 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Mannara dogs have long been bred in Sicily (Italy) to work alongside shepherds as flock guardians. This study provides a morphologic, genealogic, and genomic characterisation of the Mannara dog, useful in light of its recognition process and to improve the breed standard. Morphologic measurements of body, head, and chest were taken on 111 adult Mannara dogs. The whole population pedigree was used to calculate the inbreeding coefficient (F) and five effective population size (Ne) parameters. Twelve Mannara dogs were genotyped using the Canine 230 K SNP BeadChips and compared with Maremma sheepdog, Caucasian shepherd dog, Cane Corso Italiano, and Neapolitan mastiff for population structure, heterozygosity, and runs of homozygosity. The morphometric evaluation showed that Mannara dogs generally accords with the provisional standard and can be classified as a large/giant, meso-dolicomorphic, and mesocephalic breed. The population consists of 375 individuals, one third of which are founders and the remaining belong to 58 litters; presenting low inbreeding (F = 0.7%) and balanced sires and dams. The Ne estimates range widely: two (NeN=159 and NeFi=50) exceed the FCI limit for breed recognition and one (NeCi=25) did not. Genetically, all the included populations are well distinct, with the Maremma sheepdog being the nearest to the Mannara dog. Five Mannara have a single ancestral component, while the others show higher admixed proportions. The genomic inbreeding and heterozygosity confirm the good management of the breed. Our analyses suggest that the Mannara breed should continue the recognition process, pivotal to preserving an invaluable canine resource for the Sicilian agriculture.
The morphometric measurements of Mannara dogs generally accords with the provisional standard.
The pedigree analysis reveals that the population is well managed and meets the criteria for FCI recognition.
The Mannara dog presents a unique genomic background.
Highlights
Introduction
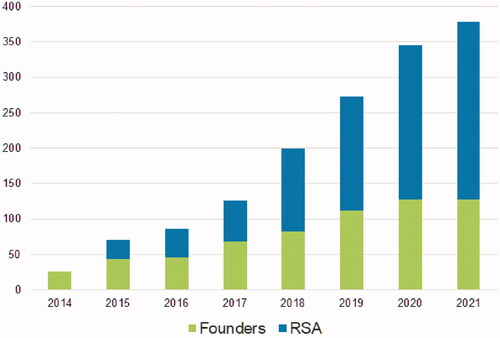
Italy boasts a great wealth of local dog breeds. Seventeen of these are officially recognised by National Agency of Italian kennel club (ENCI) and Federation Cynologique Internationale (FCI); others are in the process of being recognised, like the Oropa dog in Piedmont, the Lessina and Lagorai sheepdog in Triveneto, the Apuan Alps dog in Tuscany, the Fonni’s dog in Sardinia, the Sila sheepdog in Calabria, and the Mannara dog, also called Sicilian Mastiff, in Sicily. Mastiff-type dogs, likely ancestors of the Mannara dog, have been in Sicily since ancient times, probably as early as the Bronze age, as shown by the finding of bones in archaeological sites. It is believed that Phoenicia brought them to Sicily in the 1st millennium BC during their frequent trade in the Mediterranean area and that they are probably direct descendants of the Molossus of Epirus (Arengi Citation2011). We can find evidence of their presence in the Marmetines coins. Marmetines arrived in Sicily between the end of 4th century and the beginning of 3rd century BC, hired by Agathocles, Tyrant of Syracuse, and dominated the island for about 20 years. The coins show a mastiff guarding Adranus’ Temple, in the province of Catania. Over time, the breed has been influenced by North African nomad Berber shepherds’ dogs, imported to Sicily probably during the Sicilian wars between the end of 4th century and the beginning of the 3rd century BC and then in 878 AD during Arab rule, which influenced Sicilian agriculture for over two centuries. Since ancient times, Mannara dogs have been meant to guard the ‘mannara’ (from arabic ‘manzrah’: closed area), which in the local dialect refers to a traditional enclosure where sheep and goats are usually penned at night. The ‘mannara’ was generally made of a circular dry-stone wall, a metre and a half high, on which people usually put branches of spiny broom and blackthorn in order to protect the herd from predators, mainly wolves (extinguished since 1935 in Sicily). Chicoli (Citation1870) in his ‘Reproduction, Breeding, and Improvement of Domestic Animals in Sicily’ claimed that shepherds chose a specific local dog breed, called sheepdog (large dogs with woolly coat), to guard and protect the herd from the aggression of carnivorous animals (specifically wolves). Moreover, in order to prevent wolves from choking dogs, shepherds used to create specific spiked leather collars (Figure ). Even Migneco (Citation1987) talks about a sheepdog in Sicily: height over the average, stocky with a big head and squared muzzle, small slightly drop ears, coarse and curly long-haired coat, long tail up to the hock. Their coat was faded black and they were ferocious and always won against wolves. The breed has been preserved in the Island, integrated in the local agricultural system where cattle breeders selected them on a functional basis. However, only in January 2010, thanks to the foundation of SAMANNARA society, their true recovery path started. In 2013, after a first regional census, SAMANNARA submitted the first provisional Standard to ENCI (ENCI Citation2013). This Standard describes the breed and classifies it in the FCI group 2: Pinscher and Schnauzer – Molossoid and Swiss Mountain and Cattledogs, Section 2a: Mastiff type, N° 903. In October 2014, ENCI established a specific breeding book, called RSA (Open Additional Register) where dogs are registered if, after a zootechnical analysis by a committee of expert judges designated by ENCI, they comply with the characteristics described by the provisional Standard (founders) or if they were born from already enrolled dogs. Furthermore, from 29 September 2020, the Mannara dog, together with the other Italian breeds in the process of being recognised, can participate in competitive dog shows in Italy. So far, the Mannara dogs enrolled in RSA are 375, 124 of which are founders (April, 2021). Figure shows the annual trend of registration to the RSA book.
Figure 1. Photo of a Sicilian family with their 4-month-old Mannara dog wearing the typical spiked leather collar (Palermo, 1968).

Figure 2. Annual Mannara dog population from 2014, year of the establishment of the studbook (RSA), to present. Green = founders enrolled in the RSA after being phenotypically evaluated by breed expert judges; blue = registered offspring of dogs already in possession of the pedigree.
Given the historical importance of the Mannara dog, as well as the role it still plays in the Sicilian animal husbandry, it is important not only to acknowledge the work of all the breeders that contributed to the creation and the improvement of the population, but also to ensure that these dogs continue to stand alongside the shepherds working as guardians. For these reasons, we characterise the Mannara dog from a morphological, genealogical, and genomic perspective. This information can be used to define a definitive breed standard and, ultimately, to provide evidence for its official recognition as a breed.
Materials and methods
This study was performed according to the ethical principles that have their origins in the Italian Veterinarians’ Ethical Code (Passantino Citation2007) and the Italian and European regulations on animal welfare (Directive 2010/63/EU Citation2010). Experimental protocol was approved by the Ethical committee of the Department of Veterinary Science of the University of Messina, Italy (code 040/2020).
Morphometric traits
A total of 111 adult Mannara dogs, 60 males and 51 females, were measured and evaluated during the official meetings to be enrolled in the additional Italian Kennel Club (ENCI) studbook for the dog breeds with limited diffusion, called Open Additional Register (RSA). The sampled dogs were aged between 1 and 5 years. All the measurements and evaluations have been taken by the same commission composed of three experts, who had a common training and standard evaluation criteria.
Linear morphologic evaluation was preceded by individual descriptions concerning the subject’s personal data (date of birth and microchip number), body coat (hair and colour), bite, and teeth number.
Linear measures such as height at the withers, body length, chest width, chest height, and chest length were taken using a measuring stick. Other linear measures such as chest and pastern circumference were measured using a graduated plastic tape, while the head length, cranium length, and bizygomatic distance were measured using a calliper. Morphometric variables and reference point for measurements are depicted in Figure .
Figure 3. Representation of the ideal Mannara dog, by Paolo Maranto, FCI dog judge and ENCI board member of the SAMANNARA kennel club. The reference points for the body (A) and head (B) measurements taken in the present study are represented.
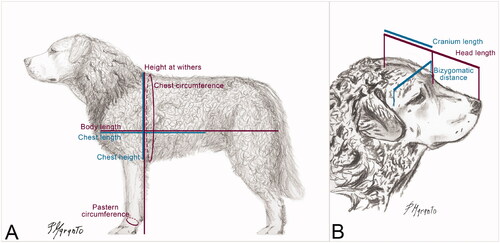
Furthermore, the following morphological relation indices were calculated as described by Bonetti (Citation1995) and Oğrak et al. (Citation2014): the ratio between bizygomatic distance and cranium length (Cephalic index), the ratio between body length and chest circumference (Body index), the ratio between chest width and chest height (Thoracic index), and the ratio between pastern circumference and height at withers (Bone index).
All dogs were weighed using a specific digital scale (Steinberg systems, mod. SBS-PS-150), which has a weighing accuracy of 50 g with a maximum load of 150 kg. The birth weight of 50 puppies (30 males and 20 females) was measured as well.
Pedigree analyses
The pedigrees of the whole population of Mannara dogs were analysed using the free R package optiSel (Wellmann Citation2019). Pedigree depth was evaluated in terms of number of fully traced generations (i. e., in which all the ancestors are known) and number of maximum generations traced (i. e., in which at least one ancestor is known). Pedigree completeness was defined as the proportion of known ancestors in each generation x in dogs with a number of maximum generations traced ≥ x. A generation interval (GI) was calculated for all the four pathways (mm = sons of sires, mf = daughters of sires, fm = sons of dams, and ff = daughters of dams), as the average age of dogs at birth of their offspring. Pedigree-based inbreeding coefficient (F) was defined as the proportion of homozygous genes identical by descent, and average relatedness (AR) as the probability of a randomly chosen allele in the whole pedigree population to belong to a given animal.
The effective population size (Ne), namely the size of a random mating population that shows the same decrease of genetic diversity than the population under study, was calculated in different ways (Leroy et al. Citation2013) on a reference population (n = 311) consisting of all the puppies born over a period of five years (2016-2021) and their ancestors over at least three generations (FCI General Assembly Citation2019). The classic Ne based on sex ratio (Nes) was computed with the formula: where M and F are the number of males and female in the population, respectively. A second method was based on the variance of progeny size and derived Nev from the formula:
(Hill Citation1972). In this formula, σ2 and σ are the observed variance and covariance, respectively, of progeny size in the different pathways; Mr and Fr are the mean annual numbers of new parents; and T is the generation interval referred to the useful offspring (i.e., which in turn reproduce) only. A method based on individual inbreeding rate (Fi) and number of equivalent complete generations traced (EqGi) was used to calculate
being the average of each individual’s
(Gutiérrez et al. Citation2009). The coancestry rate (
) between two individuals (i and j) was calculated as:
was averaged and used to obtain
(Cervantes et al. Citation2011). For NeFi and NeCi, only dogs with EqGi ≥ 1 were included. The native effective size (NeN) evaluates the Ne at the time when the individuals were born and was calculated by optiSel according to Wellmann and Bennewitz (Citation2011) formula:
where
estimates the rate of increase in mean coancestry per generation, D being the decreasing differentiable function that approximates the population’s gene diversity and
its derivative.
Genomic analyses
The genomic study included 63 unrelated dogs belonging to the following breeds: n. 5 Caucasian shepherd dog (CAUC), n. 20 Cane Corso Italiano (CCIT), n. 12 Mannara dog (MANN), n. 20 Maremma sheepdog (MARM), and n. 12 Neapolitan mastiff (NEAP).
Individual blood samples from the Mannara dogs were collected and approximately 3 mL were placed in a sterile tube with ethylenediamine tetra-acetic acid (EDTA) and immediately refrigerated/frozen until the analysis. DNeasy Blood & Tissue Kit (QIAGEN®, Germany) was used to extract the DNA. Concentration and purity of all the DNA samples were assessed using NanoDrop 1000 spectrophotometer (Thermo Scientific®, USA). The genotyping was performed in outsourcing with Canine 230 K SNP BeadChips on an iScan System (Illumina®, USA). Data of the other dogs used for the comparison came from previous studies (Parker et al. Citation2010; Talenti et al. Citation2018).
Using PLINK 1.9 software (Purcell et al. Citation2007), all the genotypes underwent a screening that retained only individuals with minimum call rates of 95% and SNPs located on autosomes characterised by minimum call rates of 95% and minor allele frequency (MAF) of 1%.
PLINK 1.9 was also used to perform a multidimensional scaling analysis (MDS), which allowed us to visualise the overall genetic distances among the dogs included in this study. Reynolds distances were computed using an in-house script (Reynolds et al. Citation1983).
The genetic admixture of all the individuals was analysed with ADMIXTURE 1.3 (Alexander and Lange Citation2011). The number of genetic clusters (K) ranged from 2 to 7. The lowest cross-validation value (cv) defined the best fitting K. Individual ancestry fractions (Q-values) were indagated.
PLINK 1.9 allowed us to calculate Expected Heterozygosity (He), Observed Heterozygosity (Ho), and Wright’s Fixation Index (FIS) in each breed. Moreover, a sliding window approach was used to estimate Runs of Homozigosity (ROH) in all the subjects. A ROH was called if: homozyg-window-snp ≥50, homozyg-window-het = 0, homozyg-window-missing ≤5, homozyg-snp ≥50, homozyg-kb ≥1000, homozyg-density ≥50, and homozyg-gap ≤100. The ROH-based inbreeding coefficient (FROH) was calculated as described by McQuillan et al. (Citation2008): in which
is the total length of all ROH above a specified minimum length and
is the length of the autosomal genome covered by the chip SNPs (McQuillan et al. Citation2008; Sams and Boyko Citation2019). This parameter was calculated both for the total ROH and for five different classes of ROH length: from 1 to 2 Mb, from 2 to 4 Mb, from 4 to 8 Mb, from 8 to 16 Mb, and over 16 Mb.
Results
Morphometric traits
On the basis of the mean and range weight across all adult individuals (Table ), Mannara dog breed was assigned to the large and giant size class in according to Salt et al. (Citation2017) size dog classification and categorisation. As regards the birth weight, the mean ± sd is of 720 ± 53 g in male puppies and 580 ± 110 g in female ones.
Table 1. Mean ± standard deviation, minimum, and maximum values of the individual somatic measurements and conformation indices for adult male and female Mannara dogs.
In general, the evaluation of the morphological traits indicates sexual dimorphism in the breed, as it is known for the domestic dog species (Sutter et al. Citation2008) and according to the provisional standard. In fact, males and females show different size and proportions as evidenced by the conformation indices (Table ). The relative calculated indices made it possible to classify this canine population in the meso-dolichomorph type, although a certain variability emerged within the sample studied, which also recorded the presence of subjects attributable to both the mesomorphic and dolichomorphic type.
The shape of the skull is the most important criterion for determining standard dog breeds. For this reason, the shape of the dog's skull was studied with the Cephalic index, whose average value was in line with the provisional breed standard, ‘not less than 53′ (ENCI Citation2013). A Cephalic index greater than 55 indicates a brachycephalic dog; between 50 and 55 indicates a mesocephalic dog; less than 50 indicates a dolichocephalic dog (Solaro 1958; Bonetti Citation1995). The head must in any case be moderately massive, broad, of the typical truncated cone shape (locally called ‘cannon’), and with slightly diverging craniofacial axes (Figure ).
Figure 4. Ideal head of Mannara dogs, with the typical ‘cannon’ shape. Photos by courtesy of the breeders.

As for the Body index values, the males were longer than the females and classified as dolichomorphic, while the females as mesophorphic (Table ). Body indices greater than 85 indicate that the animal is long; between 71 and 84 indicate that the animal is average; and less than 70 indicate that the animal is short (Bonetti Citation1995). The long animal is best suited for speed, the short animal for strength, with the medium animal showing intermediate skills.
The average Thoracic index values result superimposable in both sexes, though with a wide variability (Table ) probably attributable to the different age of the animals (1–5 years old) and consequently to the development of their thorax. Within the mesomorphic type, it is possible to discriminate animals as ‘trotter’ or ‘galloping’ type if presenting an higher or lower Thoracic index value, respectively (Bonetti Citation1995).
No specimens have been recorded with a natural lack of a tail, which is hanging in repose and carried high as a scimitar when the dog is in attention. Regarding the bite, a frequency of 50% of scissor bite, of 33% of slightly undershot (but no more than 5 mm), and of 17% of level bite was registered.
The coat, regardless of the colour, is medium long with woolly and dense undercoat, slightly or very wavy up to curly (Figure ). The coat on the muzzle and the limbs is typically short, although the males present a longer and thicker coat around the neck forming a mane.
Figure 5. Typical Mannara dogs. (A) Seven-month-old female Mannara dog, with red and white coat. (B) Adult female Mannara dog with brindle tricolour coat. (C) Adult female Mannara dog with black and white (locally called ‘nun’) coat. (D) Seven-month-old male Mannara dog with black and tan coat (locally called ‘four-eyes’). (E) Adult male Mannara dog, with black and white (locally called ‘nun’) coat, while working with a sheep herd. Photos by courtesy of the breeders.

The most common coat colour in our samples is black at different grades of intensity, with light brown markings on the eyes and muzzle (i.e., black and tan, locally called ‘four-eyes’ due to the light markings above the eyes) or with white markings on the muzzle, throat and chest, feet, and tip of the tail (locally called ‘nun’ for the similarity with the colours of the dress). The red, liver (with various dilutions), and brindle coat colours are also observed and admitted by the standard. The only colour that represents a disqualification flaw is solid white, not present in any of the included dogs.
Pedigree analyses
As reported in Table , the whole population of Mannara dogs consists of 375 dogs, with almost the same number of males and females. Of these, 20% had offspring and no statistically significant differences were found between the number of male and female parents. Their offspring size, namely the total number of progenies, is not normally distributed and ranges from 1 to 41, with a mean ± standard deviation of 6.69 ± 5.58 and a median of 6.00. Of the four pathways, fm has the lower mean (3.02 ± 2.71) and mf the higher one (3.74 ± 3.08), but there are no significant differences among the different pathways. Excluding the single outlier sire, who has an offspring size of 41, male dogs’ offspring size would become 6.4 ± 3.4 with a median of 6.00. On average, each litter is composed of 4.32 ± 2.27 dogs, with a median of 4.00, and 58 litters in total are registered. The mean number of litters from each dog used for breeding is 1.57, ranging from 1 to 9, with no significant differences between the number of times that males (1.74) and females (1.44) reproduced. The GI for male parents (2.74 ± 1.52) is slightly higher than for female ones (2.34 ± 1.42), but it does not significantly differ among the four pathways; a GI of 2.51 was obtained averaging the values for the four pathways. The distribution of the age of the parents at the moment of the birth of their offspring is represented in Figure .
Figure 6. Boxplot representing the age of the parents (males and females) at the moment of the birth of their offspring. The box represents the values from the first to the third quartile; the middle line represents the median and the X the mean; the vertical line extends from the minimum to the maximum value, excluding outliers, which are represented as individual dots.
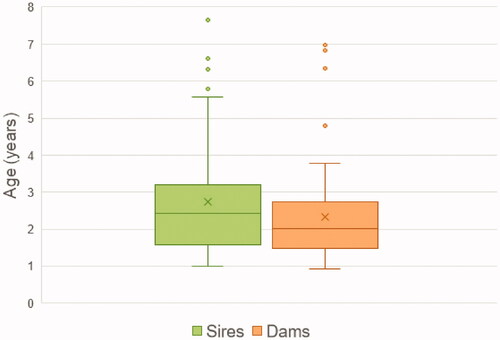
Table 2. Results of Mannara dogs’ pedigrees analyses. Founders were defined as dogs with no pedigree information.
Details about pedigrees’ depth are given in Table . No pedigree information is found for 33% of the dogs, which are considered as founders. For 53% of the population, both parents are known. At least one grandparent is reported in 19% of pedigrees, and all the four of them in 14%; the completeness of the second generation is 70%. The maximum number of generations we could trace is five.
Table 3. Pedigrees’ depth. In fully traced generations, all the ancestors are known; the number of maximum generations traced refers to the ones in which at least one ancestor is known.
Mean pedigree inbreeding (F) is 0.7% ± 3.3%, ranging from 0.0 to 25.0%; however, for 96% of the dogs, F is comprised between 0 and 5.0%. The mean value of average relatedness (AR) is 2.6%. Since F and AR calculation depends on pedigree information, a limited pedigree depth can affect the results.
The effective population size was calculated on a reference population that consisted of 311 dogs. The five methods we used for calculating Ne give very different results: Nes is equal to 233.1 ± 0.002; NeN to 158.5; NeFi to 50.0 ± 0.01; NeCi to 25.2 ± 0.02; and Nev to 3.4 ± 0.2.
Genomic analyses
After the screening, 1 CAUC and 24256 SNPs were excluded, so the analyses were performed on a subset composed of 73 samples and 122075 SNPs.
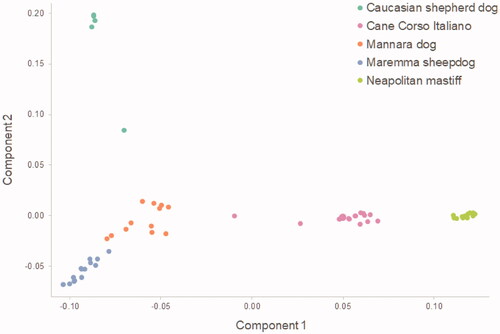
The plot representing the first two components of MDS is shown in Figure . Along the first component axis, MARM + CAUC, MANN, CCIT, and NEAP are well separated. The second one better distinguishes among CAUC, MANN, and MARM. Mannara dogs are located in a middle position, among CCIT, CAUC, and MARM, the latter being the nearest breed.
Figure 7. Plot of the multidimensional scaling analysis (MDS). Each dot represents a subject and each colour a breed.
The aforementioned results are confirmed by Reynolds distances analysis (Table S1, Figure ): MARM is the nearest breed to MANN (0.17), followed by CCIT (0.18).
Figure 8. Reynolds distance-based phylogenetic tree. CAUC: Caucasian shepherd dog; MANN: Mannara dog; MARM: Maremma sheepdog; CCIT: Cane Corso Italiano; NEAP: Neapolitan mastiff.
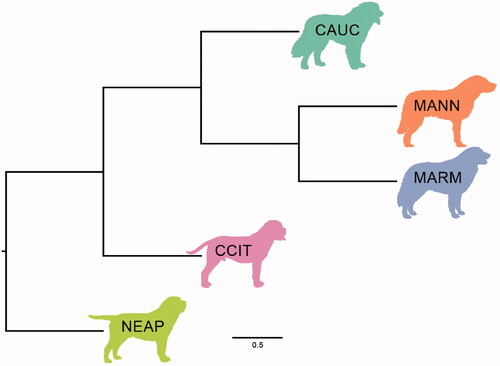
The best fitting admixture models were obtained for K = 4 (cv = 0.64) and K = 5 (cv = 0.65) and the latter distinguished a cluster for each breed; for this reason, and given the minimum difference in the cv values, the representation of the model K = 5 is shown in Figure , while all the models can be found in Supplementary materials (Figure S1). The admixture model shows that MANN is divided in two groups: five individuals have a Q-value >99.9% for their own cluster, whilst 7 have a mean Q-value = 32.5% (with a minimum value of 21.8%), being admixed on average with 36.0% MARM, 16.3% CCIT, 11.1% CAUC, and 4.1% NEAP. It should be noted that the pattern of the more mixed MANN is distinguishable from all the other breeds as well.
Figure 9. Admixture analysis representation (K = 5). Each cluster is represented by a different colour. CAUC: Caucasian shepherd dog; CCIT: Cane Corso Italiano; MANN: Mannara dog; MARM: Maremma sheepdog; NEAP: Neapolitan mastiff.
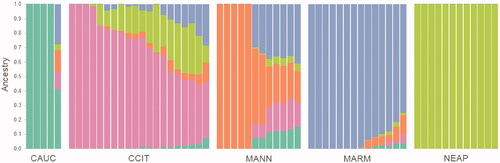
The ROH-based inbreeding (FROH) was calculated for each breed as the mean FROH of the individuals of that breed (Table S2, Figure , and Figure S2). The whole sample’s mean (± SD) FROH is 0.15 ± 0.06, the lowest value belonging to MANN (0.10) and the highest to NEAP (0.25). Mannara dog’s FROH increases with the increasing of the length of ROH, which means that inbreeding has been intensified over time, except for the longest class of ROH, which shows the lowest FROH.
Figure 10. Bar chart representing each breed’s FROH in five different length classes; the longer shorter is the ROH, the older is the inbreeding event that originated it. The sum of each class’s FROH corresponds to the overall FROH of the breed.
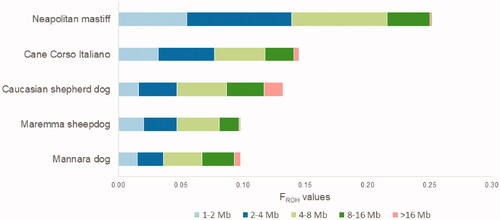
Table S3 shows He, Ho, and FIS in all the breeds. The greatest differences between Ho and He are related with CAUC (0.06) and CCIT (-0.01). With regard to MANN, a difference of 0.002 and a FIS of 0.003 were found.
Discussion
Mannara dogs have accompanied and assisted Sicilian shepherds for a long time, but only in 2014 they received a specific breeding book (RSA). The next step would be the international recognition by the FCI and the establishment of a supplementary registry of recognised dogs (RSR). For this reason, this study aims to provide a detailed characterisation of the breed on many different levels.
The morphologic examination of 111 Mannara dogs showed that, on average, the indications of the provisional breed standard are satisfied (ENCI Citation2013): the dogs can be classified as large/giant (mean weight was 41 kg for males and 32 kg for females), with a meso-dolichomorphic body (mean body index was 85 for males and 90 for females) and a mesocephalic head (mean cephalic index was 53 for both the sexes). A certain degree of variability was observed, particularly in the Thoracic index, probably due to the different age of animals (1-5 years old) and therefore their development: due to the diversity of breeds with very different shapes and sizes, growth patterns are also noticeably different, with very small dog breeds reaching maturity between 8 and 12 months of age and larger breeds taking up to 24 months to reach the adult body weight and conformation (Hawthorne et al. Citation2004; Lewis Citation2019). The average birth weights measured on 50 puppies, equal to 720 g for males and 580 g for females, are in accordance with published data referred to large/giant dog breeds (Groppetti et al. Citation2017; Schrank et al. Citation2019).
The second part of the study focussed on investigating the pedigrees of the whole population. At the present time (April, 2021), a total of 375 dogs are enrolled in the RSA, a third of which are founders. There are 58 registered litters, consisting on average of 2-6 dogs each, born to 34 sires and 41 dams. The mean age of the sires and dams at the birth of their offspring is 2.7 and 2.3 years, respectively.
The pedigree of most of the non-founder dogs has only one complete generation, but both the grandparents are known for 20% of non-founder dogs, with some individuals having their ancestry known up to the fifth generation.
The pedigree-based inbreeding (F) is still low in the breed, being on average equal to 0.7% and exceeding the recommended limit of 6.5% (European Commission Citation2020) only in a small number of dogs (3%). Internationally recognised breed with similar F values are reported in the literature (Leroy et al. Citation2009; Shariflou et al. Citation2011; Wijnrocx et al. Citation2016). However, the assumption that the founders are unrelated surely implies an underestimation of F; for this reason, genomic information is of great importance, especially when studying small, recent populations with shallow pedigrees. Moreover, contrary to what reported in many breeds (Calboli et al. Citation2008; Lewis et al. Citation2015), the numbers of male and female breeding Mannara dogs are almost equivalent, and only one sire produced a high number of offspring. This dog has been widely used as a breeder due to his typicality and compliance with the standard, in order to fix some peculiar features in the future generations; nevertheless, breeders have been advised to avoid the excessive use of sires in order to maintain a wide genetic pool, towards an optimal contribution of all the breeding dogs.
The FCI procedure for the international recognition of a new breed (FCI General Assembly Citation2019) proposes a pedigree-based effective population size (Ne) of at least 50-100 as an important condition that a dog population should meet in order to be officially recognised; however, it does not provide any indication about how this parameter should be calculated. Consistently to the data already reported in the literature, the application of five different formulas (Leroy et al. Citation2013) to the Mannara dog population led to very different results, ranging from 233, obtained using the traditional Nes formula, to 3, derived from the Nev, which is based on the variance of progeny size; however, it should be remarked that the first is based on demographic information only, while the latter would require more pedigree information to be considered accurate. Given the limited pedigree depth, NeN = 159, NeFi = 50, and NeCi = 25 are more suitable methods to estimate Ne in this population. Both NeN and NeCi gave a size of at least 50, the minimum value required by FCI. It should also be noted that different studies report similar or even smaller Ne for many recognised breeds (Leroy et al. Citation2009; Shariflou et al. Citation2011; Wijnrocx et al. Citation2016). In our opinion, Mannara dog meets the condition to be declared an internationally recognised new dog breed.
Finally, we performed a genomic analysis on a sample of 12 Mannara dogs, which were compared with four other breeds, chosen as possible contributors to the development of the Mannara dog in the light of their similar history, appearance, function, and/or geographic proximity. Given the canine breed structure, leading to a limited within-breed variability, even a small number of dogs are representative of the genetic variability of the breeds (Parker Citation2012); in fact, the sample size of each breed we used is superimposable to those reported in other studies of the canine breeds’ genomic background (Parker et al., Citation2017 ; Talenti et al. Citation2018). The evaluation of the Mannara dog’s genomic inbreeding (FROH) and heterozygosity confirms the genealogic results, showing that this population has been well managed: no problems related to excessive consanguinity or loss of heterozygosity have been found and, at the same time, the population structure analysis indicates that all the selected breeds represent well distinguished populations. The admixture investigation shows that some Mannara dogs can be assigned to their specific cluster with a Q-value > 99%, forming a “seed” of genetic uniqueness, while others show a genetic pattern that include a mix of the other breeds, with a prevalence of Maremma sheepdog. It is worth noting that, even in these latter dogs, the genetic admixture shows no similarities with the other populations. These results are consistent to those reported in Talenti et al. (Citation2018), in which acceptable purebred metric values were found for the Mannara dog, but in the phylogeny analysis it clustered together with Maremma sheepdog, and was therefore regarded as ‘variety’ on par with other officially recognised and widespread Italian canine breeds. It can be argued that the contribution of the Maremma sheepdogs to the Mannara dog breed depends on the fact that, for a long time, they have been the only recognised and widespread Italian flock guardian dogs available for those shepherds who had the necessity to protect their herds from wild predators. Notably, breeders have adopted a much stricter mating scheme since the establishment of the Mannara dog’s RSA in 2014, avoiding any cross with other breeds.
Breeders should be advised on the best mating scheme that would allow them to maintain a low inbreeding while increasing the number of generations and families of the breed; traditionally, FCI recognition required a minimum of eight family groups born over a period of five years and not sharing ancestors throughout three generations (FCI General Assembly Citation2019). ENCI deserves acknowledgement for the efforts that has made to support the breeders, paying particular attention to the context in which Mannara dogs are bred: thanks to a roving commission of expert judges, it has been possible to evaluate and enrol founder dogs compliant with the standard as well as register a great number of litters. The continuation and the enhancing of this program will be of great help to keep track of all the changes in the Mannara dog population. These results open to future studies involving a larger number of representative individuals, and confirm once more that the pairing of genomic and classic management tools can drive the breeders in the selection of dogs that are not only phenotypically in standard, but also representative of the breed’s genomic background, preserving both their health and genetic uniqueness.
Conclusions
Mannara dog breed is old, but still fundamental for the Sicilian shepherds: thanks to its robustness and attitude to work as flock guardian, it represents an invaluable resource that should be protected and enhanced. In this respect, its official recognition as a breed and the conferment of a specific studbook (RSR) are to be considered crucial.
The results here presented demonstrate that Mannara dog is characterised by a unique genomic background and meets the demographic requirements for continuing the ENCI and FCI process of recognition. Moreover, data useful for a better delineation of the breed standard are given. In order to maintain and improve the excellent results achieved so far, the breeders should keep the focus on the management of this population and implement further tools, such as the genomic evaluation of the dogs. In this way, they will preserve Mannara dogs’ genetic variability while producing dogs with excellent attitude, phenotype, and genetic background as well as a complete pedigree.
Ethical approval
This study was performed according to the ethical principles that have their origins in the Italian Veterinarians' Ethical Code (Passantino 2007) and the Italian and European regulations on animal welfare (Directive 2010/63/EU 2010). Experimental protocol was approved by the Ethical committee of the Department of Veterinary Science of the University of Messina, Italy (code 040/2020).
Supplemental Material
Download MS Word (495.7 KB)Acknowledgments
A special thanks goes to Dr. Andrea Talenti for the insightful suggestions and the proofreading. We are grateful to all the dogs’ breeders and owners, particularly Florindo Arengi, Francesca Greco, Dino Miceli, and Gaspare Tumminia. We also thank the SAMANNARA Kennel Club that supported us during this study, particularly the president Fabrizio La Rocca and the clerk and treasurer Giorgio Maddalena, and ENCI judging commission members Riccardo Di Carlo, Massimo Inzoli, Antonino La Barbera, and Paolo Maranto. The paper is born within the framework of the programmatic initiatives of the ASPA Commission for the Breeding and Feeding of Companion Animals.
Disclosure statement
The authors declare no competing interests.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
- Alexander DH, Lange K. 2011. Enhancements to the ADMIXTURE algorithm for individual ancestry estimation. BMC Bioinformatics. 12(1):246.
- Arengi F. 2011. Indagine sul cane di mannara [Investigation into the Mannara dog]. Enna: Maurizio Vetri Editore. Italian.
- Bonetti F. 1995. Zoognostica del cane [Dog Zoognostic]. Bologna: Editrice San Giorgio. Italian.
- Calboli FCF, Sampson J, Fretwell N, Balding DJ. 2008. Population structure and inbreeding from pedigree analysis of purebred dogs. Genetics. 179(1):593–601.
- Cervantes I, Goyache F, Molina A, Valera M, Gutiérrez JP. 2011. Estimation of effective population size from the rate of coancestry in pedigreed populations. J Anim Breed Genet. 128(1):56–63.
- Chicoli N. 1870. Riproduzione, allevamento e miglioramento degli animali domestici in Sicilia [Reproduction, Breeding, and Improvement of Domestic Animals in Sicily]. Palermo: Stamperia di Giovanni Lorsnaider. Italian.
- Directive 2010/63/EU 2010. Directive 2010/63/EU of the European Parliament and of the Council of 22 September on the protection of animals used for scientific purposes (Text with EEA relevance). 2010. OJ. L276:33–79.
- ENCI 2013. Standard provvisorio del Cane di Mannara [Mannara Dog’s Provisional Standard]. Italian.
- European Commission 2020. Responsible Dog Breeding Guidelines 2020.
- FCI General Assembly 2019. FCI procedure for the international recognition of a new breed.
- Groppetti D, Pecile A, Palestrini C, Marelli SP, Boracchi P. 2017. A national census of birth weight in purebred dogs in Italy. Animals. 7(12):43.
- Gutiérrez JP, Cervantes I, Goyache F. 2009. Improving the estimation of realized effective population sizes in farm animals. J Anim Breed Genet. 126(4):327–332.
- Hawthorne AJ, Booles D, Nugent PA, Gettinby G, Wilkinson H. 2004. Body-weight changes during growth in puppies of different breeds. J Nutr. 134(8 Suppl):2027S–2030S.
- Hill WG. 1972. Effective Size of Populations with Overlapping Generations. Theor Popul Biol. 3(3):278–289.
- Leroy G, Mary-Huard T, Verrier E, Danvy S, Charvolin E, Danchin-Burge C. 2013. Methods to estimate effective population size using pedigree data: examples in dog, sheep, cattle and horse. Genet Sel Evol. 45(1):1–10.
- Leroy G, Verrier É, Meriaux JC, Rognon X. 2009. Genetic diversity of dog breeds: within-breed diversity comparing genealogical and molecular data. Anim Genet. 40(3):323–332.
- Lewis G. 2019. Musculoskeletal development of the puppy. Anim Ther Mag. 15:41–44.
- Lewis TW, Abhayaratne BM, Blott SC. 2015. Trends in genetic diversity for all Kennel Club registered pedigree dog breeds. Canine Genet Epidemiol. 2(:13–10.
- McQuillan R, Leutenegger A-L, Abdel-Rahman R, Franklin CS, Pericic M, Barac-Lauc L, Smolej-Narancic N, Janicijevic B, Polasek O, Tenesa A, et al. 2008. Runs of homozygosity in European populations. Am J Hum Genet. 83(3):359–372.
- Migneco M. 1987. Considerazioni ed appunti sul cane Cirneco [Considerations and Notes on Cirneco Dog]. Catania: Stabilimento Tipografico M. Galati. Italian.
- Oğrak YZ, Yoldaş A, Uroseviç M, Drobnjak D. 2014. Some morphological traits of Tarsus Çatalburun breed of Turkish hunting dog. EurasianJVetSci. 30(1):25–29.
- Parker HG, Dreger DL, Rimbault M, Davis BW, Mullen AB, Carpintero-Ramirez G, Ostrander EA. 2017. Genomic Analyses Reveal the Influence of Geographic Origin, Migration, and Hybridization on Modern Dog Breed Development. Cell Rep. 19(4):697–708. doi:https://doi.org/10.1016/j.celrep.2017.03.079. 28445722
- Parker HG. 2012. The history and relationships of dog breeds. In: Ostrander EA, Ruvinsky A, editors. Genet dog 2nd ed. Croydon: CAB International. p. 38–56.
- Parker HG, Shearin AL, Ostrander EA. 2010. Man's best friend becomes biology's best in show: genome analyses in the domestic dog. Annu Rev Genet. 44(1):309–336.
- Passantino A. 2007. Ethical aspects for veterinarians regarding antimicrobial drug use in Italy. Int J Antimicrob Agents. 29(3):240–244.
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PIW, Daly MJ, et al. 2007. PLINK: a tool set for whole-genome association and population-based linkage analyses . Am J Hum Genet. 81(3):559–575.
- Reynolds J, Weir BS, Cockerham CC. 1983. Estimation of the coancestry coefficient: basis for a short-term genetic distance. Genetics. 105(3):767–779.
- Salt C, Morris PJ, German AJ, Wilson D, Lund EM, Cole TJ, Butterwick RF. 2017. Growth standard charts for monitoring bodyweight in dogs of different sizes. PLoS One. 12(9):e0182064.
- Sams AJ, Boyko AR. 2019. Fine-scale resolution of runs of homozygosity reveal patterns of inbreeding and substantial overlap with recessive disease genotypes in domestic dogs. G3. 9(1):117–123.
- Schrank M, Mollo A, Contiero B, Romagnoli S. 2019. Bodyweight at birth and growth rate during the neonatal period in three canine breeds. Animals. 10(1):8.
- Shariflou MR, James JW, Nicholas FW, Wade CM. 2011. A genealogical survey of Australian registered dog breeds. Vet J. 189(2):203–210.
- Sutter NB, Mosher DS, Gray MM, Ostrander EA. 2008. Morphometrics within dog breeds are highly reproducible and dispute Rensch's rule. Mamm Genome. 19(10-12):713–723.
- Talenti A, Dreger DL, Frattini S, Polli M, Marelli S, Harris AC, Liotta L, Cocco R, Hogan AN, Bigi D, et al. 2018. Studies of modern Italian dog populations reveal multiple patterns for domestic breed evolution. Ecol Evol. 8(5):2911–2925.
- Wellmann R. 2019. Optimum contribution selection for animal breeding and conservation: the R package optiSel. BMC Bioinformatics. 20(1):25–25.
- Wellmann R, Bennewitz J. 2011. Identification and characterization of hierarchical structures in dog breeding schemes, a novel method applied to the Norfolk Terrier. J Anim Sci. 89(12):3846–3858.
- Wijnrocx K, François L, Stinckens A, Janssens S, Buys N. 2016. Half of 23 Belgian dog breeds has a compromised genetic diversity, as revealed by genealogical and molecular data analysis. J Anim Breed Genet. 133(5):375–383.
