Abstract
To investigate the effects of dietary betaine supplementation on the growth performance, digestive function, intestinal integrity, immunity, and antioxidant capacity of yellow-feathered broilers, a total of 400 one-day-old female yellow-feathered broilers were randomly allocated into 5 dietary treatments with 8 replicates of 10 chicks each, and fed a basal diet supplemented with 0 (control group), 125, 250, 500 and 1000 mg/kg betaine for 74 days, respectively. During the 1–37 days and 1–74 days, betaine linearly increased (p < .05) average daily gain, and decreased (p < .05) daily feed intake and feed conversion ratio. At 37 days, betaine linearly increased (p < .05) the apparent utilisation of crude protein, dry matter, and ether extract, jejunal digesta amylase and trypsin activities, villus height of jejunum and ileum, and ileal secretory immunoglobulin A content, glutathione peroxidase activity, and claudin-1 mRNA abundance, and linearly decreased (p < .05) serum D-lactate content and diamine oxidase activity, jejunal malondialdehyde content and nuclear factor kappaB mRNA abundance, ileal toll-like receptor 4 and myeloid differentiation factor 88 mRNA abundance. At 74 days, betaine linearly increased (p < .05) pancreatic lipase activity, jejunal glutathione peroxidase activity, and ileal glutathione content, and linearly decreased (p < .05) serum diamine oxidase activity and ileal malondialdehyde content. In conclusion, dietary supplementation with betaine improved growth performance, digestive function, intestinal mucosal barrier integrity, immune function, and antioxidant capacity of yellow-feathered broilers, and its optimal dosage in this study is 1000 mg/kg.
Betaine improved growth performance.
Betaine increased digestive function.
Betaine enhanced intestinal antioxidant capacity.
Betaine improved intestinal integrity and barrier function.
Betaine improved intestinal immunity.
HIGHLIGHTS
Introduction
The yellow-feathered broiler is a slow-growing Chinese local breed that is highly accepted by Chinese consumers due to its better meat quality (Xie et al. Citation2020). China is the world’s second-largest producer of broilers, with an annual output of nearly five billion yellow-feathered broilers. With the increasing consumption of chicken meat, intensive rearing system has been introduced into broilers production. Modern intensive rearing for broilers cause various stresses, especially in the intestine, which would, in turn, lead to an increased nutrient consumption and poor feed conversion efficiency (Wang et al. Citation2019). The gut of broiler chickens has digestive, absorptive, metabolic, immunological, and endocrinological functions (Perry Citation2006). Intestinal health plays an important role in the maintenance of systemic health and the production efficiency of broilers (Oviedo-Rondon, Citation2019). Broilers were often fed antibiotics to promote intestinal health, but banning antibiotics could cause problems such as poor intestinal resistance against pathogenic bacterial invasion (Tsiouris Citation2016). To address these problems, researchers have suggested a myriad of nutritional strategies to improve intestinal health of broilers (Wang et al. Citation2019; Zou et al. Citation2019; Zhang et al. Citation2020). Among them, betaine has received considerable attention because of its physiological and nutritional functions.
Betaine is the trimethyl derivative of the amino acid glycine, which can act as methyl group donor and organic osmolyte (Eklund et al. Citation2005). This chemical, as a ‘methyl-donor’, is involved in the synthesis of many substances such as methionine, carnitine and creatine, and participates in protein and energy metabolism (Eklund et al. Citation2005). Owing to its osmoprotective properties, betaine can protect intestinal epithelial cell proteins and enzymes from environmental stress, and help to maintain the integrity of the intestinal structure when broilers are subjected to immunological stress (Kettunen et al. Citation2001b; Klasing et al. Citation2002; Metzler-Zebeli et al. Citation2009). Maintenance and improvement of gut health are essential for nutrients digestibility and productivity of animals. Integrity, immunity, and antioxidant capacity are widely believed to be the important indicators of intestinal health. When the antioxidant capacity of the intestinal mucosa decreases, oxidative stress can induce intestinal cell apoptosis, increase permeability, and abnormal inflammation (Da Silva et al. Citation2019). It had been demonstrated that the inclusion of betaine could improve the intestinal integrity and barrier function of weaned piglets (Wang et al. Citation2020). Betaine also boost cellular antioxidant defense and prevent oxidative stress (Ganesan et al. Citation2010). In addition, studies have reported that betaine could ameliorate the small intestine tissue damage in the acute liver failure mice by alleviating the inflammatory response (Chen et al. Citation2020a). Existing results suggest that betaine has a positive influence on intestinal health, however, it remained elusive whether betaine could enhance intestinal integrity and immunity of broilers. Therefore, the current experiment was conducted to investigate the effects of dietary betaine supplementation on the growth performance, digestive function, intestinal integrity, immunity, and antioxidant capacity of yellow-feathered broilers.
Materials and methods
Animals and treatment
This study was performed according to the Institutional Animal Care and Use Committee of Nanjing Agricultural University.
A total of 400 one-day-old female yellow-feathered broilers with an average initial weight of 39.0 ± 0.1 g were allocated into 5 treatments of 8 replicates (cages) with 10 birds per cage for a 74-day experimental period. Female broilers were selected in this study based on the consumption habits of Chinese people. Birds were raised in 3-layer steel cages (120 × 60 × 50 cm) in a temperature-controlled room under 23:1 h light/dark cycle and were allowed free access to mash feed and water. The room temperature was maintained at 32–34 °C for the first 3 days and then gradually reduced by 3 °C per week to a final temperature of 20 °C. The dietary treatments were basal diet supplemented with 0 (control), 125, 250, 500, and 1000 mg/kg betaine, respectively. The ingredient composition and nutrient content of basal diets were presented in Table . Betaine anhydrous (96%, chemically synthesised) was obtained from Yixing Skystone Feed Co., Ltd. (Yixing, Jiangsu, China). Broilers were weighed after feed deprivation for 12 h and average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR) were calculated at the end of each experimental period (37 and 74 days).
Table 1. Composition and nutrient level of basal diet (as-fed basis).
Sample collection
At the 37 days and 74 days of the age, 8 broilers per group (one bird per replicate) from each treatment were randomly selected. Blood samples were collected from the wing vein. The serum was separated after centrifugation at 3000 g for 15 min at 4 °C, stored at −20 °C for further analysis. Broilers were euthanized by cervical dislocation. The jejunum (anterior of Meckel’s diverticulum) and ileum (posterior to Meckel’s diverticulum) were then gingerly separated. The pancreas and the digesta samples from jejunum were collected and stored at −20 °C for further analysis. Approximately 2-cm length of the proximal jejunum and ileum was removed and flushed with ice-cold phosphate buffer saline at pH 7.4 and immediately placed in 4% paraformaldehyde for histological analyses. The jejunal and ileal mucosae were gently scraped by a glass microscope slide from the rest of the jejunum and ileum. The intestinal mucosae were stored at −80 °C for further analysis.
Apparent nutrient utilisation analysis
From 35 to 37 days and 72 to 74 days of the experiment, the faecal samples were collected from each replicate. The feed and faecal samples were oven-dried and ground to pass through a 1.0 mm screen to get a uniform particle size before further analysis. The apparent utilisation of dry matter (DM), crude protein (CP) and ether extract (EE) of the feed and faecal samples were analysed using AOAC procedures (2000). Acid-insoluble ash (AIA) was determined using the method of Choct and Annison (Citation1992). The apparent metabolic rate of nutrients was calculated according to Zhou et al. (Citation2014).
Digestive enzyme activities analysis
The protein concentrations, amylase, lipase, and trypsin activities of the pancreas and jejunal digesta samples were assayed using standard kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Intestinal morphology analysis
The section-making process of jejunum and ileum consisted of serial dehydration, clearing, and embedding in paraffin. About 5-μm-thick tissue sections (three cross sections from each sample) were cut by a microtome (Leica Instruments Ltd., Shanghai, China) and were fixed on slides. A routine staining procedure was carried out using haematoxylin and eosin. Villus height and crypt depth of 10 well-oriented villi per segment were measured using a Nikon ECLIPSE 80i light microscope equipped with a computer-assisted morphometric system (Nikon Corporation, Tokyo, Japan). Villus height was measured from the tip of the villus to the villus-crypt junction, whereas crypt depth was defined as the depth of the invagination between adjacent villi.
Serum D-lactate and diamine oxidase analysis
The D-lactate (D-LA) concentration and the diamine oxidase (DAO) activity in the serum were determined using corresponding commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), as per the manufacturer’s instructions.
Intestinal mucosal immune and antioxidant capacity analysis
The total protein, secretory immunoglobulin A (SIgA), malondialdehyde (MDA), and glutathione (GSH) contents, and total antioxidant capacity (T-AOC), and glutathione peroxidase (GSH-Px) activities in the jejunal and ileal mucosa were measured using corresponding commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) in accordance with the manufacturer's instructions.
Messenger RNA quantification
The total RNA of intestinal mucosae was isolated using TRIzol (TaKaRa Biotechnology, Dalian, China). Concentration and purity of RNA were measured from OD260/280 readings (ratio > 1.8) using ND-2000 microspectrophotometer (Thermo Scientific, Wilmington, USA). After determining the RNA concentration, 1 μg of total RNA was reverse-transcribed into complementary DNA using the PrimeScriptTM RT reagent kit (TaKaRa Biotechnology, Dalian, China). Complementary DNA was diluted 10× before real-time PCR. Real-time PCR was performed using the TB Green Premix Ex T aq (TaKaRa Biotechnology, Dalian, China) on the QuantStudio 5 Real-Time PCR System (Thermo Scientific, Wilmington, USA). The β-actin gene was selected to be the housekeeping gene to normalise the expression of the other target genes. The sequences of primers (toll-like receptor 4 (TLR4), myeloid differentiation factor 88 (MyD88), TNF receptor associated factor 6 (TRAF-6), nuclear factor kappaB (NF-κB), interleukin 1 beta (IL-1β), claudin-1 (CLDN1) and β-actin) were synthesised by Sangon Biotech (Sangon Biotech Co., Ltd., Shanghai, China), and shown in Table . Relative gene expression levels were analysed by the 2−ΔΔCt method after normalisation against β-actin.
Table 2. Primer sequences used for RT-qPCR.
Statistical analysis
Data were analysed by one-way analysis of variance (ANOVA) using SPSS statistical software (SPSS 20.0, SPSS, Chicago, USA). Differences among treatments were examined using Tukey’s multiple range tests, which were considered significant at p < .05. The linear and quadratic effects of dietary betaine levels were tested using polynomial contrasts.
Results
Growth performance
During 1–37 days (Table ), betaine supplementation linearly (p < .05) increased ADG and decreased ADFI and FCR. Compared with the control group, betaine supplementation, irrespective of its supplemental levels, significantly (p < .05) increased ADG and reduced FCR, and dietary supplementation with betaine at a level of 500 mg/kg significantly (p < .05) decreased ADFI in broilers. However, no significant differences were observed in ADG, ADFI or FCR (p < .05) among the groups during the 38–74 days. In whole experimental period (1–74 days), there were a linear (p < .05) increase in ADG and decrease in ADFI and FCR with increasing level of betaine. Compared with the control group, feeding a basal diet supplemented with 1000 mg/kg betaine significantly (p < .05) increased ADG of broilers. Similarly, supplementing 500 mg/kg betaine significantly (p < .05) decreased ADFI of broilers, and FCR was significantly (p < .05) reduced by the supplementation of 500 or 1000 mg/kg betaine.
Table 3. Effect of betaine on growth performance of yellow-feathered broilers.
Apparent nutrient utilisation
During 35–37 days (Table ), betaine linearly (p < .05) increased the apparent utilisation of EE and linearly and quadratically (p < .05) elevated the apparent utilisation of DM and CP. Compared with the control group, dietary betaine supplementation significantly (p < .05) increased the apparent utilisation of DM. Apparent utilisation of EE in 1000 mg/kg betaine-supplemented group and CP in betaine-treated group were higher than their counterparts receiving a basal diet (p < .05). However, dietary supplemental with betaine had no significant effect (p > .05) on the apparent utilisation of DM, CP or EE in broilers from 72 to 74 days.
Table 4. Effect of betaine on apparent nutrient utilisation of yellow-feathered broilers (%).
Enzyme activities in pancreas and jejunal digesta
As shown in Table , dietary supplementation with betaine linearly (p < .05) increased activities of amylase and trypsin in jejunal digesta at 37 days. The addition of 1000 mg/kg betaine significantly (p < .05) increased trypsin activity of jejunal digesta when compared with the control group. At 74 days, betaine linearly (p < .05) increased lipase activity of pancreas and linearly and quadratically (p < .05) increased amylase activity of jejunal digesta. Dietary betaine supplemented at a dosage of 1000 mg/kg significantly (p < .05) increased lipase activity of pancreas, and feeding a basal dietsupplemented betaine significantly (p < .05) increased amylase activity of jejunal digesta in comparison with the control group.
Table 5. Effect of betaine on the digestive enzyme activities of pancreas and jejunal digesta in yellow-feathered broilers.
Intestinal morphology
As presented in Table , the villus height of jejunum and ileum was linearly (p < .05) elevated with the increasing level of betaine at both 37 days and 74 days. Compared with the control group, the supplementation of 1000 mg/kg betaine remarkably (p < .05) enhanced villus height in the ileum at 37 days. The inclusion of betaine linearly (p < .05) increased crypt depth in jejunum and ileum at 74 days. Betaine supplementation at a dosage of 500 mg/kg significantly (p < .05) increased villus height in the jejunum, and the highest level of dietary betaine (1000 mg/kg) supplementation significantly (p < .05) increased villus height in the ileum when compared with the control group. However, the ratio of villus height to crypt depth in the jejunum and ileum were not altered (p > .05) by the supplementation of betaine.
Table 6. Effect of betaine on intestinal morphology of yellow-feathered broilers.
Serum D-LA content and DAO activity
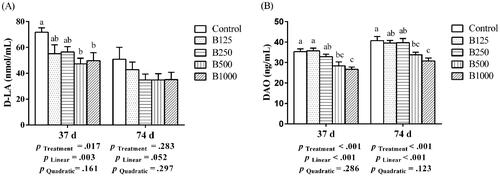
Dietary betaine supplementation linearly (p < .05) reduced serum D-LA content at 37 days and serum DAO activity at both 37 days and 74 days (Figure ). Compared with the control group, the inclusion of 500 and 1000 mg/kg betaine significantly (p < .05) decreased serum D-LA content at 37 days, but a similar result in D-LA concentration was not found at 74 days (p > .05). Dietary 500 and 1000 mg/kg betaine supplementation significantly (p < .05) reduced serum DAO activity when compared with the control group at 37 days and 74 days.
Figure 1. Effect of betaine on serum D-LA content (A) and DAO activity (B) of yellow-feathered broilers at 37 days and 74 days of age. B125, B250, B500, B1,000, basal diet supplemented with 125, 250, 500, 1000 mg/kg betaine, respectively. D-LA: D-lactate; DAO: diamine oxidase. Results are presented as means ± SEM (n = 8). a,b,cBars marked with different superscripts are significantly different (p <.05).
Intestinal mucosal SIgA concentration and antioxidant capacity
In the jejunal mucosa, betaine administration linearly (p < .05) reduced MDA content and increased GSH level at 37 days (Table ). The supplementation with 1000 mg/kg betaine significantly (p < .05) decreased MDA content, and dietary 250 and 1000 mg/kg betaine supplementation significantly (p < .05) elevated GSH content when compared with the control group. At 74 days, betaine supplementation linearly (p < .05) increased GSH-Px activity. The administration of betaine at the levels of 125, 500 or 1000 mg/kg significantly (p < .05) increased GSH-Px activity as compared with the control group. However, SIgA content and T-AOC activity in intestinal mucosa were not altered (p > .05) by the betaine supplementation.
Table 7. Effect of betaine on the SIgA concentration and antioxidant capacity of jejunal mucosa in yellow-feathered broilers.
In the ileal mucosa, betaine supplementation linearly (p < .05) increased SIgA content and GSH-Px activity at 37 days (Table ). The SIgA level of broilers given a basal diet supplemented with 125, 500 or 1000 mg/kg betaine was significantly (p < .05) higher than their counterparts fed a basal diet, and the activity of GSH-Px was significantly (p < .05) increased by the inclusion of 1000 mg/kg betaine when compared with the control group. At 74 days, with increasing supplemental levels of betaine, MDA content was linearly (p < .05) reduced, while GSH content was linearly (p < .05) increased. Compared with the control group, dietary 1000 mg/kg betaine supplementation significantly (p < .05) decreased MDA content, and T-AOC activity was significantly (p < .05) increased when supplemental level of betaine was 500 mg/kg.
Table 8. Effect of betaine on the SIgA concentration and antioxidant capacity of ileal mucosa in yellow-feathered broilers.
Intestinal mucosal gene expressions
The jejunal mucosa gene expression levels were shown in Table . At 37 days, betaine supplementation linearly (p < .05) downregulated mRNA abundance of TRAF-6 and NF-κB. Dietary betaine supplementation at a level of 1000 mg/kg significantly (p < .05) downregulated mRNA abundance of TRAF-6 and NF-κB in comparison with the control group. At 74 days, betaine supplementation linearly (p < .05) downregulated mRNA abundance of TRAF-6. Compared with the control group, the supplementation with 1000 mg/kg betaine significantly (p < .05) downregulated mRNA expression level of TRAF-6. However, treatments did not alter (p > .05) the mRNA expressions of TLR4, MyD88, IL-1β, and CLDN1 intestinal mucosa.
Table 9. Effect of betaine on the gene expression of jejunal mucosa in yellow-feathered broilers.
As for ileal mucosa (Table ), betaine linearly (p < .05) downregulated mRNA abundance of TLR4 and MyD88 and upregulated mRNA expression level of CLDN1 at 37 days. Compared with the control group, dietary 1000 mg/kg betaine supplementation significantly (p < .05) downregulated the mRNA abundance of TLR4 and MyD88 and upregulated mRNA expression level of CLDN1. At 74 days, the increasing level of betaine supplementation (p < .05) downregulated IL-1β mRNA abundance. The inclusion of 500 and 1000 mg/kg betaine significantly (p < .05) downregulated the mRNA expression level of IL-1β when compared with the control group. However, the mRNA abundances of TRAF-6 and NF-κB in intestinal mucosa had no significant influence (p > .05) among groups.
Table 10. Effect of betaine on the gene expression of ileal mucosa in yellow-feathered broilers.
Discussion
Our study showed that dietary supplementation with 500 and 1000 mg/kg betaine significantly increased ADG and decreased FCR and ADFI of yellow-feathered broilers. The effects of dietary betaine in improving growth performance of broilers have been reported previously (Rao et al. Citation2011; Chen et al. Citation2020b). Moreover, Sun et al. (Citation2019) observed that the optimal level of betaine supplementation for growth performance was 500 or 1000 mg/kg in yellow-feathered broilers. The beneficial effects of betaine on broilers’ growth performance may be due to its osmotic properties and its role as a methyl group donor (Metzler-Zebeli et al. Citation2009). The utilisation of nutrients is closely associated with the growth performance of broilers. In this study, broilers fed diets containing betaine had increased apparent utilisation of DM, CP, and EE from 34 to 37 days, which was in agreement with previous studies that broilers ingesting diets supplemented with betaine had improved utilisation of DM, CP, and EE (El-Hussein et al. Citation2007; Ratriyanto and Indreswari Citation2014). Betaine, as an osmoprotectant, has effects on supporting intestinal cell growth, enhancing intestinal cell activity, and improving intestinal morphology, thereby eventually improving nutrient digestibility (Kettunen et al. Citation2001b; Eklund et al. Citation2005). Digestive enzymes are important factors affecting the nutrient utilisation of the intestine, and they are always employed to evaluate the digestive capacity of broilers. The pancreas is an exocrine gland and an important digestive organ of animal. The jejunum is the main place of digestion and absorption in the small intestine (Ouhida et al. Citation2000). In the present study, diets supplemented with 1000 mg/kg betaine improved the activities of amylase and trypsin of jejunal digesta and the activity of pancreatic lipase, which might contribute to the improvement of nutrient digestibility. These results were partially in agreement with findings of Wang et al. (Citation2020), who found that betaine supplementation significantly enhanced activitity of digestive enzyme in weaned pigs. Studies have reported that betaine could promote the affinity of enzymes and substrates, thereby improving the catalytic efficiency of enzymes (Wang et al. Citation2020). The results of this study showed that dietary betaine supplementation increased digestive enzyme activities, promoted absorption of nutrients, thereby, improved the growth performance.
The integrity of intestinal morphology can be used as a potential indicator of intestinal health status. The higher villus heights of the jejunal and ileal indicated that the function of the intestine enhanced (Ruttanavut and Yamauchi Citation2010). In the current study, the incorporation of 1000 mg/kg betaine in the diet increased villus height of the jejunum and ileum, which was consistent with the results of Sun et al. (Citation2019), who found that diet supplemented with betaine increased the ileal villus height in yellow-feathered broilers. Furthermore, the similar effects were reported by Xu and Yu (Citation2000) and Wang et al. (Citation2020) in weaned piglets. These results indicated that betaine had a beneficial effect on intestinal development, which might be due to the fact that betaine could increase the ability of intestinal cells to bind water, promote epithelial cell formation (Kettunen et al. Citation2001a), and enhance tensile strength in chickens (Remus and Quarles Citation2000).
D-LA is an end product of intestinal bacterial fermentation (Mileti et al. Citation2009). DAO is an intracellular enzyme that is secreted by intestinal epithelial cells (Luk et al. Citation1980). The increase in the concentration of D-LA and the activity of DAO in blood could reflect the degree of damage to the intestinal mucosa and intestinal permeability (Mileti et al. Citation2009; Liu et al. Citation2020). The intestinal mucosal barrier is the first barrier against a hostile environment, mainly formed by the tight junctions of epithelial cells that mainly consists of occludin, claudin1 and zonula occludens-1. In present study, broilers given diets supplemented betaine decreased D-LA content and DAO activity in serum, and increased the expression of CLDN1 mRNA in ileum mucosa, indicating that betaine could exert a beneficial effect on intestinal barrier function. This result was in accordance with the finding of Wang et al. (Citation2020), who reported that betaine could improve the intestinal integrity and barrier function by reducing plasma DAO activity, upregulated expression of tight junction proteins, and improving intestinal morphology in weaned piglets. In addition, Wu et al. (Citation2020) observed that betaine improved intestinal permeability by enhancing the mRNA expression of CLDN1 in intestinal porcine epithelial cells subjected to lipopolysaccharide treatment. Therefore, these results suggested that betaine could maintain intestinal integrity and barrier function, which, in turn, helped to improve the digestion and absorption of nutrients.
The SIgA is the main antibody type in the intestinal mucosa, and is the first line of defense of humoral immune responses to protect the gut epithelium from pathogenic microorganisms and toxic compounds (Corthesy Citation2013). In the current study, betaine supplementation boosted the content of SIgA in ileal mucosa of broilers. This result was in accordance with the finding of Hamidi et al. (Citation2010), who pointed that the IgA content of gut tissue of broilers in coccidiosis condition was increased by added betaine in dietary. Likewise, Sun et al. (Citation2020) reported that the supplementation of betaine increased immunoglobulin M content in fish intestine. Betaine could improve cellular immunity by increasing macrophage phagocytosis, enhancing the number of epithelial lymphocytes, and thicking the lamina propria thickness (Ovington et al. Citation1995; Hamidi et al. Citation2009), thereby enhancing the body's immune response. This may be one of the reasons for the increase in SIgA content.
Oxidative stress can lead to inflammatory disease (Zhang et al. Citation2013), and disrupt mucosal barrier integrity and function (Banan et al. Citation2000). MDA is the main end product by oxidative degradation, which could reflect the degree of lipid peroxidation (Latha et al. Citation2017). T-AOC is one of the important indicators reflecting total antioxidant capacity (Ghiselli et al. Citation2000). GSH-Px as an antioxidant enzyme in cell can break down hydrogen peroxide and lipid oxidation products into water and oxygen (Ceballos-Picot et al. Citation1996). GSH is an important small antioxidant molecule widely distributed among living cells and body fluids (Kerio et al. Citation2011). In the present study, the incorporation of 1000 mg/kg betaine decreased MDA content and increased GSH content and T-AOC and GSH-Px activities in the small intestinal mucosa, indicating that betaine inclusion improved the antioxidant capacity of small intestine in broiler chickens. A similar result was reported by Alirezaei et al. (Citation2012), who found that supplementation of betaine in diets improved antioxidant defense in the breast muscles of broiler chickens. Chen et al. (Citation2020b) reported that the inclusion of betaine reduced MDA content and enhanced GSH-Px activity and GSH content of breast muscle in transported broilers. The antioxidant effect of betaine may be associated with S-adenosylmethionine and GSH, which participate in enhancing the antioxidant defense mechanism of cells and protect cells from oxidative damage (Ganesan et al. Citation2010; Alirezaei et al. Citation2011).
TLRs, as pattern recognition receptor, play an important role in the intestinal mucosal immune system by mediating signal transduction. Members of the TLRs family are potent activators of MyD88, and the MyD88-dependent pathway leads to activation of NF-κB via TRAF6 (Vallabhapurapu and Karin Citation2009), ultimately leading to the synthesis and release of a number of pro-inflammatory mediators (Chow et al. Citation1999). Current results revealed that 1000 mg/kg betaine downregulated the mRNA expression of TLR4, MyD88, IRAK-4, NF-κB, and IL-1β in the intestines of broilers. The results were in agreement with the findings of Yang et al. (Citation2018), who found that betaine downregulated the NF-κB signalling pathway or inhibited the inflammatory reaction to alleviate monocrotaline-induced pulmonary hypertension in rat. Additionally, Wu et al. (Citation2020) demonstrated that betaine reduced the mRNA expression of pro-inflammatory cytokine interleukin 6 in intestinal porcine epithelial cells treatmented with lipopolysaccharide, indicating that betaine could exert anti-inflammatory activity. Betaine can attenuate inflammation by increasing the synthesis of S-adenosylmethionine (Purohit et al. Citation2007). An elevation of S-adenosylmethionine can prevent the induction of inducible nitric oxide synthase, attenuate the production of NF-κB, and increase the production of glutathione which in turn is involved in NF-κB suppression (Detopoulou et al. Citation2008; Dou et al. Citation2018). Studies have shown that the barrier and integrity of the intestine may be regulated by pro-inflammatory factors which can downregulate the transmembrane tight junction protein expression, thereby increasing the intestinal permeability (Mankertz et al. Citation2000). Therefore, the improved intestinal barrier and integrity function might be also associated with the reduced mRNA expressions of intestinal genes that included TLR4, MyD88, IRAK-4, NF-κB, and IL-1β.
Conclusions
In this study, dietary betaine supplementation improved growth performance, digestive function, intestinal mucosal barrier integrity, immune function, and antioxidant capacity of yellow-feathered broilers. Besides, the optimal dosage of betaine in this study is 1000 mg/kg.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- AOAC. 2000. Official methods of analysis. 17th ed. Arlington (VA): Association of Official Analytical Chemists.
- Alirezaei M, Jelodar G, Niknam P, Ghayemi Z, Nazifi S. 2011. Betaine prevents ethanol-induced oxidative stress and reduces total homocysteine in the rat cerebellum. J Physiol Biochem. 67(4):605–612.
- Alirezaei M, Reza Gheisari H, Reza Ranjbar V, Hajibemani A. 2012. Betaine: a promising antioxidant agent for enhancement of broiler meat quality. Br Poult Sci. 53(5):699–707.
- Banan A, Choudhary S, Zhang Y, Fields JZ, Keshavarzian A. 2000. Oxidant-induced intestinal barrier disruption and its prevention by growth factors in a human colonic cell line: role of the microtubule cytoskeleton. Free Radic Biol Med. 28(5):727–738.
- Ceballos-Picot IÈne, Witko-Sarsat V, Merad-Boudia M, Nguyen AT, Thévenin M, Jaudon MC, Zingraff J, Verger C, Jingers P, Descamps-Latscha B. 1996. Glutathione antioxidant system as a marker of oxidative stress in chronic renal failure. Free Radic Biol Med. 21(6):845–853.
- Chen Q, Wang Y, Jiao FZ, Shi CX, Pei MH, Wang LW, Gong ZJ. 2020a. Betaine inhibits toll-like receptor 4 responses and restores intestinal microbiota in acute liver failure mice. Sci Rep. 10(1):21850.
- Chen R, Wen C, Gu Y, Wang C, Chen Y, Zhuang S, Zhou YM. 2020b. Dietary betaine supplementation improves meat quality of transported broilers through altering muscle anaerobic glycolysis and antioxidant capacity. J Sci Food Agric. 100(6):2656–2663.
- Choct M, Annison G. 1992. The inhibition of nutrient digestion by wheat pentosans. Br J Nutr. 67(1):123–132.
- Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 274(16):10689–10692.
- Corthesy B. 2013. Role of secretory IgA in infection and maintenance of homeostasis. Autoimmun Rev. 12(6):661–665.
- Da Silva EO, Gerez JR, Hohmann MSN, Verri WA, Bracarense A. 2019. Phytic acid decreases oxidative stress and intestinal lesions induced by fumonisin b1 and deoxynivalenol in intestinal explants of pigs. Toxins. 11(1):18.
- Detopoulou P, Panagiotakos DB, Antonopoulou S, Pitsavos C, Stefanadis C. 2008. Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: the ATTICA study. Am J Clin Nutr. 87(2):424–430.
- Dou X, Li S, Hu L, Ding L, Ma Y, Ma W, Chai H, Song Z. 2018. Glutathione disulfide sensitizes hepatocytes to TNFα-mediated cytotoxicity via IKK-β S-glutathionylation: a potential mechanism underlying non-alcoholic fatty liver disease. Exp Mol Med. 50(4):1–16.
- Eklund M, Bauer E, Wamatu J, Mosenthin R. 2005. Potential nutritional and physiological functions of betaine in livestock. Nutr Res Rev. 18(1):31–48.
- El-Hussein OM, Abo-El-Ell MA, Abd-Elsame MO, M. Abd-Elf M. 2007. Response of broiler chick performance to dietary betaine and folic acid at different methionine levels. Int J Poultry Sci. 6(7):515–525.
- Ganesan B, Buddhan S, Anandan R, Sivakumar R, Anbinezhilan R. 2010. Antioxidant defense of betaine against isoprenaline-induced myocardial infarction in rats. Mol Biol Rep. 37(3):1319–1327.
- Ghiselli A, Serafini M, Natella F, Scaccini C. 2000. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic Biol Med. 29(11):1106–1114.
- Hamidi H, Jahanian R, Pourreza J. 2010. Effect of dietary betaine on performance, immunocompetence and gut contents osmolarity of broilers challenged with a mixed coccidial infection. Asian J Anim Vet Adv. 5(3):193–201.
- Hamidi H, Pourreza J, Rahimi H. 2009. Dietary betaine affect duodenal histology of broilers challenged with a mixed coccidial infection. Pak J Biol Sci. 12(3):291–295.
- Kerio LC, Bend JR, Wachira FN, Wanyoko JK, Rotich MK. 2011. Attenuation of t-butylhydroperoxide induced oxidative stress in HEK 293 WT cells by tea catechins and anthocyanins. J Toxicol Environ Health. 3:367–375.
- Kettunen H, Peuranen S, Tiihonen K. 2001a. Betaine aids in the osmoregulation of duodenal epithelium of broiler chicks, and affects the movement of water across the small intestinal epithelium in vitro. Comp Biochem Physiol A Mol Integr Physiol. 129(2–3):595–603.
- Kettunen H, Tiihonen K, Peuranen S, Saarinen MT, Remus JC. 2001b. Dietary betaine accumulates in the liver and intestinal tissue and stabilizes the intestinal epithelial structure in healthy and coccidia-infected broiler chicks. Comp Biochem Physiol A Mol Integr Physiol. 130(4):759–769.
- Klasing KC, Adler KL, Remus JC, Calvert CC. 2002. Dietary betaine increases intraepithelial lymphocytes in the duodenum of coccidia-infected chicks and increased functional properties of phagocytes. J Nutr. 132(8):2274–2282.
- Latha S, Chaudhary S, Ray RS. 2017. Hydroalcoholic extract of Stevia rebaudiana bert. leaves and stevioside ameliorates lipopolysaccharide induced acute liver injury in rats. Biomed Pharmacother. 95:1040–1050.
- Liu S, Zhang D, Liu Y, Zhou D, Yang H, Zhang K, Zhang D. 2020. Circular RNA circ_0001105 protects the intestinal barrier of septic rats by inhibiting inflammation and oxidative damage and YAP1 expression. Gene. 755:144897.
- Luk GD, Bayless TM, Baylin SB. 1980. Diamine oxidase (histaminase). A circulating marker for rat intestinal mucosal maturation and integrity. J Clin Invest. 66(1):66–70.
- Mankertz J, Tavalali S, Schmitz H, Mankertz A, Riecken EO, Fromm M, Schulzke JD. 2000. Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J Cell Sci. 113(11):2085–2090.
- Mileti E, Matteoli G, Iliev ID, Rescigno M. 2009. Comparison of the immunomodulatory properties of three probiotic strains of lactobacilli using complex culture systems: prediction for in vivo efficacy. PLoS One. 4(9):e7056.
- Metzler-Zebeli BU, Eklund M, Mosenthin R. 2009. Impact of osmoregulatory and methyl donor functions of betaine on intestinal health and performance in poultry. Worlds Poult Sci J. 65(3):419–441.
- Ouhida I, Perez JF, Gasa J, Puchal F. 2000. Enzymes (β-glucanase and arabinoxylanase) and/or sepiolite supplementation and the nutritive value of maize-barley-wheat based diets for broiler chickens. Br Poult Sci. 41(5):617–624.
- Oviedo-Rondon EO. 2019. Holistic view of intestinal health in poultry. Anim Feed Sci Technol. 250:1–8.
- Ovington KS, Alleva LM, Kerr EA. 1995. Cytokines and immunological control of Eimeria spp. Int J Parasitol. 25(11):1331–1351.
- Perry GC. 2006. Avian gut function in health and disease. Wallingford (UK): Oxford Univ Press.
- Purohit V, Abdelmalek MF, Barve S, Benevenga NJ, Halsted CH, Kaplowitz N, Kharbanda KK, Liu QY, Lu SC, McClain CJ, Swanson C, et al. 2007. Role of S-adenosylmethionine, folate, and betaine in the treatment of alcoholic liver disease: summary of a symposium. Am J Clin Nutr. 86(1):14–24.
- Rao SV, Rama Raju MVLN, Panda AK, S, Poonam Sunder G. Shyam 2011. Effect of supplementing betaine on performance, carcass traits and immune responses in broiler chicken fed diets containing different concentrations of methionine. Asian Australas J Anim Sci. 24(5):662–669.
- Ratriyanto A, Indreswari RS. 2014. Effects of protein levels and supplementation of methyl group donor on nutrient digestibility and performance of broiler chickens in the tropics. Int J Poultry Sci. 13(10):575–581.
- Remus JC, Quarles CL. 2000. The effect of betaine on lesion scores and tensile strength of coccidia-challenged broilers. Poult Sci. 79(1):118.
- Ruttanavut J, Yamauchi K. 2010. Growth performance and histological alterations of intestinal villi in broilers fed dietary mixed minerals. Asian J Anim Sci. 4(3):96–106.
- Sun CY, Liu WC, Xiao M, Zhao ZH, An LL. 2019. Effects of graded levels of betaine supplementation on growth performance and intestinal morphology in indigenous young yellow feather broilers. Pak J Zool. 51(6):2323–2328.
- Sun H, Jiang WD, Wu P, Liu Y, Jiang J, Yang QH, Kuang SY, Tang L, Zhou XQ, Feng L. 2020. Betaine supplementations enhance the intestinal immunity of on-growing grass carp (Ctenopharyngodon idella): partly related to TOR and NF-kappa B signaling pathway. Aquaculture. 518:734846.
- Tsiouris V. 2016. Poultry management: a useful tool for the control of necrotic enteritis in poultry. Avian Pathol. 45(3):323–325.
- Vallabhapurapu S, Karin M. 2009. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 27(1):693–733.
- Wang HC, Li SS, Xu SY, Feng J. 2020. Betaine improves growth performance by increasing digestive enzymes activities, and enhancing intestinal structure of weaned piglets. Anim Feed Sci Technol. 267:114545.
- Wang Y, Wang Y, Wang B, Mei X, Jiang S, Li W. 2019. Protocatechuic acid improved growth performance, meat quality, and intestinal health of Chinese yellow-feathered broilers. Poult Sci. 98(8):3138–3149.
- Wu J, He C, Bu J, Luo Y, Yang S, Ye C, Yu S, He B, Yin Y, Yang X. 2020. Betaine attenuates LPS-induced downregulation of Occludin and Claudin-1 and restores intestinal barrier function. BMC Vet Res. 16(1):75.
- Xie WY, Pan NX, Zeng HR, Yan HC, Wang XQ, Gao CQ. 2020. Comparison of nonlinear models to describe the feather growth and development curve in yellow-feathered chickens. Animal. 14(5):1005–1013.
- Xu ZR, Yu DY. 2000. Effect of betaine on digestive function of weaned piglets. Chin J Vet Sci. 20:201–204.
- Yang JM, Zhou R, Zhang M, Tan HR, Yu JQ. 2018. betaine attenuates monocrotaline-induced pulmonary arterial hypertension in rats via inhibiting inflammatory response. Molecules. 23(6):1274.
- Zhang C, Wang C, Chen K, Zhao X, Geng Z. 2020. Effect of l-theanine on growth performance, intestinal development and health, and peptide and amino acid transporters expression of broilers. J Sci Food Agric. 100(4):1718–1725.
- Zhang L, Liu C, Li D, Zhao Y, Zhang X, Zeng X, Yang Z, Li S. 2013. Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88. Int J Biol Macromol. 54:270–275.
- Zhou P, Tan YQ, Zhang L, Zhou YM, Gao F, Zhou GH. 2014. Effects of dietary supplementation with the combination of zeolite and attapulgite on growth performance, nutrient digestibility, secretion of digestive enzymes and intestinal health in broiler chickens. Asian Australas J Anim Sci. 27(9):1311–1318.
- Zou X, Ji J, Qu H, Wang J, Shu DM, Wang Y, Liu TF, Li Y, Luo CL. 2019. Effects of sodium butyrate on intestinal health and gut microbiota composition during intestinal inflammation progression in broilers. Poult Sci. 98(10):4449–4456.
