Abstract
The objective of this study was to examine the effects of different stocking densities on Matou goats. Thirty-six female Matou goats were randomly assigned into three experimental treatments including the low density (LD, 0.67 goat/m2), the medium density (MD, 1 goat/m2) and the high density (HD, 2 goats/m2) group. Compared with LD group, the average daily weight gain (ADG) of HD group decreased (p < .05). The length of duodenal and ileal villi in HD group was significantly lower than that in LD group (p < .05). The rumen pH and concentration of total volatile fatty acid (VFA), propionate butyrate and ammonia-nitrogen increased in HD group (p < .05). The acetate/propionate ratio was the highest in MD group (p < .05). The mRNA expression level of agouti-related protein gene (AgRP) in hypothalamus of LD was higher than that in HD group (p < 0.05). Proopiomelanocortin (POMC) mRNA expression level was the highest in MD group (p<.05) and the lowest in HD group (p < .05). An increase in growth hormone receptor (GHR), insulin-like growth factor 1 (IGF1) and claudin-1 mRNA expression level were noticed in HD group (p<.05). Compared with LD group, the serum total protein (TP), albumin (Alb), globulin (Glb) IgA and IgG levels in HD group decreased (p < .05). Glucocorticoid (GCS), β-endorphin (β-EP) and cortisol levels in serum were also significantly increased in HD group compared with LD group (p < .05). These results showed that stocking density affected rumen environment and high stocking density resulted in a decrease in growth, digestive and immune function of Matou goats.
Introduction
Matou goat is one of outstanding indigenous goat breeds in China, mainly fed in Hubei and Hunan provinces, famous for its fast growth and good meat quality (Moaeen-Ud-Din et al. Citation2008). With the development of animal husbandry in China, the production of local breeds has attracted more and more attention. Although mutton is not the most consumed meat in the world, the production of mutton has been on the rise from 1994 to 2018 (FAOSTAT Citation2020).
Proper feeding regime, correct breed selection and proper breeding density are all factors affecting economic benefits. In addition, many variables can affect nutritional efficiency, among which stocking density is one of them (Gharaghani et al. Citation2015). With the increase of mutton consumption, many farmers increase their productivity and make full use of the limited feeding area by increasing the stocking density to increase their income. However, it is unclear which stocking density is the best to growth of goats.
It has been found that the daily weight gain and feed intake of broilers decreased with the increase of stocking density in the study of broilers (Tong et al. Citation2012; Gholami et al. Citation2020). When exposed to high indoor stocking density, broilers’ movement is greatly restricted, resulting in less standing, more lying down and less preening (Sanchez-Casanova et al. Citation2019). It has been shown that low stocking density improves horses’ body condition score, coat cleanliness and coat volume and increases performance in sport, play and self-grooming, while high stocking density leads to reduced resting and feeding time (Raspa, Tarantola, Bergero, Bellino, et al. Citation2020; Raspa, Tarantola, Bergero, Nery, et al. Citation2020). Studies of dairy cows have shown similar results (Huzzey et al. Citation2006; Fregonesi et al. Citation2007).
Gastrointestinal digestive function had also been found to be somewhat affected by stocking density. González et al. (Citation2008) found that with the increase of stocking density, the rumen lactate concentration of cows increased. Clemmons et al. (Citation2020) found that differences in stocking density led to differences in some of the rumen microbial species, and these differences may affect animal production. Moreover, inappropriate stocking density induced physiological stress in animals (Huzzey et al. Citation2012; Fustini et al. Citation2017). Stress can increase the permeability of rumen, resulting in the destruction of the tight junction of gastrointestinal epithelial cells and affecting the function of gastrointestinal mucosal barrier (Soderholm and Perdue Citation2001).
In addition, high stocking densities increased the risk of disease (Lean et al. Citation2008). In broilers that were not treated with antibiotics, the faecal moisture content of broilers that were fed with high density was significantly higher than that of broilers that were fed with low density, indicating that the increased density of broilers aggravated the threat of intestinal diseases to broilers (McKeith et al. Citation2020). Compared with low-density organic turkeys, high-density turkeys were more likely to develop drug resistance and significantly reduced lysozyme and serum bactericidal activity (Mughini-Gras et al. Citation2020). The risk of heifers’ rumen acidosis and liver abscesses may increase with increasing breeding density (González et al. Citation2008).
As mentioned above, stocking density has effects on animals such as broilers, horses and heifers. There have also been studies related to goat stocking density. Study by Utsumi et al. (Citation2010) showed that high stocking density promoted the feeding behaviour of goats. Chojnacki et al. (Citation2014) found female goats conceived at high feeding densities were more prone to prenatal stress and their offspring were more prone to be fearful. However, due to the absence of research on the impact of different stocking densities on the growth of goats, farmers mostly rely on their personal experience, resulting in stocking density to ensure its effective production. Therefore, it is necessary to understand the impact of stocking density on goat growth in order to get better production efficiency. In this study, we evaluated the effects of stocking density on growth performance, gastrointestinal function and immune performance of Matou goats to provide further information.
Methods
Experimental design, goats and diets
The experimental protocol was ratified by the Animal Ethic Committee of Huazhong Agricultural University, Hubei, China, and the experiment performed with respect to the International Guidelines for research involving animals. The experimental site is Jinxin Agricultural Development Co., LTD., Enshi City, Hubei Province.
Thirty-six healthy 3-month-old female Matou goats with body weight of 22.63 ± 2.89 kg (mean ± SD) were randomly divided into three groups (12 goats in each group): low stocking density (LD) group, medium stocking density (MD) group and high stocking density (HD) group. Each group was assigned to a separate shed for a 10-d pre-experiment and 30- experiment. The stocking density of LD group was 0.67 goat/m2, and the movable area of the shed is 18 m2; the stocking density of MD group was 1 goat/m2, and the movable area of the shed is 12 m2; the stocking density of HD group was 2 goats/m2, and the movable area of the shed is 6 m2. Goats were mainly raised in shed. In order to conform to the state of most goats in China and to satisfy the habits of goats, they were grazed in pasture of the company from 10 AM to 2 PM every day. Weight of goats was measured every 10 d.
The experimental diet was prepared according to the nutritional needs of goats with initial weight of 20 kg and daily weight gain of 0.10 kg/d by referring to feeding standard of meat-producing sheep and goats of China (NY/T 816-2004). Goats were free to drink and eat. The ratio of concentrate to roughage was 40:60, which were fed every morning and evening (Table ).
Table 1. Ingredients and chemical composition of the diets (DM basis, %).
Determination of growth and slaughter performance
During the experiment, feed intake of each pen was recorded every day, and the weight of each goat was recorded every 10 d to calculate average daily weight gain (ADG). At the end of the experiment (day 30), three Matou goats from each group were selected randomly for slaughter. Organs (livers, kidney, spleen, lung and heart) were detached, cleaned and reported as a proportion of live goat’s body weight at slaughter.
Measurement of morphological parameters in small intestine
After the slaughter, 3 cm duodenum, jejunum and ileum from mid-duodenum, -jejunum and -ileum were collected, which were obtained and to measure the crypt depth (CD), crypt width and villus height. Samples were rinsed using saline solution before fixing in 4% paraformaldehyde for 24–48 h. The paraffin-coated samples were stained by hematoxylin-eosinstained before microscopic testing. The images were collected by O1ympus and analysed by Image-Pro Plus 5.02 software.
Measurement of rumen fermentation performance
After the slaughter, the pH of the rumen fluid was immediately determined with a pH metre. An aliquot of 10 mL rumen fluid was centrifuged at 10,000 xg at 4 °C for 15 min. 5 mL supernatant was mixed with 1 mL 25% metaphosphate and then stored at −20 °C. The content of volatile fatty acid (VFA) in each group was analysed using gas chromatography (GC7890A, Agilent, Santa Clara, CA) according to Wu et al. (Citation2013). After centrifugation for 10 min at 4000 xg, 2 mL supernatant was isolated and then 8 mL 0.2 mol/L hydrochloric acid was added and shaken until mixed. The concentration of ammonia-nitrogen was determined by spectrophotometer (UV-2600, Shimadzu Instrument Co., LTD, Suzhou, China) according to Wu et al. (Citation2013).
Quantitative analysis of RT-PCR
The hypothalamus tissues and rumen tissues of the Matou goat were collected and stored at −80 °C in liquid nitrogen for the determination of the mRNA expression of the feeding-related genes and rumen-related genes.
The total RNA was isolated using a total DNA extraction kit (Wuhan Sevier Biological Technology Co. LTD, Wuhan, China). The quantity and quality of the purified RNA were detected using spectrophotometer (NanoDrop-2000, Thermo, Waltham, MA). Purified RNA was diluted to make its final concentration to 200 ng/μL. The cDNA was transcribed according to the instructions of RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, MA). The reaction conditions were kept at 42 °C for 60 min and the enzyme was inactivated at 80 °C for 5 min. An aliquot of the cDNA samples was mixed with 25 μL FastStart Universal SYBR® Green Master (Rox) (Roche, Basel, Switzerland). The primers used for amplification are shown in Table . All primer sequences were synthesised by Wuhan Servicebio technology CO., LTD (Wuhan, China). Mixtures were incubated in a real-time PCR system (ABI StepOne Plus, Applied Biosystems, Foster City, CA). The thermal cycling programing was performed as follows: initial denaturation of 10 min at 95 °C; then 40 cycles at 95 °C for 15 s and at 60 °C for 60 s. The dissolution curve was measured from 75 °C and increased 1 °C every 20 s until 95 °C. Results (fold changes) were expressed as 2−ΔΔCT with ΔΔCT= (CT target gene of sample to be tested – CT internal control gene of sample to be tested) – (CT target gene of control sample – CT internal control gene of control sample). In this study, the first goat in LD group as standard, thus leading to a relative expression of 1 = 2°.
Table 2. Primers for qPCR assay.
Measurement of blood metabolites
After 30-d treatment, six Matou goats were selected from each group, blood was collected from the jugular vein. The samples were centrifuged at 3000 r/min for 15 min to obtain serum. Levels of albumin (Alb), globulin (Glb) and total protein (TP) in the serum were analysed by automatic biochemical analyser (Wuhan Shengshida Medical Equipment Co. LTD, Wuhan, China). Using ELISA kits (Beinley Biotechnology Co., LTD., Wuhan, China), levels of immunoglobulin M (IgA), immunoglobulin M (IgM), immunoglobulin G (IgG), stress-related hormones glucocorticoid (GCS), β-endorphin (β-EP) and antioxidant indexes total antioxidant capacity (T-AOC), malondialdehyde (MDA) and superoxide dismutase (SOD) in serum were measured using automatic microplate reader (Labsystems Multiskan MS, Helsinki, Finland).
Statistical analysis
All the data obtained were checked for normality and then subjected to analysis using one-way ANOVA analysis of SPSS version 26.0 software (SPSS Inc., Chicago, IL). Differences among groups were examined by Tukey’s multiple range test. For statistical purposes, all the comparisons were considered significant at p < .05. The results were expressed as mean ± SEM.
Results
Growth performance and slaughter performance
The results of the growth performance of Matou goats in response to different stocking densities are summarised in Table . As the increase of stocking density, ADG tended to decrease. ADG in the HD group was lower than that in the LD group (151.97 ± 22.41 vs. 57.67 ± 25.54, p < .05). No differences were observed in slaughter performance and organ index among different treatments (p> 0.05).
Table 3. Effects of stocking density on production performance.
Morphological parameters of duodenum, jejunum and ileum
As seen in Table , duodenal villus length (VL) in LD group and MD group was higher than that in HD group (p < .05), and the ileal VL in the LD group was significantly higher than that in the MD group and the HD group (p < .05). No differences were observed in CD and VL:CD among these groups (p>.05).
Table 4. Effects of stocking density on morphological parameters of duodenum, jejunum and ileum.
Rumen fermentation parameters
Table shows rumen fermentation performance in different groups. Compared to LD, rumen pH in HD group was decreased (p < .05) and in MD group was increased (p<.05). The ammonia-nitrogen concentration increased as the increase of stocking density (p < .05). The total VFA in LD group and MD group were lower than that in HD group (p<.05). The level of acetate was not affected by treatments. The level of propionate in rumen fluid of MD group was lower than that in the HD group (p < .05) and the contents of butyrate in LD group and MD group were significantly lower than those in HD group (p < .05). The level of acetate/propionate in rumen fluid in the MD group was higher than that of LD group and HD group (p<.05).
Table 5. Effects of stocking density on rumen fermentation parameters.
Expression of the feeding-related genes and rumen-related genes
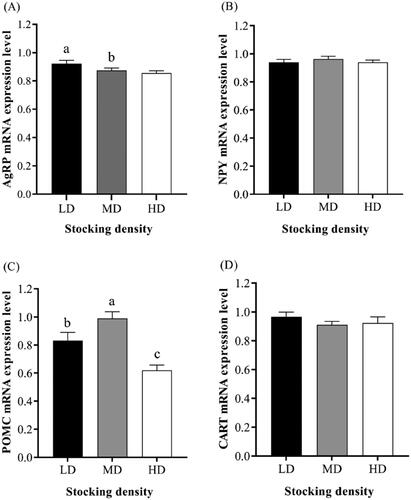
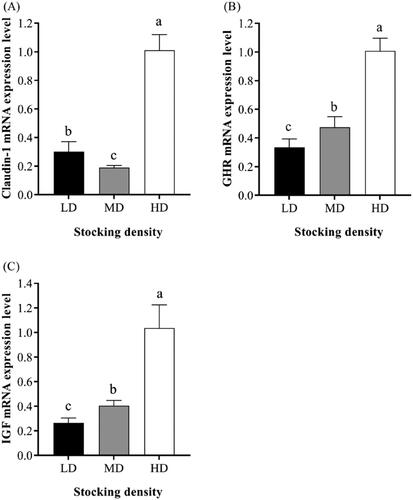
Figure shows the mRNA expression levels of feeding-related genes in the hypothalamus at different stocking densities. A depression in mRNA expression level of agouti-related protein gene (AgRP) in the HD group was noticed, compared to the LD group (0.92 ± 0.02 vs. 0.86 ± 0.05, p<.05). Moreover, compared to the LD group, mRNA expression level of proopiomelanocortin (POMC) in the MD group increased and in the HD group decreased. As shown in Figure , mRNA expression levels of claudin-1, growth hormone receptor (GHR) and insulin-like growth factor 1 (IGF1) in the rumen of HD were higher than those of MD and LD groups (p < .05).
Blood metabolites parameters
Table shows parameters of blood metabolism in different stocking densities. As seen in results, TP, Alb and Glb in the LD group were significantly higher than those in the HD group (p < .05). The levels of IgG and IgM in the LD group were higher than those in the MD group (p < .05). Interestingly, the levels of IgG and IgM in the HD group were also increased compared with the MD group (p < .05). A depression in IgA was noticed as stocking density increased (p < 0.05). GCS and β-EP in the LD group were lower than those in the HD group (p < .05). Cortisol levels in the HD group were significantly higher than those in the LD group (p < .05) and those in the MD group were significantly higher than those in the HD group (p<.05). The level of adrenocorticotropic hormone (ACTH) in serum was not affected by treatments (p > .05). The effect between different stocking densities on serum antioxidant capacity was also not significant (p > 0.05).
Table 6. Effects of stocking density on protein content, antioxidant capacity and stress hormone content of serum.
Discussion
The results of previous studies on the effect of stocking density on growth performance were quite different. Tong et al. (Citation2012) showed that with the increase of stocking density, the average daily gain and feed intake of broiler chickens decreased, but the carcase weight did not change significantly. Gholami et al. (Citation2020) showed that carcase weight of broiler chickens decreased with increasing stocking density. González et al. (Citation2008) showed that stocking density had no effect on heifers’ ADG. The differences in results of these studies may be due to differences in animal species and stocking density in the experiments. In our study, the ADG of goats in HD group have a significant decline, but feed intake and slaughter performance of each group did not change significantly.
Some hypothalamic neurons can integrate the body's appetite-related information and make appropriate adjustments to maintain the homeostasis regulation related to eating behaviour (Ferro Cavalcante et al. Citation2020). Hypothalamus is the main region for the integration and regulation of various appetite signals, among which NPY and AgRP are important appetite promoters and POMC and CART are important appetite suppressors (Morton et al. Citation2006; Fukushima et al. Citation2015; Ferro Cavalcante et al. Citation2020). In addition, POMC is a precursor of endogenous opioid peptide, which can produce ACTH and β-EP (Brenner et al. Citation1998). In this study, mRNA expression level of AgRP was a potential factor affecting the appetite of the goats, although there was no significant change in intake at different stocking densities. Contrary to expectations, results showed that the highest stocking density induced a significant decrease in POMC mRNA expression. This result is inconsistent with the changes in POMC mRNA expression under stress state found in previous studies (Baubet et al. Citation1994; Yamano et al. Citation2004).
The integrity of the intestinal barrier plays a vital role in maintaining the normal function of the intestinal tract. The VL, CD and VL:CD are important indicators reflecting the development status of the intestinal tract and the function of digestion and absorption (Jazi et al. Citation2018). In this experiment, VL in the duodenum and ileum decreased significantly in the HD group. These results are in agreement with those reported by Schroder et al. (Citation1995) who showed that when the stocking density increases, the gastrointestinal circulation blood flow decreases, producing a large number of oxidised free radicals, which damage the gastrointestinal villi. Moreover, RT-Q-PCR results showed that mRNA expression level of claudin-1 in rumen tissues of HD group was also higher than that of LD group, and mRNA expression levels of GRH and IGF1 in rumen tissues increased with the increase of stocking density. Claudin-1 has been reported to repair gastrointestinal barrier function (Han et al. Citation2020). GHR acts as a receptor for GH, which can mediate Janus kinase 2 (JAK2) and other signal pathways to participate in the regulation of body metabolism (Chandrashekar et al. Citation1999). IGF1 is an important factor regulating the growth and development of the body. It plays an important role in individual metabolic growth, cell proliferation and differentiation, bone growth and other aspects. Studies have shown that IGF1 can promote DNA synthesis of goat rumen epithelial cells in vitro (Shen et al. Citation2004) and promote gastric injury repair and gastrointestinal development (Coerper et al. Citation2001). So our study further confirms the conclusion that the gastrointestinal barrier function is impaired in the HD group. In short, stress may occur and the growth of villi in the duodenum and ileum was inhibited when stocking density increased.
Ammonia-nitrogen concentration in the rumen was an index to evaluate the degradation rate of feed protein and the rate of rumen microbial protein synthesis (Mcdonald et al. Citation1988). The change of rumen pH is closely related to the production and consumption rate of ammonia-nitrogen in rumen. In this experiment, we found that the concentration of ammonia-nitrogen in rumen increased significantly with the increase of stocking density, but the rumen pH of HD was the lowest and that of MD was the highest. Inconsistent changes in rumen pH and ammonia-nitrogen levels suggested complex effects of stocking density on rumen environment.
VFAs are the end products of dietary degradation by rumen microorganisms and the main energy source of ruminants. The composition of VFAs is related to the structure of diet and rumen microorganisms (Wang et al. Citation2020). Previous studies have shown that the decrease of CH4 production in the rumen was often associated with higher concentration of propionate and lower concentration of acetate (van Gastelen et al. Citation2015), which indicates that the energy conversion efficiency also changes. In this study, we found that acetate/propionate ratio in the MD group was higher than that in the LD group and HD group, which means goats in the MD group have a better energy conversion efficiency. In addition, an increase in the butyrate is usually accompanied by an increase in the rumen protozoa number (Morgavi et al. Citation2012; van Gastelen et al. Citation2015). We speculated that the high stocking density caused changes in rumen circulating blood flow, rumen permeability and digestion and absorption capacity of goats in each group. These changes result in inconsistent rumen fermentation environment and changes in rumen microbial composition, leading to changes in some fermentation indexes. However, the specific reasons and the situation of microflora need to be further studied.
Previous studies have shown that high stocking density can easily lead to animal stress (Huzzey et al. Citation2012; Fustini et al. Citation2017). This conclusion was also supported by increased levels of β-EP and GCS in serum as stocking density increased in our study. β-EP is an important opioid peptide that regulates the effects of stress on mental states (McGonigle et al. Citation2016). GCS, a steroid hormone, can regulate the metabolism of protein, fat, sugar and other nutrients in the body. As an important regulator of the hypothalamus–pituitary–adrenal (HPA) axis, GCS can regulate the metabolic balance of the body and promote the stress response of the body, so GCS is also known as ‘stress hormone’ (Zulkifli and Siegel Citation1995). Cortisol acts through the HPA axis to promote protein degradation and catabolism (Yoshioka et al. Citation2005). When animals are stressed, cortisol levels in the body increase (Sharma et al. Citation2013). Interestingly, results from our study indicate that with the increase of stocking density, the contents of TP, Alb and Glb in serum gradually decreased. However, the MD group had the highest cortisol level of the three groups. The level of IgA in serum decreased with increasing stocking density. However, the MD group had the lowest IgG and IgM levels of the 3 groups. These results suggest that stress is not the only factor causing changes in protein metabolism and immune performance in different treatments of goats so that further research may be needed.
Conclusion
In this study, different stocking densities had effects on Matou goats. Overall, high stocking density had adverse effects on ADG, intestinal morphological parameters and immune performance. The variation of rumen fermentation parameters was complex, which might mean that the composition of rumen microorganisms varied with different stocking densities. Results from the present study highlight the importance of density to Matou goats being raised. Further research is needed to determine the optimal stocking density and potential effects of stocking density on rumen microorganisms.
Ethical approval
The research protocol was approved by the Animal Ethic Committee of Huazhong Agriculture University.
Acknowledgements
The authors are grateful to the Jinxin Agricultural Development Co., Ltd. (Enshi, Hubei province, China) for allowing us to use their animals. We also thank the Department of Animal Nutrition and Feed Science of Huazhong Agricultural University for its assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data and materials supporting the results or analyses presented in this paper are available upon reasonable request.
Additional information
Funding
References
- Baubet V, Fèvre-Montange M, Gay N, Debilly G, Bobillier P, Cespuglio R. 1994. Effects of an acute immobilization stress upon proopiomelanocortin (Pomc) messenger-Rna levels in the mediobasal hypothalamus - a quantitative in-situ hybridization study. Brain Res Mol Brain Res. 26(1–2):163–168.
- Brenner I, Shek PN, Zamecnik J, Shephard RJ. 1998. Stress hormones and the immunological responses to heat and exercise. Int J Sports Med. 19(2):130–143.
- Chandrashekar V, Bartke A, Coschigano KT, Kopchick JJ. 1999. Pituitary and testicular function in growth hormone receptor gene knockout mice. Endocrinology. 140(3):1082–1088.
- Chojnacki RM, Vas J, Andersen IL. 2014. The effects of prenatal stocking densities on the fear responses and sociality of goat (Capra hircus) kids. PLoS One. 9(4):e94253.
- Clemmons BA, Campbell MA, Schneider LG, Grant RJ, Dann HM, Krawczel PD, Myer PR. 2020. Effect of stocking density and effective fiber on the ruminal bacterial communities in lactating Holstein cows. PeerJ. 8:e9079..
- Coerper S, Wolf S, von Kiparski S, Thomas S, Zittel TT, Ranke MB, Hunt TK, Becker HD. 2001. Insulin-like growth factor I accelerates gastric ulcer healing by stimulating cell proliferation and by inhibiting gastric acid secretion. Scand J Gastroenterol. 36(9):921–927.
- FAO (Food and Agriculture Organization of the United Nations). 2020. FAOSTAT. http://www.fao.org/faostat/zh/#data/QA.
- Ferro Cavalcante TC, de Farias Campina RC, de Souza JA, Marcelino da Silva AA, Lopes de Souza S. 2020. Hypothalamic peptide and nutrient sensors gene expression in the hypothalamus of neonatal rat. Brain Res Bull. 164:214–220.
- Fregonesi JA, Tucker CB, Weary DM. 2007. Overstocking reduces lying time in dairy cows. J Dairy Sci. 90(7):3349–3354.
- Fukushima A, Hagiwara H, Fujioka H, Kimura F, Akema T, Funabashi T. 2015. Sex differences in feeding behavior in rats: the relationship with neuronal activation in the hypothalamus. Front Neurosci. 9:88.
- Fustini M, Galeati G, Gabai G, Mammi LE, Bucci D, Baratta M, Accorsi PA, Formigoni A. 2017. Overstocking dairy cows during the dry period affects dehydroepiandrosterone and cortisol secretion. J Dairy Sci. 100(1):620–628.
- Gharaghani H, Shariatmadari F, Torshizi MA. 2015. Effect of fennel (foeniculum vulgare mill.) used as a feed additive on the egg quality of laying hens under heat stress. Rev Bras Cienc Avic. 17(2):199–207.
- Gholami M, Chamani M, Seidavi A, Sadeghi AA, Aminafschar M. 2020. Effects of stocking density and environmental conditions on performance, immunity, carcase characteristics, blood constitutes, and economical parameters of cobb 500 strain broiler chickens. Ital J Anim Sci. 19(1):524–535.
- González LA, Ferret A, Manteca X, Ruíz-de-la-Torre JL, Calsamiglia S, Devant M, Bach A, 2008. Performance, behavior, and welfare of Friesian heifers housed in pens with two, four, and eight individuals per concentrate feeding place. J Anim Sci. 86(6):1446–1458.
- Han R, Wang L, Zhao ZG, You LJ, Pedisic S, Kulikouskaya V, Lin ZQ. 2020. Polysaccharide from Gracilaria Lemaneiformis prevents colitis in BALB/c mice via enhancing intestinal barrier function and attenuating intestinal inflammation. Food Hydrocolloid. 109:106048. [accessed 2020 December 31]:[13 p.].
- Huzzey JM, DeVries TJ, Valois P, von Keyserlingk MAG. 2006. Stocking density and feed barrier design affect the feeding and social behavior of dairy cattle. J Dairy Sci. 89(1):126–133.
- Huzzey JM, Nydam DV, Grant RJ, Overton TR. 2012. The effects of overstocking Holstein dairy cattle during the dry period on cortisol secretion and energy metabolism. J Dairy Sci. 95(8):4421–4433.
- Jazi V, Foroozandeh AD, Toghyani M, Dastar B, Rezaie Koochaksaraie R, Toghyani M. 2018. Effects of Pediococcus acidilactici, mannan-oligosaccharide, butyric acid and their combination on growth performance and intestinal health in young broiler chickens challenged with Salmonella typhimurium. Poultry Sci. 97(6):2034–2043.
- Lean IJ, Westwood CT, Playford MC. 2008. Livestock disease threats associated with intensification of pastoral dairy farming. N Z Vet J. 56(6):261–269.
- Mcdonald CL, Rowe JB, Gittins SP, Smith JAW. 1988. Feed additives for attracting sheep to eat a pelleted diet during assembly for live export. Aust J Exp Agric. 28(6):719–723.
- McGonigle CE, Nentwig TB, Wilson DE, Rhinehart EM, Grisel JE. 2016. β-endorphin regulates alcohol consumption induced by exercise restriction in female mice . Alcohol. 53:51–60.
- McKeith A, Loper M, Tarrant KJ. 2020. Research note: stocking density effects on production qualities of broilers raised without the use of antibiotics. Poult Sci. 99(2):698–701.
- Moaeen-Ud-Din M, Yand LG, Chen SL, Zhang ZR, Xiao JZ, Wen QY, Dai M. 2008. Reproductive performance of Matou goat under sub-tropical monsoonal climate of Central China. Trop Anim Health Prod. 40(1):17–23.
- Morgavi DP, Martin C, Jouany JP, Ranilla MJ. 2012. Rumen protozoa and methanogenesis: not a simple cause-effect relationship. Br J Nutr. 107(3):388–397.
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. 2006. Central nervous system control of food intake and body weight. Nature. 443(7109):289–295.
- Mughini-Gras L, Di Martino G, Moscati L, Buniolo F, Cibin V, Bonfanti L. 2020. Natural immunity in conventionally and organically reared turkeys and its relation with antimicrobial resistance. Poult Sci. 99(2):763–771.
- Raspa F, Tarantola M, Bergero D, Bellino C, Mastrazzo CM, Visconti A, Valvassori E, Vervuert I, Valle E. 2020. Stocking density affects welfare indicators in horses reared for meat production. Animals. 10(6):1103.
- Raspa F, Tarantola M, Bergero D, Nery J, Visconti A, Mastrazzo CM, Cavallini D, Valvassori E, Valle E. 2020. Time-Budget of horses reared for meat production: influence of stocking density on behavioural activities and subsequent welfare. Animals. 10(8):1334.
- Sanchez-Casanova R, Sarmiento-Franco L, Segura-Correa J, Phillips CJC. 2019. Effects of outdoor access and indoor stocking density on Bbehaviour and stress in broilers in the subhumid tropics. Animals. 9(12):1016.
- Schroder J, Wardelmann E, Winkler W, Fandrich F, Schweizer E, Schroeder P. 1995. Glutamine dipeptide-supplemented parenteral nutrition reverses gut atrophy, disaccharidase enzyme activity, and absorption in rats . JPEN J Parenter Enteral Nutr. 19(6):502–506.
- Sharma S, Ramesh K, Hyder I, Uniyal S, Yadav VP, Panda RP, Maurya VP, Singh G, Kumar P, Mitra A, et al. 2013. Effect of melatonin administration on thyroid hormones, cortisol and expression profile of heat shock proteins in goats (Capra hircus) exposed to heat stress. Small Ruminant Res. 112(1–3):216–223.
- Shen Z, Seyfert HM, LöHrke B, Schneider F, Zitnan R, Chudy A, Kuhla S, Hammon HM, Blum JW, Martens H, et al. 2004. An energy-rich diet causes rumen papillae proliferation associated with more IGF type 1 receptors and increased plasma IGF-1 concentrations in young goats. J Nutr. 134(1):11–17.
- Soderholm JD, Perdue MH. 2001. Stress and the gastrointestinal tract II. Stress and intestinal barrier function. Am J Physiol-Gastr L. 280(1):G7–G13.
- Tong HB, Lu J, Zou JM, Wang Q, Shi SR. 2012. Effects of stocking density on growth performance, carcass yield, and immune status of a local chicken breed. Poultry Sci. 91(3):667–673.
- van Gastelen S, Antunes-Fernandes EC, Hettinga KA, Klop G, Alferink SJJ, Hendriks WH, Dijkstra J. 2015. Enteric methane production, rumen volatile fatty acid concentrations, and milk fatty acid composition in lactating Holstein-Friesian cows fed grass silage- or corn silage-based diets. J Dairy Sci. 98(3):1915–1927.
- Wang LJ, Zhang GN, Li Y, Zhang YG. 2020. Effects of high forage/concentrate diet on volatile fatty acid production and the microorganisms involved in VFA production in cow rumen. Animals. 10(2):223.
- Wu DQ, Tang SX, He ZX, Odongo EN, Tan ZL, Han XF, Zhou CS, Kang JH, Wang M. 2013. Oleic and linoleic acids alter fermentation characteristics, methane and fatty acid isomers production during in vitro incubation with mixed ruminal microbes. J Food Agric Environ. 11(2):464–469.
- Utsumi SA, Cibils AF, Estell RE, Baker TT, Walker JW. 2010. One-seed juniper sapling use by goats in relation to stocking density and mixed grazing with sheep. Rangeland Ecol Manag. 63(3):373–386.
- Yamano Y, Yoshioka M, Toda Y, Oshida Y, Chaki S, Hamamoto K, Morishima I. 2004. Regulation of CRF, POMC and MC4R gene expression after electrical foot shock stress in the rat amygdala and hypothalamus. J Vet Med Sci. 66(11):1323–1327.
- Yoshioka G, Imaeda N, Ohtani T, Hayashi K. 2005. Effects of cortisol on muscle proteolysis and meat quality in piglets. Meat Sci. 71(3):590–593.
- Zulkifli I, Siegel PB. 1995. Is there a positive side to stress. World Poultry Sci J. 51(1):63–76.