ENA) repository with the number PRJEB43707. The other derived data supporting the findings of this study are available from the corresponding author [PB] on request.
Funding
Project F61–Reg.(UE)1305/2013–PSR 2014/2020 DGR Emilia-Romagna n.227/2017– FOCUS AREA 3A–Operazione 16.2.01 (Coordinator: Agricola Tre valli). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional information
Funding
References
- Auldist DE, Morrish L, Eason P, King RH. 1998. The influence of litter size on milk production of sows. Anim Sci. 67(2):333–337.
- Azad MAK, Liu G, Bin P, Ding S, Kong X, Guan G, Yin Y. 2020. Sulfur-containing amino acid supplementation to gilts from late pregnancy to lactation altered offspring's intestinal microbiota and plasma metabolites. Appl Microbiol Biotechnol. 104(3):1227–1242.
- Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J Stat Softw. 67:1–48.
- Berry AS, Pierdon MK, Misic AM, Sullivan MC, O’Brien K, Chen Y, Baldassano RN, Parsons TD, Beiting DP. 2020. Remodeling of the maternal gut microbiome during pregnancy is shaped by parity. bioRxiv.
- Bourne FJ, Curtis J. 1973. The transfer of immunoglobulins IgG, IgA and IgM from serum to colostrum and milk in the sow. Immunology. 24(1):157–162.
- Cabrera RA, Lin X, Campbell JM, Moeser AJ, Odle J. 2012. Influence of birth order, birth weight, colostrum and serum immunoglobulin G on neonatal piglet survival. J Anim Sci Biotech. 3:1–10.
- Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 13(7):581–583.
- Carter SD, Hill GM, Mahan DC, Nelssen JL, Richert BT, Shurson GC. 2000. Effects of dietary valine concentration on lactational performance of sows nursing large litters. J Anim Sci. 78(11):2879–2884.
- Chalvon-Demersay T, Luise D, Floc’h L, Tesseraud S, Lambert W, Bosi P, Trevisi P, Beaumont M, Corrent E. 2021. Functional amino acids in pigs and chickens: implication for gut health. Front Vet Sci. 8:663727.
- Che L, Xu M, Gao K, Wang L, Yang X, Wen X, Xiao H, Jiang Z. 2020. Effects of dietary valine supplementation during late gestation on the reproductive performance and mammary gland development of gilts. J Anim Sci Biotechnol. 11:15.
- Che L, Xu M, Gao K, Wang L, Yang X, Wen X, Xiao H, Jiang Z, Wu D. 2019. Valine supplementation during late pregnancy in gilts increases colostral protein synthesis through stimulating mTOR signaling pathway in mammary cells. Amino Acids. 51(10–12):1547–1559.
- Chen X, Xu J, Ren E, Su Y, Zhu W. 2018. Co-occurrence of early gut colonization in neonatal piglets with microbiota in the maternal and surrounding delivery environments. Anaerobe. 49:30–40.
- Craig A, Henry W, Magowan E. 2016. Effect of phase feeding and valine-to-lysine ratio during lactation on sow and piglet performance. J Anim Sci. 94(9):3835–3843.
- Csapo J, Martin TG, Csapo-Kiss ZS, Hazas Z. 1996. Protein, fats, vitamin and mineral concentrations in porcine colostrum and milk from parturition to 60 days. Int Dairy J. 6(8–9):881–902.
- Dai ZL, Zhang J, Wu G, Zhu WY. 2010. Utilization of amino acids by bacteria from the pig small intestine. Amino Acids. 39(5):1201–1215.
- Declerck I, Dewulf J, Sarrazin S, Maes D. 2016. Long-term effects of colostrum intake in piglet mortality and performance. J Anim Sci. 94(4):1633–1643.
- Dixon P. 2003. VEGAN, a package of R functions for community ecology. J Veg Sci. 14(6):927–930.
- Eguchi-Ogawa T, Toki D, Wertz N, Butler JE, Uenishi H. 2012. Structure of the genomic sequence comprising the immunoglobulin heavy constant (IGHC) genes from Sus scrofa. Mol Immunol. 52(3–4):97–107.
- Elliott RF, Vander Noot GW, Gilbreath RL, Fisher H. 1971. Effect of dietary protein level on composition changes in sow colostrum and milk. J Anim Sci. 32(6):1128–1137.
- Fan ZY, Yang XJ, Kim J, Menon D, Baidoo SK. 2016. Effects of dietary tryptophan: lysine ratio on the reproductive performance of primiparous and multiparous lactating sows. Anim Reprod Sci. 170:128–134.
- Feyera T, Højgaard CK, Vinther J, Bruun TS, Theil PK. 2017. Dietary supplement rich in fiber fed to late gestating sows during transition reduces rate of stillborn piglets. J Anim Sci. 95(12):5430–5438.
- Feyera T, Skovmose SJ, Nielsen SE, Vodolazska D, Bruun TS, Theil PK. 2021. Optimal feed level during the transition period to achieve faster farrowing and high colostrum yield in sows. J Anim Sci. 99(2):skab040.
- Feyera T, Theil PK. 2017. Energy and lysine requirements and balances of sows during transition and lactation: a factorial approach. Livest Sci. 201:50–57.
- Gao K, Li G, Zhu C, Wang L, Yang X, Wen X, Wu Z, Jiang Z. 2019. Effect of optimizing dietary valine-to-lysine ratio in late gestation or lactation on biochemical indices and performance of lactating primiparous sows. Anim Feed Sci Technol. 253:13–21.
- Göransson L. 1990. The effect of late pregnancy feed allowance on the milk composition of the sow's colostrum and milk. Acta Vet Scand. 31(1):109–115.
- Gourley KM, Swanson AJ, DeRouchey JM, Tokach MD, Dritz SS, Goodband RD, Woodworth JC. 2020. Effects of increased lysine and energy feeding duration prior to parturition on sow and litter performance, piglet survival, and colostrum quality. J Anim Sci. 98(5):skaa105.
- Gourley KM, Swanson AJ, Royall RQ, DeRouchey JM, Tokach MD, Dritz SS, Goodband RD, Hastad CW, Woodworth JC. 2020. Effects of timing and size of meals prior to farrowing on sow and litter performance. Transl Anim Sci. 4(2):txaa066–736.
- Greiner L, Graham A, Goncalves M, Orlando U, Touchette KJ. 2019. Evaluation of the optimal standardized ileal digestible valine: lysine ratio in lactating sow diets1. J Anim Sci. 97(7):2965–2971.
- Harper H, Silva G, Peterson BA, Hanson A, Hamilton DN, Vier CM, Soto JS, Lu N, Orlando UA. 2021. 124 Effects of different feeding levels prior to farrowing on sow and litter performance. J Anim Sci. 99(1):53–54.
- Heine W, Radke M, Wutzke KD, Peters E, Kundt G. 1996. Alpha-lactalbumin-enriched low-protein infant formulas: a comparison to breast milk feeding. Acta Paediatr. 85(9):1024–1028.
- Hurley WL. 2015. Composition of sow colostrum and milk. In: Farmer C, editor. The gestating and lactating sow. Wageningen, The Netherlands: Academic Publishers; p. 193–230.
- Krogh U, van Vliet S, Bruun TS, Feyera T, Hinrichsen T, Pedersen TF, Theil PK. 2020. Impact of dietary protein to energy ratio and two different energy levels fed during late gestation on plasma metabolites and colostrum production in sows. Livest Sci. 234:103999.
- Le Floc’h N, Wessels A, Corrent E, Wu G, Bosi P. 2018. The relevance of functional amino acids to support the health of growing pigs. Animal Feed Sci Technol. 245:104–116.
- Libal GW, Uttecht DJ, Hamilton CR. 1997. Tryptophan needs of lactating sows fed diets supplemented with crystalline lysine. J Anim Sci. 75(2):417–422.
- Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15(12):550.
- Luise D, Bertocchi M, Bosi P, Correa F, Spinelli E, Trevisi P. 2020. Contribution of L-Arginine supplementation during gestation on sow productive performance and on sow microbial faecal profile. Ital J Anim Sci. 19(1):330–340.
- McMurdie PJ, Holmes S. 2013. Phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLOS One. 8(4):e61217.
- Miao J, Adewole D, Liu S, Xi P, Yang C, Yin Y. 2019. Tryptophan supplementation increases reproduction performance, milk yield, and milk composition in lactating sows and growth performance of their piglets. J Agric Food Chem. 67(18):5096–5104.
- Mosnier E, Dourmad JY, Etienne M, Le Floc’h N, Père MC, Ramaekers P, Sève B, Van Milgen J, Meunier-SalaüN MC. 2009. Feed intake in the multiparous lactating sow: its relationship with reactivity during gestation and tryptophan status. J Anim Sci. 87(4):1282–1291.
- NRC. 2012. Nutrient requirements of swine. Washington (DC): National Research Council, National Academies Press.
- Paulicks BR, Pampuch FG, Roth-Maier DA. 2004. Selected parameters of protein and serotonin metabolism in milk and blood of lactating sows in response to the dietary tryptophan supply. Proc Soc Nutr Physiol. 13:50.
- Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. 2013. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41:D590–D596.
- Quesnel H. 2011. Colostrum production by sows: variability of colostrum yield and immunoglobulin G concentrations. Animal. 5(10):1546–1553.
- Quesnel H, Farmer C, Devillers N. 2012. Colostrum intake: influence on piglet performance and factors of variation. Livest Sci. 146(2–3):105–114.
- Rezaei R, Wu Z, Hou Y, Bazer FW, Wu G. 2016. Amino acids and mammary gland development: nutritional implications for milk production and neonatal growth. J Anim Sci Biotech. 7:20.
- Rooke JA, Bland IM. 2002. The acquisition of passive immunity in the new-born piglet. Liv Prod Sci. 78(1):13–23.
- Sawanon S, Koike S, Kobayashi Y. 2011. Evidence for the possible involvement of Selenomonas ruminantium in rumen fiber digestion. FEMS Microbiol Lett. 325(2):170–179.
- Spring S, Premathilake H, Bradway C, Shili C, DeSilva U, Carter S, Pezeshki A. 2020. Effect of very low-protein diets supplemented with branched-chain amino acids on energy balance, plasma metabolomics and fecal microbiome of pigs. Sci Rep. 10(1):15816–15859.
- Strathe AV, Bruun TS, Zerrahn JE, Tauson AH, Hansen CF. 2016. The effect of increasing the dietary valine-to-lysine ratio on sow metabolism, milk production, and litter growth. J Anim Sci. 94(1):155–164.
- Tabeling R, Schwier S, Kamphues J. 2003. Effects of different feeding and housing conditions on dry matter content and consistency of faeces in sows. J Anim Physiol Anim Nutr. 87(3–4):116–121.
- Tailford LE, Crost EH, Kavanaugh D, Juge N. 2015. Mucin glycan foraging in the human gut microbiome. Front Genet. 6:81.
- Theil PK. 2015. Transition feeding of sows. In: Farmer C, editor. The gestating and lactating sow. Wageningen, The Netherlands: Wageningen Academic Publishers; p. 415–424.
- Trevisi P, Corrent E, Mazzoni M, Messori S, Priori D, Gherpelli Y, Simongiovanni A, Bosi P. 2015. Effect of added dietary threonine on growth performance, health, immunity and gastrointestinal function of weaning pigs with differing genetic susceptibility to Escherichia coli infection and challenged with E. coli K88ac. J Anim Physiol Anim Nutr. 99(3):511–520.
- Trevisi P, Luise D, Correa F, Bosi P. 2021. Timely control of gastrointestinal eubiosis: a strategic pillar of pig health. Microorganisms. 9(2):313.
- Trevisi P, Luise D, Won S, Salcedo J, Bertocchi M, Barile D, Bosi P. 2020. Variations in porcine colostrum oligosaccharide composition between breeds and in association with sow maternal performance. J Anim Sci Biotech. 11(1):1–11.
- Vadmand CM, Krogh U, Hansen CF, Theil PK. 2015. Impact of sow and litter characteristics on colostrum yield, time for onset of lactation, and milk yield of sows. J Anim Sci. 93(5):2488–2500.
- Wang X, Qiao SY, Liu M, Ma YX. 2006. Effects of graded levels of true ileal digestible threonine on performance, serum parameters and immune function of 10–25 kg pigs. Anim Feed Sci Technol. 129(3–4):264–278.
- Wang W, Zeng X, Mao X, Wu G, Qiao S. 2010. Optimal dietary true ileal digestible threonine for supporting the mucosal barrier in small intestine of weanling pigs. J Nutr. 140(5):981–986.
- Wu G, Knabe DA. 1994. Free and protein-bound amino acids in sow's colostrum and milk. J Nutr. 124(3):415–424.
- Yuan TL, Zhu YH, Shi M, Li TT, Li N, Wu GY, Bazer FW, Zang JJ, Wang FL, Wang JJ. 2015. Within-litter variation in birth weight: impact of nutritional status in the sow. J Zhejiang Univ Sci B. 16(6):417–435.
- Zhang H, Chen Y, Li Y, Zhang T, Ying Z, Su W, Zhang L, Wang T. 2019. l-Threonine improves intestinal mucin synthesis and immune function of intrauterine growth-retarded weanling piglets. Nutrition. 59:182–187.