Abstract
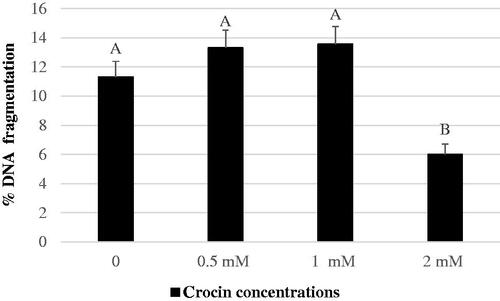
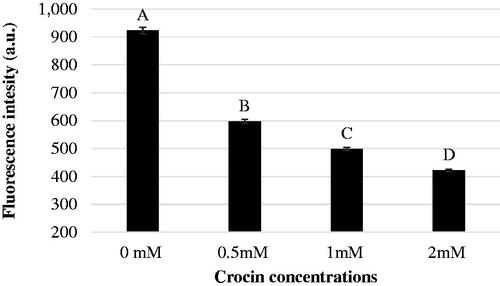
This work aimed to evaluate the effect of crocin on frozen-thawed sperm quality in buffalo. Spermatozoa were incubated in Tyrode’s Albumin Lactate Pyruvate medium supplemented with 0, 0.5, 1, and 2 mM crocin for 2 h. Sperm motility was evaluated by phase-contrast microscopy, viability and acrosome integrity by Trypan blue Giemsa staining, and membrane functional integrity by the hypoosmotic swelling test. The DNA fragmentation was evaluated by Tunel and ROS levels by spectrofluorometric analysis. The treatment with 2 mM of crocin increased (P < .05) sperm membrane functional integrity compared to the control group (59.1 ± 1.6 vs 53.3 ± 1.5) and reduced sperm DNA fragmentation, compared to the other groups (11.3 ± 1.1, 13.3 ± 1.2, 13.6 ± 1.2 and 6.0 ± 0.7, respectively in 0, 0.5, 1 and 2 mM crocin; P < .01). Finally, a dose-dependent decrease (P < .01) in superoxide anion production in the presence of crocin was observed, as indicated by Dihydroethidium values (922.6 ± 13.0, 596.8 ± 7.4, 498.9 ± 5.3 and 421.4 ± 5.0 a.u., respectively in 0, 0.5, 1 and 2 mM crocin; P < .01). The results of this study demonstrated a positive effect of 2 mM crocin on frozen-thawed buffalo sperm, as indicated by the improvement of sperm membrane integrity and the reduction of DNA fragmentation and ROS levels.
Crocin improves buffalo sperm quality.
Crocin improves sperm membrane integrity and reduces DNA fragmentation.
Crocin decreases oxidative stress in buffalo sperm.
Highlights
Keywords:
Introduction
Presently, the livestock productivity of buffalo deeply relies on genetic improvement carried out through the use of advanced reproductive biotechnologies. The accessibility of genetic material from bulls of high merit is critical to enhancing breeding selection. Semen cryopreservation allows the long-term storage and transport of germplasm and is critical for the diffusion of artificial insemination (AI) and in vitro embryo production (IVEP). However, the cryopreservation process impairs sperm cell function, potentially leading to a reduction in fertility (Bailey et al. Citation2000). Sperm sensitivity to cooling is largely attributed to the lipid composition of the sperm plasma membrane (Bailey et al. Citation2000). Differences in fatty acid composition and sterol levels of the cell membrane have been associated with the tolerance of sperm cells to cold shock and cryopreservation (Waterhouse et al. Citation2006). The lower cholesterol: phospholipids ratio (Rajoriya et al. Citation2014) and the high proportion of polyunsaturated fatty acids in the sperm membrane make buffalo spermatozoa largely sensitive to oxidative stress during the freezing-thawing process (Andrabi Citation2009). It is known that the manipulation of sperm cells can result in excessive production of reactive oxygen species (ROS), exposing the gametes to significant oxidative damages (Agarwal and Majzoub Citation2017). Oxidative stress promotes toxic lipid peroxides production generally associated with decreased sperm cells functions related to the integrity of plasma membrane, sperm-oocyte fusion, and DNA integrity (Agarwal and Majzoub Citation2017).
Antioxidants have long been utilised in the management of male subfertility as they can counterbalance the elevated levels of ROS inducing a high state of oxidative stress. More specifically, natural antioxidants have been included in the semen extender to effectively prevent cryopreservation-induced oxidative stress in several species, including buffalo (Longobardi et al. Citation2017a, Citation2017b; Del Prete et al. Citation2019). However, thawing and washing of cryopreserved sperm during in vitro fertilisation (IVF) results in the removal of seminal antioxidants (Agarwal et al. Citation2006) and increased ROS production, influencing sperm DNA fragmentation and fertilising ability (Simões et al. Citation2013). Therefore, antioxidants can be used to protect frozen-thawed sperm from thawing-induced oxidative stress, to improve in vitro fertilising ability (Chi et al. Citation2008; Gualtieri et al. Citation2014; Sapanidou et al. Citation2015).
Saffron (Crocus sativus L.) and its active constituents have a wide range of activities including antioxidant, anti-cancer, anticonvulsant, anti-inflammatory, and anti-atherosclerotic properties (Nam et al. Citation2010). Crocin, a water-soluble carotenoid pigment of saffron, can scavenge free radicals and protect membranes from oxidative stress (Singla and Giliyaru Citation2011). In the ram, crocin addition to the semen extender, prior cryopreservation decreased sperm DNA fragmentation, without improving other fertility parameters (Mata-Campuzano et al. Citation2015). Moreover, treating frozen-thawed sperm with crocin enhanced sperm quality parameters by preventing oxidative stress and DNA fragmentation in deer and cattle (Domínguez-Rebolledo et al. Citation2010; Sapanidou et al. Citation2015). It was also demonstrated that supplementation of the in vitro fertilisation medium by crocin significantly increased the blastocysts yields (Sapanidou et al. Citation2015). A thorough search of the relevant literature yielded no related article on the impact of crocin on cryopreserved buffalo sperm. Therefore, this work aimed to evaluate whether incubation of frozen-thawed sperm with crocin would improve the post-thaw sperm quality in buffalo, by preventing the uncontrolled overproduction of ROS related to thawing and centrifugation procedures. In particular, sperm motility, viability, acrosome and membrane integrity, as well as DNA fragmentation and intracellular ROS levels were evaluated.
Materials and methods
Unless otherwise stated, reagents were purchased from Sigma-Aldrich-Merck (Milano, Italy). The RNA-free DNase and RNAse A were obtained from Roche Diagnostics Corporation (Indianapolis, IN, USA). The experiments were performed on frozen-thawed sperm routinely produced in the Semen Collection Centre and did not require the approval of the ethics committee.
Experimental design
Four healthy Italian Mediterranean Buffalo (Bubalus bubalis) bulls (4–6 years) maintained at an authorised National Semen Collection Centre (Centro Tori Chiacchierini, Civitella D’Arna, Italy; authorisation numbers: PG0001C for Italy and IT014 for Europe) under uniform management conditions, routinely used for semen collection twice per week, were selected for the trial. Ejaculates (eight/bull; n = 32) were collected using an artificial vagina pre-warmed to 42 °C and only the ejaculates with ≥70% motility were utilised. The collection and freezing of semen were performed under commercial conditions as previously described (Longobardi et al. Citation2017a). Frozen-thawed buffalo sperm were selected by BoviPure gradients (according to the manufacturer’s instructions, Nidacon, Sweden) and incubated in a Tyrode’s albumin lactate pyruvate (TALP) medium supplemented with 0.1 mM hypotaurine, 0.2 mM penicillamine, and 0.01 mM heparin (IVF medium) for 2 h at 38.7 °C with 0 (control, without crocin), 0.5, 1 and 2 mM of crocin in a controlled gas atmosphere of 5.5% CO2 in humidified air. Immediately after thawing and after 2 h incubation sperm motility, viability, acrosome and membrane integrity, as well as DNA fragmentation were evaluated. Finally, ROS levels were estimated in all groups after 2 h incubation.
Assessment of sperm motility
Total sperm motility was assessed by an experienced laboratory technician under positive phase‐contrast microscopy (magnification: X 40; Nikon E200) on a clean and dry glass slide overlaid with a coverslip and maintained on a thermo-regulated stage at 37 °C. Based on the mass activity, semen motility was evaluated as previously described by Vale (Citation1997) in buffalo, with slight modifications, i.e. graded into scores from 1 to 10 (Longobardi, Citation2015).
Assessment of sperm viability and acrosome integrity by Trypan Blue/Giemsa technique
Sperm viability and acrosome integrity were assessed by Trypan Blue/Giemsa technique as reported by Boccia et al. (Citation2007) with slight modifications. Briefly, on a clean slide, 5 μL of semen and 5 μL 0.27% Trypan blue were spread, fixed in paraformaldehyde solution 2% in PBS for 2 min and stained with 7.5% Giemsa overnight. Sperm cells were microscopically evaluated (magnification: X 40; Nikon E200) and differentiated as acrosome intact live (AIL), acrosome intact dead (AID), acrosome-lost live (ALL), and acrosome-lost dead (ALD). Only spermatozoa displaying both head and tail viable (pink colored) were recorded as live, while those with either the head or the tail unviable (black-dark violet-colored) were recorded as dead. A total of 200 spermatozoa were analysed per slide (2 slides/sample).
Assessment of sperm membrane functional integrity
Sperm membrane functional integrity was assessed by the hypoosmotic swelling (HOS) test, as previously described (Correa and Zavos Citation1994). Fifty μL of semen were mixed with 500 μL of a hypo-osmotic solution (0.73 g sodium citrate and 1.35 g fructose in 100 mL of distilled water, 150 mOsm) and incubated at 37 °C for 45 min. A drop of diluted semen was placed on a clean slide and covered with a coverslip. A total of 200 spermatozoa per slide (2 slides/sample). Were counted in different fields (magnification: X 100) under phase-contrast microscope (Nikon E200) and the percentage of spermatozoa positive to HOS test (having coiled tails), i.e. those with intact membrane, was determined.
Evaluation of DNA fragmentation by Tunel assay
The sperm DNA fragmentation was determined by Tunel assay using a commercially available kit (In Situ Cell Death Detection Kit, fluorescein, Roche, Indianapolis, IN, USA) as previously described (Longobardi et al. Citation2020). A total of 200 spermatozoa per slide (2 slides/sample) were analysed in each sample by using a fluorescent microscope (Eclipse E-600; Nikon, Japan).
Evaluation of intracellular ROS levels
ROS levels were determined by spectrofluorometric analysis evaluating Dihydroethidium (DHE) fluorescence spectra post-incubation, as previously described (Longobardi et al. Citation2017b). Cell permeant reagent DHE is a fluorogenic dye that exhibits blue-fluorescence in the cytosol until oxidised, where it intercalates within the cell's DNA, staining its nucleus a bright fluorescent red. After 2 h incubation, the absorbance of 0 (control), 0.5, 1, and 2 mM crocin samples was monitored at 570 nm, using a plate reader (GloMax®-Multi Detection System-Promega, Milano). ROS levels were evaluated as arbitrary units of fluorescent signals. The fluorescence spectra were recorded in triplicate for five replicates.
Statistical analysis
Differences in sperm motility, viability, acrosome and membrane integrity, DNA fragmentation, and ROS production among groups were analysed by ANOVA, with the Tukey test used for post-hoc comparisons. Based on the skewness and kurtosis statistics, the variables were judged to be normally distributed (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp). A value of P < .05 was considered statistically significant.
Results
The analysis of sperm quality parameters at thawing was carried out to assess the quality of semen at the beginning of the trial. The parameters recorded for sperm motility, AIL, ALL, membrane integrity, as well as the DNA fragmentation, showed a good semen quality (on average 70 ± 0.2; 89.2 ± 1.0; 2.13 ± 1.0, 60.6 ± 1.8; 14.8 ± 1.7, respectively).
Concerning post-incubation groups, sperm motility, viability, acrosome, and functional membrane integrity are shown in Table . In particular, sperm motility increased (P < .01) with 1 mM crocin compared to the control group. However, no differences were found among groups in sperm viability and acrosome integrity, which remained very high after 2 h of incubation in all groups (Table ). Interestingly, the treatment of semen with the highest concentration (2 mM) of crocin increased (P < .05) the percentage of spermatozoa with intact membrane compared to the control group, while intermediate values were observed with 0.5 and 1 mM crocin (Table ).
Table 1. Percentages of sperm motility, acrosome intact live (AIL), acrosome-lost live (ALL), and functional membrane integrity (HOS+) of buffalo frozen-thawed sperm after 2 h incubation with different crocin concentrations.
In addition, the incubation of semen with 2 mM crocin resulted in a reduction (P < .01) of the percentage of spermatozoa with DNA fragmentation, compared to the control and the other groups (Figure ). Finally, a dose-dependent decrease (P < .01) in superoxide anion production in the presence of crocin was observed, as indicated by DHE values (Figure ).
Discussion
This work hypothesized that incubation of frozen-thawed buffalo sperm with the antioxidant crocin would improve sperm quality parameters by protecting sperm cells from thawing-induced oxidative damages. The rational was based on the high sensitivity of buffalo sperm to oxidative damages during freezing-thawing, due to the high concentration of long-chain polyunsaturated fatty acids in the sperm membrane (Rajoriya et al. Citation2014; Longobardi et al. Citation2017a). Recent findings have shown that crocin, under in vitro conditions, improved sperm quality parameters by preventing DNA fragmentation in bovine and deer sperm (Domínguez-Rebolledo et al. Citation2010; Sapanidou et al. Citation2015). To the best of our knowledge, this is the first report that investigated the effect of crocin on buffalo frozen-thawed sperm. The results of this study demonstrated that the exposure of frozen-thawed buffalo sperm to 2 mM crocin improves sperm quality, as indicated by increased membrane integrity, reduced DNA fragmentation and intracellular ROS levels, confirming the hypothesis.
To evaluate the effects of crocin on sperm quality a dose-response trial was carried out, showing that the most effective concentration was the higher tested (2 mM). However, an improvement of post-thawing sperm motility was recorded with 1 mM concentration, in agreement with a previous study in cattle (Sapanidou et al. Citation2015). Similarly, in other studies, the positive effect of carotenoids on sperm quality parameters, such as motility and viability, has been reported in humans, mice, and rabbits (Abdullaev Jafarova et al. Citation2002; Tsantarliotou et al. Citation2013; Mardani et al. Citation2014). In our study, however, no change in viability was observed under the different experimental conditions; this parameter remained particularly high after the incubation time, indicating the good quality of the starting semen material.
The most interesting results of this study concern sperm membrane integrity and DNA fragmentation rate. The assessment of structural and functional integrity of plasma membrane is useful for predicting sperm fertilising ability (Brito et al. Citation2003), as the sperm plasma membrane is the primary site of injury in cryopreserved spermatozoa (Hammerstedt et al. Citation1990). In the present study, sperm membrane integrity significantly improved compared to the control when sperm were incubated with 2 mM crocin. This result is in agreement with a recent study, in which crocin was effective to protect the integrity of the bovine sperm membrane, although at a lower concentration (Sapanidou et al. Citation2015). The differences in dose-response between the two studies are probably due to species-specific features.
In our work crocin, at the highest concentration tested, also significantly reduced DNA fragmentation compared to the control and the other groups. Similar results have been reported in the previously mentioned study on bovine spermatozoa, in which DNA fragmentation was reduced after 2 h incubation with 1 and 2 mM crocin (Sapanidou et al. Citation2015). A reduction in the DNA fragmentation index was also observed in goat and ram frozen-thawed sperm (Mata-Campuzano et al. Citation2015; Longobardi et al. Citation2020). These results are relevant because of the adverse effect of sperm DNA damage on the outcomes of in vitro fertilisation (Simões et al. Citation2013). Oxidative stress is the major cause of DNA fragmentation in spermatozoa (Twigg et al. Citation1998), as confirmed by the positive correlation existing between ROS production and DNA fragmentation (Simões et al. Citation2013). Furthermore, it is known that freezing and thawing procedures result in excessive ROS production, a major factor impairing sperm function (Senger Citation1980; Rastegarnia et al. Citation2013). Accordingly, in addition to increased membrane integrity and reduced DNA fragmentation, treatment of frozen-thawed buffalo sperm with 2 mM crocin was effective at counteracting the thawing-induced oxidative stress, as shown by decreased levels of superoxide anion. Likewise, a drop in ROS levels were also detected in bovine and goat sperm treated with crocin (Sapanidou et al. Citation2015; Longobardi et al. Citation2020). In contrast, in the red deer crocin failed to decrease ROS production in sperm, while it was effective on sperm cells exposed to exogenous oxidative stress (Domínguez-Rebolledo et al. Citation2010). It is worth pointing out that in the present study crocin decreased ROS production in a dose-dependent manner, while the effect on DNA fragmentation, as well as on membrane integrity, was only recorded at the highest concentration. This may suggest that a reduction of superoxide anion levels under a certain threshold is necessary to observe beneficial effects on chromatin and membrane integrity, the main targets of ROS attack (Agarwal et al. Citation2006; Simões et al. Citation2013).
It is known that antioxidants can be used to counteract the adverse impact of high ROS concentrations to improve semen parameters. In previous studies, it was demonstrated that the addition of natural antioxidants to the extender prior to freezing improved buffalo sperm quality (Longobardi et al. Citation2017a, Citation2017b). However, to enhance in vitro fertilising capacity of buffalo frozen sperm for Assisted Reproductive Technologies, a more valid strategy is to use antioxidants after thawing, to prevent excessive ROS production due to this procedure, as washing results in the removal of additives, including antioxidants, from the extender (Tsantarliotou and Sapanidou Citation2018). Crocin, a carotenoid constituent of saffron, is a forceful scavenger of free radicals, especially superoxide anions, and induces glutathione synthesis, protecting the cell from oxidative damages (Mokhber Maleki et al. Citation2016). Therefore, the beneficial effects of crocin on buffalo thawed sperm quality parameters, such as membrane integrity and DNA fragmentation, may be related to its ability to modulate the redox balance and counteract the overproduction of intracellular ROS.
Conclusions
In conclusion, the results of this study demonstrated a positive effect of crocin on frozen-thawed buffalo sperm. The dose-response trial showed that the optimal concentration of crocin is the highest tested, i.e. 2 mM, as indicated by the improvement of sperm membrane integrity and the reduction of DNA fragmentation rate. In addition, treatment with crocin resulted in a dose-dependent decrease of superoxide anion production. However, an increase in motility was recorded after incubating spermatozoa with 1 mM of crocin. These preliminary data suggest deepening the molecular mechanism of action of crocin and its potential dose-dependent effect on the procedure of in vitro fertilisation and subsequent embryonic development.
Disclosure statement
The authors declare no relevant financial or non-financial conflict of interest.
Data availability statement
All data relevant to the study are included in the article.
Additional information
Funding
References
- Abdullaev Jafarova F, Caballero-Ortega H, Riverón-Negrete L, Pereda-Miranda R, Rivera-Luna R, Manuel Hernández J, Pérez-López I, Espinosa-Aguirre JJ. 2002. In vitro evaluation of chemopreventive potential of saffron. Rev Invest Clin. 54(5):430–436.
- Agarwal A, Majzoub A. 2017. Laboratory tests for oxidative stress. Indian J Urol. 33(3):199–206.
- Agarwal A, Said TM, Bedaiwy MA, Banerjee J, Alvarez JG. 2006. Oxidative stress in an assisted reproductive techniques setting. Fertil Steril. 86(3):503–512.
- Andrabi SM. 2009. Factors affecting the quality of cryopreserved buffalo (Bubalus bubalis) bull spermatozoa. Reprod Domest Anim. 44(3):552–569.
- Bailey JL, Bilodeau JF, Cormier N. 2000. Semen cryopreservation in domestic animals: a damaging and capacitating phenomenon. J Androl. 21(1):1–7.
- Boccia L, Di Palo R, De Rosa A, Attanasio L, Mariotti E, Gasparrini B. 2007. Evaluation of buffalo semen by Trypan blue/Giemsa staining and related fertility in vitro. Ital J Anim Sci. 6 (sup2):739–742.
- Brito LF, Barth AD, Bilodeau-Goeseels S, Panich PL, Kastelic JP. 2003. Comparison of methods to evaluate the plasmalemma of bovine sperm and their relationship with in vitro fertilization rate. Theriogenology. 60(8):1539–1551.
- Chi HJ, Kim JH, Ryu CS, Lee JY, Park JS, Chung DY, Choi SY, Kim MH, Chun EK, Roh SI. 2008. Protective effect of antioxidant supplementation in sperm-preparation medium against oxidative stress in human spermatozoa. Hum Reprod. 23(5):1023–1028.
- Correa JR, Zavos PM. 1994. The Hypoosmotic swelling test: its employment as an assay to evaluate the functional integrity of the frozen-thawed bovine sperm membrane. Theriogenology. 42(2):351–360.
- Del Prete C, Stout T, Montagnaro S, Pagnini U, Uccello M, Florio P, Ciani F, Tafuri S, Palumbo V, Pasolini MP, et al. 2019. Combined addition of superoxide dismutase, catalase and glutathione peroxidase improves quality of cooled stored stallion semen. Anim Reprod Sci. 210:106195.
- Domínguez-Rebolledo AE, Fernández-Santos MR, Bisbal A, Ros-Santaella JL, Ramón M, Carmona M, Martínez-Pastor F, Garde J. 2010. Improving the effect of incubation and oxidative stress on thawed spermatozoa from red deer by using different antioxidant treatments. Reprod Fertil Dev. 22(5):856–870.
- Gualtieri R, Barbato V, Fiorentino I, Braun S, Rizos D, Longobardi S, Talevi R. 2014. Treatment with zinc, d-aspartate and co-enzyme Q10 protects bull sperm against damage and improved their ability to support embryo development. Theriogenology. 82(4):592–598.
- Hammerstedt RH, Graham JK, Nolan P. 1990. Cryopreservation of mammalian sperm: what we ask them to survive. J Androl. 11(1):73–88.
- Longobardi V. 2015. Innovative strategies to improve fertility of buffalo semen [PhD dissertation]. Napoli (IT): Federico II University of Naples.
- Longobardi V, Salzano A, Campanile G, Marrone R, Palumbo F, Vitiello M, Zullo G, Gasparrini B. 2017a. Carnitine supplementation decreases capacitation-like changes of frozen-thawed buffalo spermatozoa. Theriogenology. 88(88):236–243.
- Longobardi V, Zullo G, Cotticelli A, Salzano A, Albero G, Navas L, Rufrano D, Claps S, Neglia G. 2020. Crocin improves the quality of cryopreserved goat semen in different breeds. Animals. 10(6):1101.
- Longobardi V, Zullo G, Salzano A, De Canditiis C, Cammarano A, De Luise L, Puzio MV, Neglia G, Gasparrini B. 2017b. Resveratrol prevents capacitation-like changes and improves in vitro fertilizing capability of buffalo frozen-thawed sperm. Theriogenology. 88:1–8.
- Mardani M, Vaez A, Razavi S. 2014. Effect of saffron on rat sperm chromatin integrity Iran. J Reprod Med. 12:343–350.
- Mata-Campuzano M, Álvarez-Rodríguez M, Álvarez M, Tamayo-Canul J, Anel L, de Paz P, Martínez-Pastor F. 2015. Post-thawing quality and incubation resilience of cryopreserved ram spermatozoa are affected by antioxidant supplementation and choice of extender. Theriogenology. 83(4):520–528.
- Mokhber Maleki E, Eimani H, Bigdeli MR, Golkar Narenji A, Abedi R. 2016. Effects of crocin supplementation during in vitro maturation of mouse oocytes on glutathione synthesis and cytoplasmic maturation. Int J Fertil Steril. 10:53–61.
- Nam KN, Park Y-M, Jung H-J, Lee JY, Min BD, Park S-U, Jung W-S, Cho K-H, Park J-H, Kang I, et al. 2010. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol. 648(1–3):110–116.
- Rajoriya JS, Prasad JK, Ghosh SK, Ramteke S, Barik NC, Das GK, Pande M. 2014. Cholesterol loaded cyclodextrin increases freezability of buffalo bull (Bubalus bubalis) spermatozoa by increasing cholesterol to phospholipid ratio. Vet World. 7(9):702–706.
- Rastegarnia A, Shahverdi A, R, Topraggaleh T, Ebrahimi B, Shafipour V. 2013. Effect of different thawing rates on post-Thaw viability, kinematic parameters and chromatin structure of buffalo (Bubalus bubalis) spermatozoa. Cell J. 14(4):306–313.
- Sapanidou V, Taitzoglou I, Tsakmakidis Ι, Kourtzelis I, Fletouris D, Theodoridis A, Zervos I, Tsantarliotou M. 2015. Antioxidant effect of crocin on bovine sperm quality and in vitro fertilization. Theriogenology. 84(8):1273–1282.
- Senger PL. 1980. Handling frozen bovine semen – factors which influence viability and fertility . Theriogenology. 13(1):51–62.
- Simões R, Feitosa WB, Siqueira AF, Nichi M, Paula-Lopes FF, Marques MG, Peres MA, Barnabe VH, Visintin JA, Assumpção MEO. 2013. Influence of bovine sperm DNA fragmentation and oxidative stress on early embryo in vitro development outcome. Reproduction. 146(5):433–441.
- Singla RK, Giliyaru VB. 2011. Crocin: an overview indo glob. J Pharm Sci. 1(4):281–286.
- Tsantarliotou MP, Poutahidis T, Markala D, Kazakos G, Sapanidou V, Lavrentiadou S, Zervos II, Taitzoglou I, Sinakos Z. 2013. Crocetin administration ameliorates endotoxin-induced disseminated intravascular coagulation in rabbits. Blood Coagul Fibrinolysis. 24(3):305–310.
- Tsantarliotou MP, Sapanidou V. 2018. The importance of antioxidants in sperm quality and in vitro embryo production. J Vet Androl. 3(1):1–12.
- Twigg J, Fulton N, Gomez E, Irvine DS, Aitken RJ. 1998. Analysis of the impact of intracellular reactive oxygen species generation on the structural and functional integrity of human spermatozoa: lipid peroxidation, DNA fragmentation and effectiveness of antioxidants. Hum Reprod. 13(6):1429–1437.
- Vale WG. 1997. Sperm cryopreservation. Third course on biotechnology of reproduction in buffaloes, Caserta, Italy. In Bubalus Bubalis J Buffalo Sci Tech. 4:129–140.
- Waterhouse KE, Hofmo PO, Tverdal A, Miller RR. 2006. Within and between breed differences in freezing tolerance and plasma membrane fatty acid composition of boar sperm. Reproduction. 131(5):887–894.