?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this study, nano-silymarin (NSM) was produced and compared with powdered (PSM) and lecithinized silymarin (LSM) on performance and egg quality in late-phase laying hens. Egg quality was assessed using fresh eggs or those stored under different temperatures (25 or 5 °C) for a period of 28 days. Seventy 80-weeks Lohmann LSL-Lite hens were allocated to 7 treatments with 10 replicates for 12 weeks. Treatments included: (1) control diet without silymarin, (2) daily intake of 100 mg PSM/kg body weight (BW), (3) daily intake of 200 mg PSM/kg BW, (4) daily intake of 100 mg NSM/kg BW, (5) daily intake of 200 mg NSM/kg BW, (6) daily intake of 100 mg LSM/kg BW, and (7) daily intake of 200 mg LSM/kg BW. Scanning electron microscopy showed that NSM was produced with size ranges between 5.84-52.86 nm. Bird study showed that egg production, egg weight, and egg mass increased and feed conversion ratio decreased than the control by silymarin supplementation in any form (p < .05). Egg storage increased yolk weight, yolk and albumen pH, and malondialdehyde content and decreased albumen weight and Haugh unit than the fresh egg (p < .05). Silymarin treatment improved Haugh unit, nutrient digestibility, intestinal morphology, and pH of digesta (p < .05). Moreover, the effects of silymarin were more pronounced when converted to NSM or LSM and offered at the highest level (p < .05). Overall, diet supplementation with 200 mg/kg BW of NSM or LSM to improve hen performance and egg quality is asserted by our results.
Egg storage increased the percentage of yolk, yolk and albumen pH, and malondialdehyde concentration.
Egg storage decreased the percentage of albumen and Haugh unit.
Silymarin improved Haugh unit and apparent ileal nutrient digestibility.
Silymarin improved intestinal morphology and pH of digesta.
Silymarin as LSM and NSM had better effects on performance, diet digestibility, and intestinal health.
Highlights
Introduction
Silymarin, a purified extract from the seeds of the milk thistle (Silybum marianum [L.] Gaertn.), contains a mixture of flavonolignans like silybinin, isosilybin, silydianin, and silychristin with silybin being the main active ingredient (Federico et al. Citation2017). Several previous studies have focussed on the bioactivity of these molecules, showing that they exert inhibitory effects against hepatitis C virus (Wagoner et al. Citation2010; Liu et al. Citation2019) and antimicrobial effects against different harmful bacteria such as Staphylococcus aureus, Escherichia coli, and Campylobacter jejuni (Evren and Yurtcu Citation2015; Radhika et al. Citation2017; Kareem et al. Citation2020; Jahanian et al. Citation2021), thus making them good candidates for maintaining the health or management of the liver and gastrointestinal injuries. Silymarin also acts as a powerful scavenger of reactive free radicals and inhibits fat and protein peroxidation by enhancing the activity of endogenous antioxidant enzymes and non-enzymatic antioxidants (vitamins E and C) in the liver and muscle tissues (El-Gazayerly et al. Citation2014; Alhidary et al. Citation2017). Abou-Shehema et al. (Citation2016) showed that 5 mg/kg of silymarin in the diet improved antioxidant status, liver function, and lipid profile in laying hens under summer conditions, whereas Quarantelli et al. (Citation2009) found improvements in egg-laying rate and nutrient contents of the eggs when they included 200 mg/kg of silymarin in the diet. Both studies provided evidence for improved ovarian activity by feeding silymarin (Quarantelli et al. Citation2009; Abou-Shehema et al. Citation2016). The effectiveness of dietary silymarin (40 and 80 mg/kg) on improving the chemical composition and oxidative stability of thigh and breast meat of broiler chickens has also been reported (Schiavone et al. Citation2007). Thus, silymarin may also be a valuable antioxidant to improve the quality of fresh and stored eggs.
It should be noted that egg storage is a common practice in the advanced business egg industry, despite the fact that this can negatively influence the internal and shell quality of eggs to depend on hen age, temperature, and storage duration (Akyurek and Okur Citation2009; Attia et al. Citation2020). Moreover, egg yolk is prone to oxidation during storage due to a high amount of unsaturated fatty acids (Abreu et al. Citation2014), thus the requirement for antioxidants is increasing. Many researchers have reported the use of herbs and spices as antioxidant components to prevent lipid oxidation in eggs (Botsoglou et al. Citation2005; Radwan Nadia et al. Citation2008; Loetscher et al. Citation2014; Goliomytis et al. Citation2019; Mohajer et al. Citation2021). However, the usefulness of these treatments seems to be affected by the bioavailability of their active compounds (Krause and Ternes Citation2000; Lorenzo et al. Citation2019). Therefore, the low intestinal absorption (20–50%) of silymarin (Rathore et al. Citation2020) may limit its application in the above field.
There are various methods to increase the solubility and bioavailability of a low water-soluble drug. The bioavailability of silybin augmented tenfold when complexed with phosphatidylcholine (Morazzoni et al. Citation1993). Tedesco et al. (Citation2004) showed that silymarin-phospholipid complex protected broiler chickens against the harmful effects of aflatoxin B1. Soy lecithin is a mixture of various phospholipids such as phosphatidylcholine, phosphatidylethanolamine, and phosphatidylinositol (Attia and Kamel Citation2012; Akit et al. Citation2016), which has been reported as a suitable compound to increase intestinal absorption of silymarin (Wang et al. Citation2015). Furthermore, during the last decade, nano-technological methods have been applied to improve the bioavailability of silymarin in new pharmaceutical applications (Di Costanzo and Angelico Citation2019). Kakran et al. (Citation2015) produced silymarin nano-crystals using the evaporative deposition method of nano-suspension. These researchers reported that the evaporative deposition method of nano-suspension is an effective method to make drug nano-particles with enhanced dissolution rate. Silymarin and its nano-crystals probably protect the liver against damage caused by the consumption of titanium oxide nanoparticles due to their antioxidant properties (Hajizadeh Moghaddam et al. Citation2017). Parveen et al. (Citation2011) reported that the low dose of silymarin nano-emulsion produced similar or, in most cases, higher effects than a higher dose of silymarin that indicating improved bioavailability and better absorption of the poorly water-soluble substances in the form of nano-emulsion. Nutritional and antioxidant effects of nano-silymarin and silymarin-phosphatidylcholine complex have been investigated in rodents (Ustyol et al. Citation2017; Fatehi et al. Citation2018; Di Costanzo and Angelico Citation2019). However, no data about their potential use in laying hens or other poultry have been published in the literature.
Based on this background, in this study, nano-silymarin (NSM) was produced and compared with powdered (PSM) and lecithinized silymarin (LSM) on performance, nutrient digestibility, intestinal morphology, and quality of fresh and stored eggs in laying hens.
Materials and methods
Procurement of PSM and preparation of NSM and LSM
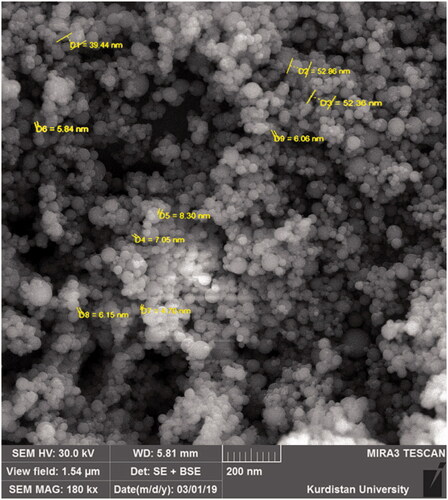
PSM with a purity of 80.2% was purchased from Zardband Pharmaceutical Company (Tehran, Iran). A ratio of 1:2 silymarin and lecithin was used to prepare LSM (Tedesco et al. Citation2004). NSM crystals were obtained using the evaporative deposition method of nano-suspension as described by Kakran et al. (Citation2015) with some modifications. Briefly, 2 mg of silymarin was dissolved in 1 mL of acetone (solvent) and placed in ultrasonication for 1 h. Then hexane (anti-solvent) was added to obtain a nano-suspension. The ratio of solvent to anti-solvent was 1:30. The resulting nano-suspension was placed in a water bath at 40 °C and set to a rotation of 90 rpm for 45 min. The solid particles were collected in the flask after evaporation and used for further analysis. The size of the particles was measured using a scanning electron microscope (TESCAN MIRA3, sro Brno, Czech Republic).
Birds, management, and dietary treatments
All procedures used in this study were approved by the guidelines of the Animal Ethics Committee at the University of Kurdistan (IR.UOK.REC.1400.002). In total, 70 Lohmann LSL-Lite hens with the same average body weight (1,650 ± 74 g) were obtained from a commercial flock (80 weeks) and allocated to 7 dietary treatments with 10 replicates. The birds were housed individually and experimental diets were fed for 12 weeks. Each cage was equipped with an individual feeder and a nipple drinker. The dietary treatments included: (1) control diet without silymarin, (2) daily nutrition of 100 mg silymarin/kg body weight (BW) as PSM [PSM100], (3) daily nutrition 200 mg silymarin/kg BW as PSM [PSM200], (4) daily nutrition of 100 mg silymarin/kg BW as NSM [NSM100], (5) daily nutrition 200 mg silymarin/kg BW as NSM [NSM200], (6) daily nutrition of 100 mg silymarin/kg BW as LSM [LSM100], and (7) daily nutrition of 200 mg silymarin/kg BW as LSM [LSM200]. The control diet (Table ) was formulated to meet the nutritional requirements of laying hens (Lohmann LSL-Classic International Citation2011). Mash feed and water were supplied ad libitum. The lighting regimen was 16 h light and 8 h dark throughout the experiment. The temperature in the house was set at 20 °C.
Table 1. Composition of the basal diet.
Performance measurements and egg quality
At the beginning and end of the experiment, the birds from each age were weighed and their body weight changes were calculated. During the experiment, daily egg numbers and egg weight were recorded for each cage. This data was used to calculate egg production and egg mass per replicate. Feed intake was manually measured on a weekly basis using an ADAM weighing balance (NBL 4602e model, Oxford, UK) with an accuracy of 0.01 g. Data on feed intake and egg mass were used to calculate the feed conversion ratio. All the eggs laid during the last 10 days of the trial were collected to evaluate the egg quality parameters (relative weights of yolk, albumen, and eggshell, pH of yolk and albumen, and Haugh unit). Eggs collected for each treatment per day were divided into three equal groups. The first group was analysed on the same day (fresh eggs) while the second and third groups were stored for 28 days at room temperature (average 25 °C) and in the refrigerator (average 5 °C), respectively, and then evaluated. Yolk malondialdehyde (MDA) concentration was determined in fresh eggs and those stored at 25 °C as described by Draper and Hadeley (Citation1990).
Intestinal morphology and pH of digesta
At the end of the experiment, 4 birds from each treatment were randomly selected and sacrificed. A portion of jejunum was obtained, fixed in 10% formalin, and transferred to the laboratory for histological study. The jejunum samples were dehydrated using increasing concentrations of ethyl alcohol (70, 90, 96, and 100%), cleared in xylene, and embedded in paraffin. The microtome (HistoLine, Pantigliate, Italy) was used to prepared 5-µm-thick sections of paraffin blocks, and then these samples were stained with haematoxylin and eosin and were evaluated using a light microscope (Olympus, Tokyo, Japan). Then cross-sections of 10 villi were randomly selected. The villus height and width, as well as the crypt depth, were evaluated by 40x magnification (Prakatur et al. Citation2019). The villus height was measured as the distance from the apex of the villus to the junction of the villus and crypt. The villus width was measured as the distance from the junction to the basement membrane of the epithelial cell at the bottom of the crypt at the bottom third of the length of the villus (Prakatur et al. Citation2019). An average value was calculated for the measurements taken from 10 villi per one sample and was expressed as the average villus height, width, and crypt depth. The villus to crypt ratio was obtained by dividing the height of the villus by the depth of the crypt. A pH metre was used to measure the pH of the ileal and jejunal contents. For this purpose, 1 g of the digesta was mixed with 10 mL of distilled deionised water, and then the pH of the samples was measured (Nisbet et al. Citation1993).
Apparent ileal nutrient digestibility
Nutrient digestibility was determined in the last 3 days of the experiment. All diets contained 3 g/kg of titanium dioxide (TiO2) as an indigestible marker. At the end of the experiment, 4 birds from each treatment were randomly selected and killed by cervical dislocation. Immediately after slaughter, the ileal contents were collected and stored at −20 °C until analysed using the methods of AOAC (Citation1990, Citation2000). Samples of diet and ileal digesta were subjected to an oven at 105 °C for 24 h to determine dry matter (method 934.01, AOAC Citation1990) and combusted at 600 °C overnight in porcelain crucibles to determine ash (method 930.05, AOAC Citation1990). Nitrogen was measured by the Kjeldahl procedure (method 984.13, AOAC Citation1990) and distillation with 0.1 N hydrochloric acids for calculation of crude protein (nitrogen × 6.25). Ether extract was analysed by 6-h Soxhlet extraction (method 920.85, AOAC Citation1990). Calcium was determined by the versenate complexometric titration method using ethylene diamine tetra-acetic acid as an indicator (method 927.02, AOAC Citation2000). Total phosphorus was determined by vanadium-molybdate (yellow) spectrometry (method 965.17, AOAC Citation2000). Concentrations of TiO2 in the diet and ileal digesta samples were determined according to a method detailed by Short et al. (Citation1996). The absorption of TiO2 samples was determined using a Jasco V-570 spectrophotometer (Jasco International Co. Ltd., Tokyo, Japan) at a wavelength of 410 nm. Nutrient digestibility (ND) was calculated using the following equation (Karami et al. Citation2020):
Statistical analysis
Data were analysed as a completely randomised design using the GLM procedure of SAS 9.1 (SAS Institute Citation2001). Firstly, Dunnett’s tests were used to identify treatments that were significantly different from the control, and then the data excluding the control were analysed as a 3 × 2 factorial arrangement of treatments using the two-way ANOVA, and form of silymarin (PSM, NSM, and LSM), added silymarin level (100 and 200 mg/kg BW), and their interaction was included in the statistical model. Significant differences among the means were tested using Tukey’s studentized range tests. The PDIFF option (Adjust = Tukey) was used to identify differences between the fresh eggs and those stored at different temperatures. All statements of significance were based on p < .05.
Results and discussion
The results of the scanning electron microscopy (Figure ) showed that NSM was produced, and the particle sizes were between 5.84 nm and 52.86 nm. These particles were obtained with a concentration of 2 mg/mL and a solvent to the anti-solvent ratio of 1:30. In the study of Kakran et al. (Citation2015), the smallest average particle size obtained was 350 nm for silymarin with a concentration of 5 mg/mL, and the ratio of solvent to anti-solvent was 1:20. Decreasing the concentration of poorly water-soluble substances along with increasing the solvent to anti-solvent ratio leads to smaller particle sizes. The reason for this can be explained by the formation of nano-particles via a homogeneous nucleus. High initial concentration and higher solvent to anti-solvent ratio may lead to higher supersaturation and the formation of the large number of nuclei (Sherif and Al-Gayyar Citation2013). An increase in the number of nuclei means an increase in the number of nuclei with a smaller size. High initial concentration, in addition to creating higher supersaturation, also leads to the aggregation of particles. Regarding the results of Kakran et al. (Citation2015), the effect of aggregation was predominant. Therefore, they reported that lower concentrations of the drug result in smaller particles. Once the nuclei are formed, they begin to grow. However, in later stages, as the solvent to anti-solvent ratio increases, the diffusion distance between the nuclei increases and the growth of the nuclei is limited (Kakran et al. Citation2015). Based on these reports, in the present study, after trial and error with several different concentrations, we used low concentrations of silymarin and a high ratio of solvent to anti-solvent to make NSM.
The effects of different forms and levels of silymarin on the performance of laying hens during the experiment are shown in Table . Feed intake and body weight change were not affected by dietary treatments. However, egg production, egg weight, and egg mass increased by feeding the PSM200, NSM100, NSM200, LSM100, and LSM200 diets compared with the control (p < .05). There were parallel decreases in feed conversion ratio with significant differences from the control in birds fed the NSM100, NSM200, and LSM200 diets (p < .05). Similarly, Quarantelli et al. (Citation2009) reported improvements in egg production and feed conversion ratio by adding 200 mg/kg of silymarin to the diet of laying hens. In other studies, Hashemi Jabar et al. (2018) reported that 30 g/kg of dietary milk thistle meal increased egg production and decreased feed intake and Šťastník et al. (Citation2019) showed that using 70 g/kg of milk thistle seed cakes in the diet improved egg mass in laying hens. Based on the findings of this study, the better performance obtained with silymarin is possibly mediated by the effects of the drug on the gut development and/or retention of dietary nutrients. Moreover, Abou-Shehema et al. (Citation2016) found that dietary silymarin (5 g/kg) increased the number of more developed ovulatory follicles, which was connected with increased concentrations of oestrogen and progesterone in the blood and inhibition of steroidogenesis in laying hens. This effect could be responsible for the higher ovarian activity (Quarantelli et al. Citation2009). Egg production and egg mass increased and the feed conversion ratio decreased further by passing from the PSM to the LSM and then the NSM diets (p < .05). Similar values were obtained with the LSM and NSM diets for egg weight that were significantly higher than those obtained with the PSM diets (p < .05). Additionally, the effects of all silymarin supplements was better at the level of 200 mg/kg BW than the level of 100 mg/kg BW (p < .05), except for egg production that showed similar values by NSM100 and NSM200. This was reflected in a significant interaction between supplementation level and form of silymarin on the egg production (p < .05). The better results obtained with NSM and LSM can be explained by the findings that nano-silymarin and silymarin-phospholipid complexes were more bioavailable as compared to the conventional drug owing to their faster release and an enhanced capacity to cross the lipid-rich biological membranes, and finally reaching the systemic circulation (Kidd Citation2009; Parveen et al. Citation2011). In addition, our results suggest that nanoparticles might be more successful in achieving these goals than the phospholipid complexes.
Table 2. The effect of different forms and levels of silymarin on the performance of laying hens.
The effects of dietary treatments on jejunal morphology and jejunal and ileal pH are shown in Table . Feeding a diet with NSM or LSM significantly increased the villus height, villus width, and villus to crypt ratio than the control (p < .05). A significant reduction in the crypt depth was also observed with the NSM200 diet (p < .05). In addition, the villus height increased and crypt depth decreased upon an increase in the silymarin level and bypassing from the PSM to the NSM and LSM diets (p < .05). The NSM diets also increased the ratio of villus height to crypt depth compared with the PSM diets (p < .05). Similar to these results, Jahanian et al. (Citation2017) showed that feeding silymarin at the level of 1,000 mg/kg of the diet increased villus height and villus to crypt ratio in aflatoxin‐challenged broiler chickens. Hashemi Jabali et al. (Citation2018) also reported that diet supplementation with 30 g/kg of milk thistle meal enhanced the villus to crypt ratio in the jejunum of laying hens. Histological indices of intestinal epithelial cells are important indicators of intestinal health and function status. A higher villus height ensures a greater count of enterocytes, which increases the production of digestive enzymes and the level of absorption per unit of the intestine, thereby leading to growth stimulation (Attia et al. Citation2017). On the other hand, the crypt is considered as the villus factory and a deeper crypt indicates a faster tissue turnover, while a higher villus to crypt ratio is usually synonymous with the healthier intestinal epithelium in chickens (Shahryari et al. Citation2021). The change in villus width may also be a reflection of differences in the intestinal immune status (Mirakzehi et al. Citation2017). Cook and Bird (Citation1973) reported a shorter villus and a deeper crypt when the total counts of pathogenic bacteria increased in the digestive tract. Therefore, the observed effects of silymarin on intestinal morphology may be resulted from the removal of invasive pathogenic microorganisms that shifts the bacterial community towards the predominance of the fermentative taxa. In vitro, silymarin components are known to inhibit the enteric pathogens due to the presence of phenolic hydroxyl groups that can interact with the bacterial membrane proteins leading to the leakage of intracellular components (Evren and Yurtcu Citation2015; Radhika et al. Citation2017; Kareem et al. Citation2020), while nano-formulations of these compounds provide a larger surface area and improve their bioavailability for exerting antimicrobial activities (Lee et al. Citation2017). In vivo, diets supplemented with silymarin decreased ileal and caecal counts of Escherichia coli and Salmonella spp. and increased caecal counts of Lactobacillus spp. in broiler chickens and ducks (Elnaggar et al. Citation2021; Jahanian et al. Citation2021). An increase in Lactobacilli relative multiplicity in the gastrointestinal tract is correlated with increased concentrations of short-chain fatty acids and a decrease in luminal pH (Kiczorowska et al. Citation2016). This view was supported by our results that all silymarin treatments decreased the jejunal and ileal pH than the control (p < .05). The intestinal pH values were, however, independent of silymarin form and level. Indeed, the superior effects of the NSM and LSM diets compared with the PSM diets or the better effect of the higher drug level can not be explained by changes in the intestinal pH. A more recent study showed that the morphological development of the intestine was associated with its antioxidant capacity in late-phase laying hens (Guo et al. Citation2020). In a study by Valenzuela et al. (Citation1989), the liver and intestine of rats showed an increase of over 50% in total glutathione content with the administration of a single dose of silymarin. Silymarin administered by gavage also had a good therapeutic effect on murine colitis, as demonstrated by lower colonic lipid peroxidation levels and microscopic evaluation of colonic damage (Esmaily et al. Citation2009). Moreover, a higher activity for liver antioxidant enzymes (superoxide dismutase, catalase, and glutathione peroxidase) was noticed when rats treated with a silymarin-phospholipid complex versus the milk thistle extract (El-Gazayerly et al. Citation2014). Confirmation of these results in poultry and how the antioxidant capacity of different silymarin forms relate to the morphological traits of the gut require further study.
Table 3. The effect of different forms and levels of silymarin on intestinal morphology and pH in laying hens.
As shown in Table , diet inclusion with silymarin had no impact on ileal dry matter, ash, calcium, and phosphorus digestibility. However, the crude protein and ether extract digestibility increased with the PSM200, NSM100, NSM200, LSM100, or LSM200 diets compared with the control (p < .05). The effect of NSM and LSM diets on improving protein digestibility was evident in comparison with PSM diets (p < .05), but changes in the digestibility of ether extract among the silymarin forms and levels were not found significant. These results are different from the findings reported in broiler chickens (Hasheminejad et al. Citation2015; Sultan et al. Citation2018). Hasheminejad et al. (Citation2015) observed decreased dry matter and calcium digestibility with diets containing 5 or 10 mg/kg of silymarin. In contrast, Sultan et al. (Citation2018) found improved digestibility values for dry matter, ash, crude protein, and ether extract when milk thistle powder was added at 10 g/kg of diet. Aside from bird strains, the level of active ingredients added to diets is likely to contribute to these differences. As mentioned above, the positive effects of moderate silymarin levels on enhanced digestion are likely to be mediated through antioxidant and antimicrobial properties that improve intestinal morphology, establish a healthier microbial population and reduce pH in the intestine (Attia et al. Citation2017, Citation2019). Such a condition reduces the competition for nutrients between the intestinal pathogens and host (Kiczorowska et al. Citation2016), enhances pancreatic secretion (Rodjan et al. Citation2018), and ultimately leads to increased digestibility and nutrient uptake by the intestinal absorptive cells (Abolfathi et al. Citation2019). In addition, silymarin in the form of NSM or LSM seems to have a higher ability to escape the destructive effects of the upper gastrointestinal tract, which increases its ability to act at target sites (Di Costanzo and Angelico Citation2019). The entrance of some phytogenic compounds into the small intestine may be essential to show their potential bioactivity and to achieve a satisfactory bioavailability (Jeong et al. Citation2005).
Table 4. The effect of different forms and levels of silymarin on nutrient digestibility in laying hens.
Table indicates the results of egg quality traits. Internal quality decreased in eggs stored for 28 days (p < .05). Eggs stored at room temperature (25 °C) showed an increase in the relative weight of yolk and a decrease in the relative weight of albumen after 28 days of storage (p < .05). Moreover, eggs stored at room or refrigeration (5 °C) temperature showed an increase in the pH of yolk and albumen and a decrease in the Haugh unit compared with the fresh ones (p < .05), while for albumen pH and Haugh unit greater changes were recorded at room temperature than at refrigerator (p < .05). Most of these changes in egg quality are related to water loss and the escape of carbon dioxide (CO2) from the albumen (Hinton et al. Citation2000; Akter et al. Citation2014). Carbonic acid, a component of the albumen buffer system, converts to water and CO2, which is lost through the shell pores (Akter et al. Citation2014). These alterations lead to a decrease in albumen weight and acidity, an increase in the pH of albumen and yolk, and degradation of an albumen protein complex (Feddern et al. Citation2017). The dense albumen becomes liquid due to the degradation of ovomucin-lysozyme complex, resulting in increased albumen pH and reduced viscosity of the albumen and Haugh unit (Kato and Sato Citation1972; Akter et al. Citation2014). Comparative outcomes were exhibited by Scott and Silversides (Citation2000) and Attia et al. (Citation2020) that egg storage increased the yolk weight and lessened the albumen weight due to the water nature of albumen. Mohiti-Asli et al. (Citation2008) also showed that both refrigeration and room storage expensed yolk and albumen pH and reduced Haugh unit after 28 days of storage.
Table 5. The effect of different forms and levels of silymarin on the quality of fresh and stored eggs at different temperatures.
All groups receiving silymarin showed an increase in the Haugh unit of eggs than the control (p < .05) except those fed the PSM100 diet, but other egg quality indices were not affected by dietary silymarin. The Haugh unit is a standard measure of albumen quality. The higher the Haugh unit value, the better the albumen quality of the eggs (Sarlak et al. Citation2021). Ovomucin is one of the albumen critical proteins to create the viscosity character of egg albumen (Omana et al. Citation2010), and the value of the Haugh unit is mainly influenced by the ovomucine content in eggs (Liu et al. Citation2020). Silymarin is a naturally derived polyphenolic antioxidant with hepatoprotective properties (Attia et al. Citation2017, Citation2019). Moreover, silymarin has an essential role in hepatic proteins synthesis via activation of DNA-dependent RNA polymerase I and thus improves liver cell regeneration (Pradhan and Girish Citation2013; Gao et al. Citation2018). Therefore, the effect of silymarin on increasing Haugh unit scores may have been mediated by its effect of silymarin on protein synthesis in the liver. Silymarin also increased the digestion of dietary protein (Table ) that may contribute to the synthesis of ovomucin (Rama Rao et al. Citation2020), although, unlike the protein digestibility, the Haugh unit was not affected by the level and form of silymarin in the diet.
The results of egg yolk MDA concentration, as a lipid oxidation index, is shown in Table . The content of MDA increased in the stored eggs compared with the fresh eggs (p < .05). These results are in concordance with Criste et al. (Citation2018) and Yang et al. (Citation2021), who reported that egg yolk MDA concentration increased during the storage. Akter et al. (Citation2014) also reported that egg yolk lipid peroxidation occurred during 7 to 28 days of storage at 23 to 31 °C, which increased MDA concentrations. In this study, silymarin at any level or form could not alter MDA contents in fresh eggs or those stored for 28 days at room temperature or in the refrigerator. This result was rather unexpected as silymarin is an antioxidant that inhibits not only free radical generation but also fat and protein peroxidation by enhancing the activity of endogenous antioxidants (El-Gazayerly et al. Citation2014; Alhidary et al. Citation2017). Schiavone et al. (Citation2007) indicated that dietary silymarin was able to improve the oxidative stability of broiler thighs and breasts. Future studies on the metabolism and transportability of silymarin to eggs are needed to understand this incompatibility.
Conclusions
Supplementing the diet of laying hens with 100 or 200 mg silymarin/kg of body weight as PSM, LSM, or NSM did not influence lipid peroxidation in egg yolks. However, the antioxidant and antimicrobial properties of silymarin could improve Haugh unit, nutrient digestibility, intestinal morphology, and ileal and jejunal pH in laying hens, which in turn could improve the bird performance. In addition, in most cases, the effects were more significant when silymarin converted to NSM or administered as LSM. To conclude, a recommendation for supplementing the laying hen diet with 200 mg/kg of silymarin as LSM or NSM is supported by the results of this study.
Ethical approval
All experimental procedures were conducted according to the international protocols and approved by Research Committee of the University of Kurdistan, Iran.
Acknowledgment
We are grateful to Dr. M. Habibian (Young Researchers and Elite Club, Islamic Azad University, Isfahan (Khorasgan) Branch, Isfahan, Iran) for assistance in editing and proof reading of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abolfathi ME, Tabeidian SA, Foroozandeh Shahraki AD, Tabatabaei SN, Habibian M. 2019. Comparative effects of n-hexane and methanol extracts of elecampane (Inula helenium L.) rhizome on growth performance, carcass traits, feed digestibility, intestinal antioxidant status and ileal microbiota in broiler chickens. Arch Anim Nutr. 73(2):88–110.
- Abou-Shehema BM, Rawia SH, Khalifah MM, Abdalla AA. 2016. Effect of silymarin supplementation on the performance of developed chickens under summer condition 2-during laying period. Egypt Poult Sci J. 36(4):1233–1249.
- Abreu VKG, Pereira ALF, De Freitas ER, Trevisan MTS, Da Costa JMC. 2014. Effect of anacardic acid on oxidative and color stability of spray dried egg yolk. LWT-Food Sci Technol. 55(2):466–471.
- Akit H, Sazili AQ, Ismail NA, Atan NA, Loh TC. 2016. Effect of dietary soy lecithin on laying performance, egg quality and meat texture of aged layer hen. Pertanika J Trop Agric Sci. 39(3):365–372.
- Akter Y, Kasim A, Omar H, Sazili AQ. 2014. Effect of storage time and temperature on the quality characteristics of chicken eggs. J Food Agric Environ. 12(3–4):87–92.
- Akyurek H, Okur AA. 2009. Effect of storage time, temperature and hen age on egg quality in free-range layer hens. J Anim Vet Adv. 8(10):1953–1958.
- Alhidary IA, Rehman Z, Khan RU, Tahir M. 2017. Anti-aflatoxin activities of milk thistle (Silybum marianum) in broiler. World Poult Sci J. 73(3):559–566.
- AOAC. 1990. Official methods of analysis of AOAC international. 15th ed. Arlington (VA): Association of Official Analytical Chemists.
- AOAC 2000. Official methods of analysis of AOAC international. 17th ed. Arlington (VA): Association of Official Analytical Chemists.
- Attia G, El-Eraky W, Hassanein E, El-Gamal M, Farahat M, Hernandez-Santana A. 2017. Effect of dietary inclusion of a plant extract blend on broiler growth performance, nutrient digestibility, caecal microflora and intestinal histomorphology. International J of Poultry Science. 16(9):344–353.
- Attia YA, Al-Harthi MA, El-Maaty HMA. 2020. Calcium and cholecalciferol levels in late-phase laying hens: effects on productive traits, egg quality, blood biochemistry, and immune responses. Front Vet Sci. 7(389):389.
- Attia YA, Hamed RS, Bovera F, Al-Harthi MA, El-Hamid A, Esposito L, Shahba HA. 2019. Milk thistle seeds and rosemary leaves as rabbit growth promoters. Anim Sci Pap Rep. 37(3):277–295.
- Attia YA, Kamel KI. 2012. Semen quality, testosterone, seminal plasma biochemical and antioxidant profiles of rabbit bucks fed diets supplemented with different concentrations of soybean lecithin. Animal. 6(5):824–833.
- Botsoglou NA, Florou-Paneri P, Nikolakakis I, Giannenas I, Dotas V, Botsoglou EN, Aggelopoulos S. 2005. Effect of dietary saffron (Crocus sativus L.) on the oxidative stability of egg yolk. Br Poult Sci. 46(6):701–707.
- Cook RH, Bird FH. 1973. Duodenal villus area and epithelial cellular migration in conventional and germ-free chicks. Poult Sci. 52(6):2276–2280.
- Criste FL, Mierlita D, Simeanu D, Boisteanu P, Pop IM, Georgescu B, Nacu G. 2018. Study of fatty acids profile and oxidative stability of egg yolk from hens fed a diet containing white lupine seeds meal. Rev Chim. 69(9):2454–2460.
- Di Costanzo A, Angelico R. 2019. Formulation strategies for enhancing the bioavailability of silymarin: the state of the art. Molecules. 24(11):2155.
- Draper HH, Hadley M. 1990. Malondialdehyde determination as index of lipid peroxidation. Meth Enzymol. 186:421–431.
- El-Gazayerly ON, Makhlouf AI, Soelm AM, Mohmoud MA. 2014. Antioxidant and hepatoprotective effects of silymarin phytosomes compared to milk thistle extract in CCl4 induced hepatotoxicity in rats. J Microencapsul. 31(1):23–30.
- Elnaggar AS, Ali RAM, El-Said EA. 2021. Physiological and immunological responses of ducks (Cairina moschata domestica) to silymarin supplementation. EPSJ. 40(4):895–913.
- Esmaily H, Hosseini-Tabatabaei A, Rahimian R, Khorasani R, Baeeri M, Barazesh-Morgani A, Yasa N, Khademi Y, Abdollahi M. 2009. On the benefits of silymarin in murine colitis by improving balance of destructive cytokines and reduction of toxic stress in the bowel cells. Cent Eur J Biol. 4(2):204–213.
- Evren E, Yurtcu E. 2015. In vitro effects on biofilm viability and antibacterial and antiadherent activities of silymarin. Folia Microbiol. 60(4):351–356.
- Fatehi D, Mohammadi M, Shekarchi B, Shabani A, Seify M, Rostamzadeh A. 2018. Radioprotective effects of Silymarin on the sperm parameters of NMRI mice irradiated with γ-rays. J Photochem Photobiol B. 178:489–495.
- Feddern V, Pra MCD, Mores R, Nicoloso RDS, Coldebella A, Abreu PGD. 2017. Egg quality assessment at different storage conditions, seasons and laying hen strains. Ciênc Agrotec. 41(3):322–333.
- Federico A, Dallio M, Loguercio C. 2017. Silymarin/silybin and chronic liver disease: a marriage of many years. Molecules. 22(2):191.
- Gao X, Xiao ZH, Liu M, Zhang NY, Khalil MM, Gu CQ, Qi DS, Sun LH. 2018. Dietary silymarin supplementation alleviates zearalenone-induced hepatotoxicity and reproductive toxicity in rats. J Nutr. 148(8):1209–1216.
- Goliomytis M, Simitzis P, Papalexi A, Veneti N, Hager-Theodorides AL, Charismiadou MA, Deligeorgis SG. 2019. Influence of citrus flavonoids on laying hen performance, inflammatory immune response, egg quality and yolk oxidative stability. Br Poult Sci. 60(3):272–278.
- Guo Y, Zhao ZH, Pan ZY, An LL, Balasubramanian B, Liu WC. 2020. New insights into the role of dietary marine-derived polysaccharides on productive performance, egg quality, antioxidant capacity, and jejunal morphology in late-phase laying hens. Poult Sci. 99(4):2100–2107.
- Hajizadeh Moghaddam A, Ahmadi Avendi E, Sayraf R, Zare M, University of Mazandaran 2017. Comparison of the therapeutic effects of silymarin and nanosilymarin on hepatotoxicity induced by titanium dioxide nanoparticles. Avicenna J Clin Med. 23(4):345–351.
- Hashemi Jabali NS, Mahdavi AH, Ansari Mahyari S, Sedghi M, Akbari Moghaddam Kakhki R. 2018. Effects of milk thistle meal on performance, ileal bacterial enumeration, jejunal morphology and blood lipid peroxidation in laying hens fed diets with different levels of metabolizable energy. J Anim Physiol Anim Nutr. 102(2):410–420.
- Hasheminejad SA, Makki OF, Nik HA, Ebrahimzadeh A. 2015. The effects of aflatoxin B1 and silymarin-containing milk thistle seeds on ileal morphology and digestibility in broiler chickens. Vet Sci Develop. 5(1):115–119.
- Hinton A, Jr Buhr RJ, Ingram KD. 2000. Physical, chemical, and microbiological changes in the crop of broiler chickens subjected to incremental feed withdrawal. Poult Sci. 79(2):212–218.
- Jahanian E, Mahdavi AH, Asgary S, Jahanian R. 2017. Effects of dietary inclusion of silymarin on performance, intestinal morphology and ileal bacterial count in aflatoxin‐challenged broiler chicks. J Anim Physiol Anim Nutr. 101(5):e43–54.
- Jahanian E, Mahdavi AH, Jahanian R. 2021. Silymarin improved the growth performance via modulating the microbiota and mucosal immunity in Escherichia coli-challenged broiler chicks: silymarin on growth performance of E. coli-challenged broiler chicks. Livest Sci. 249:104529.
- Jeong YJ, Choi YJ, Kwon HM, Kang SW, Park HS, Lee M, Kang YH. 2005. Differential inhibition of oxidized LDL-induced apoptosis in human endothelial cells treated with different flavonoids. Br J Nutr. 93(5):581–591.
- Kakran M, Sahoo GN, Li L. 2015. Fabrication of nanoparticles of silymarin, hesperetin and glibenclamide by evaporative precipitation of nanosuspension for fast dissolution. Pharm Anal Acta. 6(1):2.
- Karami M, Karimi A, Sadeghi A, Zentek J, Goodarzi Boroojeni F. 2020. Effects of phytase and benzoic acid supplementation on growth performance, nutrient digestibility, tibia mineralization and serum traits in male broiler chickens. Livest Sci. 242:104258.
- Kareem SM, Mahmood SS, Hindi NK. 2020. Effects of curcumin and silymarin on the Shigella dysenteriae and Campylobacter jejuni in vitro. J Gastrointest Canc. 51(3):824–828.
- Kato A, Sato Y. 1972. The release of carbohydrate rich component from ovomucin gel during storage. Agric Biol Chem. 36(5):831–836.
- Kiczorowska B, Al-Yasiry ARM, Samolińska W, Marek A, Pyzik E. 2016. The effect of dietary supplementation of the broiler chicken diet with Boswellia serrata resin on growth performance, digestibility, and gastrointestinal characteristics, morphology, and microbiota. Livest Sci. 191:117–124.
- Kidd PM. 2009. Bioavailability and activity of phytosome complexes from botanical polyphenols: the silymarin, curcumin, green tea, and grape seed extracts. Altern Med Rev. 14(3):226–246.
- Krause EL, Ternes W. 2000. Bioavailability of the antioxidative Rosmarinus officinalis compound carnosic acid in eggs. Eur Food Res Technol. 210(3):161–164.
- Lee J-S, Hong DY, Kim ES, Lee HG. 2017. Improving the water solubility and antimicrobial activity of silymarin by nanoencapsulation. Colloids Surf B Biointerfaces. 154:171–177.
- Liu CH, Jassey A, Hsu HY, Lin LT. 2019. Antiviral activities of silymarin and derivatives. Molecules. 4(8):1552.
- Liu X, Liu W, Deng Y, He C, Xiao B, Guo S, Zhou X, Tang S, Qu X. 2020. Use of encapsulated Bacillus subtilis and essential oils to improve antioxidant and immune status of blood and production and hatching performance of laying hens. Ital J Anim Sci. 19(1):1573–1581.
- Loetscher Y, Kreuzer M, Messikommer RE. 2014. Late laying hens deposit dietary antioxidants preferentially in the egg and not in the body. J Appl Poult Res. 23(4):647–660.
- Lohmann LSL-Classic International. 2011. Variety LSL-Lite commercial management guide [Internet]. Tierzucht (GmbH, Germany) Lohmann International. https://www.itz.de/
- Lorenzo JM, Mousavi Khaneghah A, Gavahian M, Marszałek K, Eş I, Munekata PE, Ferreira IC, Barba FJ. 2019. Understanding the potential benefits of thyme and its derived products for food industry and consumer health: from extraction of value-added compounds to the evaluation of bioaccessibility, bioavailability, anti-inflammatory, and antimicrobial activities. Crit Rev Food Sci Nutr. 59(18):2879–2895.
- Mirakzehi MT, Hosseini SJ, Saleh H. 2017. The effects of hydroalcoholic extracts of Withania somnifera root, Withania coagulans fruit and 1, 25-dihydroxycholecalciferol on immune response and small intestinal morphology of broiler chickens. J Appl Anim Res. 45(1):591–597.
- Mohajer A, Seifi S, Rezaie S, Khaniki GJ, Samarghandian S, Farkhondeh T, Sadighara P. 2021. Effect of Aloe vera extract on reducing aflatoxin B1 in eggs of laying hen and egg yolk oxidative stability. Biointerface Res Appl Chem. 11(5):12680–12688.
- Mohiti-Asli M, Shariatmadari F, Lotfollahian H, Mazuji MT. 2008. Effects of supplementing layer hen diets with selenium and vitamin E on egg quality, lipid oxidation and fatty acid composition during storage. Can J Anim Sci. 88(3):475–483.
- Morazzoni P, Montalbetti A, Malandrino S, Pifferi G. 1993. Comparative pharmacokinetics of silipide and silymarin in rats. Eur J Drug Metab Pharmacokinet. 18(3):289–297.
- Nisbet DJ, Corrier DE, DeLoach JR. 1993. Effect of mixed cecal microflora maintained in continuous culture and of dietary lactose on Salmonella Typhimurium colonization in broiler chicks. Avian Dis. 37(2):528–535.
- Omana DA, Wang J, Wu J. 2010. Ovomucin - a glycoprotein with promising potential. Trends Food Sci Technol. 21(9):455–463.
- Parveen R, Baboota S, Ali J, Ahuja A, Vasudev SS, Ahmad S. 2011. Effects of silymarin nanoemulsion against carbon tetrachloride-induced hepatic damage. Arch Pharm Res. 34(5):767–774.
- Pradhan SC, Girish C. 2013. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Ind J Med Res. 137(2):491–504.
- Prakatur I, Miskulin M, Pavic M, Marjanovic K, Blazicevic V, Miskulin I, Domacinovic M. 2019. Intestinal morphology in broiler chickens supplemented with propolis and bee pollen. Animals. 9(6):301.
- Quarantelli A, Romanelli S, Basini G, Righi F. 2009. The effects of silymarin on ovarian activity and productivity of laying hens. Ital J Anim Sci. 8(sup2):769–771.
- Radhika MI, Ezhilarasan D, Gopinath P. 2017. Antimicrobial efficacy of silymarin and silibinin against oral microorganisms. J Microbiol Infect Dis. 7(03):139–143.
- Radwan Nadia L, Hassan RA, Qota EM, Fayek HM. 2008. Effect of natural antioxidant on oxidative stability of eggs and productive and reproductive performance of laying hens. International J of Poultry Science. 7(2):134–150.
- Rama Rao SV, Prashanth K, Paul SS, Raju MV, Nagalakshmi D, Prakash B. 2020. Evaluation of feeding value of combination of alternate protein sources in White Leghorn layers. Br Poult Sci. 61(6):710–718.
- Rathore P, Arora I, Rastogi S, Akhtar M, Singh S, Samim M. 2020. Collagen nanoparticle-mediated brain silymarin delivery: an approach for treating cerebral ischemia and reperfusion-induced brain injury. Front Neurosci. 14:538404.
- Rodjan P, Soisuwan K, Thongprajukaew K, Theapparat Y, Khongthong S, Jeenkeawpieam J, Salaeharae T. 2018. Effect of organic acids or probiotics alone or in combination on growth performance, nutrient digestibility, enzyme activities, intestinal morphology and gut microflora in broiler chickens. J Anim Physiol Anim Nutr. 102(2):e931–e940.
- Sarlak S, Tabeidian SA, Toghyani M, Foroozandeh Shahraki AD, Goli M, Habibian M. 2021. Effects of replacing inorganic with organic iron on performance, egg quality, serum and egg yolk lipids, antioxidant status, and iron accumulation in eggs of laying hens. Biol Trace Elem Res. 199(5):1986–1999.
- SAS Institute. 2001. SAS/STAT User, Guide. Release Version 9.1. Cary. NC: SAS Institute Inc.
- Schiavone A, Righi F, Quarantelli A, Bruni R, Serventi P, Fusari A. 2007. Use of Silybum marianum fruit extract in broiler chicken nutrition: influence on performance and meat quality. J Anim Physiol Anim Nutr. 91(5–6):256–262.
- Scott TA, Silversides FG. 2000. The effect of storage and strain of hen on egg quality. Poult Sci. 79(12):1725–1729.
- Shahryari M, Tabeidian SA, Foroozandeh Shahraki AD, Tabatabaei SN, Toghyani M, Forouzmand M, Habibian M. 2021. Using soybean acid oil or its calcium salt as the energy source for broiler chickens: Effects on growth performance, carcass traits, intestinal morphology, nutrient digestibility, and immune responses. Anim Feed Sci Technol. 276:114919.
- Sherif IO, Al-Gayyar MM. 2013. Antioxidant, anti-inflammatory and hepatoprotective effects of silymarin on hepatic dysfunction induced by sodium nitrite. Eur Cytokine Netw. 24(3):114–121.
- Short FJ, Gorton P, Wiseman J, Boorman KN. 1996. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim Feed Sci Technol. 59(4):215–221.
- Šťastník O, Mrkvicová E, Pavlata L, Roztočilová A, Umlášková B, Anzenbacherová E. 2019. Performance, biochemical profile and antioxidant activity of hens supplemented with addition of milk thistle (Silybum marianum) seed cakes in diet. Acta Univ Agric Silvic Mendelianae Brun. 67(4):993–1003.
- Sultan A, Ahmad S, Khan S, Khan RU, Chand N, Tahir M, Shakoor A. 2018. Comparative effect of zinc oxide and silymarin on growth, nutrient utilization and hematological parameters of heat distressed broiler. Pak J Zool. 50(2):751–756.
- Tedesco D, Steidler S, Galletti S, Tameni M, Sonzogni O, Ravarotto L. 2004. Efficacy of silymarin-phospholipid complex in reducing the toxicity of aflatoxin B1 in broiler chicks. Poult Sci J. 83(11):1839–1843.
- Ustyol L, Demiroren K, Kandemir I, Erten R, Bulan K, Kaba S, Demir N, Basunlu MT. 2017. Comparative nephroprotective effects of silymarin, N-acetylcysteine, and thymoquinone against carbon tetrachloride-induced nephrotoxicity in rats. Iran Red Crescent Med J. 19(1):e37746.
- Valenzuela A, Aspillaga M, Vial S, Guerra R. 1989. Selectivity of silymarin on the increase of the glutathione content in different tissues of the rat. Planta Med. 55(5):420–422.
- Wagoner J, Negash A, Kane OJ, Martinez LE, Nahmias Y, Bourne N, Owen DM, Grove J, Brimacombe C, McKeating JA, et al. 2010. Multiple effects of silymarin on the hepatitis C virus lifecycle. Hepatology. 51(6):1912–1921.
- Wang M, Xie T, Chang Z, Wang L, Xie X, Kou Y, Xu H, Gao X. 2015. A new type of liquid silymarin proliposome containing bile salts: its preparation and improved hepatoprotective effects. PLoS One. 10(12):e0143625.
- Yang B, Zhao G, Wang L, Liu S, Tang J. 2021. Effects of the Agaricus bisporus stem residue on performance, nutrients digestibility and antioxidant activity of laying hens and its effects on egg storage. Anim Biosci. 34(2):256–264.