Abstract
The effects of lycopene on liver damage in Aflatoxin B1 (AFB1)-challenged broilers were investigated. A total of 240 Arbour Acres Broilers in five groups were fed basal diet (control), basal diet with 100 μg/kg AFB1 (AFB1), AFB1 diet with 100, 200, and 400 mg/kg lycopene (AL1, AL2, and AL3), respectively. Broilers in AFB1 group had higher hepatic activities of cytochrome P450 1A1 (CYP1A1) and cytochrome P450 2A6 (CYP2A6) than the control group (P < .05). The hepatic concentrations of AFB1-8,9-epoxide-DNA adduct (AFBO-DNA), reactive oxygen species (ROS), malondialdehyde (MDA), 4-hydroxynonenal (4-HNE), protein carbonyl (PC) and 8-hydroxydeoxyguanosine (8-OHdG) were higher in AFB1 group than the control group (P < .05). In lycopene supplementation groups, the activities of CYP1A1 and CYP2A6, and the concentrations of AFBO-DNA, ROS, MDA, and 8-OHdG were lower than AFB1 group (P < .05). The 4-HNE and PC concentrations in AL2 and AL3 groups were lower than AFB1 group (P < .05). Compared to the control group, broilers in the AFB1 group had reduced glutathione (GSH) concentration and decreased activities of glutathione-S-transferase (GST), glutamine-cysteine ligase (GCL), total superoxide dismutase (T-SOD), catalase (CAT), and glutathione peroxidase (GPx) (P < .05). Whereas the GSH concentration in AL2 group, the GST activity in AL1 and AL3 groups, the GCL and CAT activities in AL1 group, and GPx activity in AL1, AL2, and AL3 groups were higher than AFB1 group (P < .05). Therefore, lycopene alleviated AFB1-induced liver damage possibly through inhibiting cytochrome P450 isozymes and improving detoxification and antioxidant systems in broilers.
Dietary lycopene alleviated liver damage induced by aflatoxin B1 in broilers.
Lycopene inhibited crucial cytochrome 450 isozymes involved in aflatoxin B1 bioactivation.
Lycopene increased detoxification and antioxidant ability in aflatoxin B1 exposed broilers.
HIGHLIGHTS
Introduction
Mycotoxins are unavoidable in feed industry, and the health of poultry are seriously threatened by the contamination of mycotoxins (Murugesan et al. Citation2015; Peng et al. Citation2018). Aflatoxins are derivatives of dihydrofuran coumarin, which are secondary metabolites of Aspergillus fungi, A. parasitic and A. flavus. Aflatoxin B1 (AFB1), which is a hepatotoxic chemical substance, is the most common and toxic among the four prevalent forms of aflatoxins (B1, B2, G1, G2) (Saini and Kaur Citation2012). Aflatoxins were categorised as Group I carcinogens by the International Agency for Research on Cancer (IARC), and agency forming part of the WHO. AFB1 is bioactivated into toxicant AFB1-8,9-epoxide (AFBO) by cytochrome P450 (CYP450) isozymes in the phase I metabolic reaction (Diaz et al. Citation2010). During bioactivation, the reactive oxygen species (ROS) as intermediates are generated and induce oxidative stress, which is also an important pathway for AFB1 to play toxic roles (Marin and Taranu Citation2012). In the phase II detoxification reaction, with the catalysis of glutathione-S-transferase (GST), AFBO conjugates with reduced glutathione (GSH) to form non-toxic AFBO-GSH adduct (Rawal et al. Citation2010).
Studies have shown that certain bioactive substances in dietary constituents may alleviate the toxicity of AFB1 through regulating phase I and phase II metabolic reactions (Lee et al. Citation2001; El-Agamy Citation2010; Zhang et al. Citation2016; Yilmaz et al. Citation2018). Lycopene, which is a natural bioactive substance with powerful antioxidant ability, belonging to carotenoids, mainly exists in vegetables, fruits and flowers, and possesses a variety of bioactive functions against cardiovascular diseases and cancers (Nobre et al. Citation2009; Gonzalvez et al. Citation2014; Liang et al. Citation2019). It has been identified as a class A nutrient by the United Nations Food and Agriculture Organisation and World Health Organisation (Liang et al. Citation2019). Lycopene improved blastocyst rate and quality of bovine oocytes (Residiwati et al. Citation2021), increased circulating IgG concentrations of ewes and their lambs (Fallah et al. Citation2021), enhanced porcine embryonic development by reducing oxidative stress and apoptosis (Kang et al. Citation2021), and increased oxidative stability of eggs (An et al. Citation2019). Furthermore, lycopene can alleviate the damages caused by various toxic substances in humans, mice, and rats, which might be related to the regulation of CYP450 isozymes and the activation of phase II detoxification and antioxidant systems (Boeira et al. Citation2014; Takeshima et al. Citation2014; Xia et al. Citation2016; Xu et al. Citation2017; Yilmaz et al. Citation2018).
In our previous studies, lycopene had beneficial effects on the growth performance and intestinal morphology and function of broilers (Sarker et al. Citation2021; Wan et al. Citation2021). We hypothesise that lycopene could alleviate AFB1 induced damages by regulating pivotal CYP450 isozymes and phase II detoxification and antioxidant systems in broilers. Therefore, the present study was conducted to further investigate the effects of dietary lycopene supplementation on activities of CYP450 isozymes and detoxification and antioxidant systems in AFB1 challenged broilers.
Materials and methods
Ethics statement
All animal experiments were approved by Ethical Committee and conducted in accordance with the Institutional Animal Care and Use Committee of Yangzhou University (Permit number: SYXK(Su)2016-0020, Yangzhou, China).
Animal, diet, and management
Completely randomised design was used in the experiment. A total of 240 Arbour Acres broilers in the five groups (five groups × six replicates per group × eight broilers per replicate) were fed basal diet (control), basal diet supplemented with 100 μg/kg AFB1 (AFB1), basal diet supplemented with 100 μg/kg AFB1 and 100 mg/kg lycopene (AL1), basal diet supplemented with 100 μg/kg AFB1 and 200 mg/kg lycopene (AL2), and basal diet supplemented with 100 μg/kg AFB1 and 400 mg/kg lycopene (AL3), respectively. The basal corn-soybean meal diet was formulated based on the recommendation of NRC (Citation1994) to meet the nutrient requirements of Arbour Acres broilers (Table ). Lycopene (purity ≥80%) and AFB1 (purity ≥98%) were obtained from Shanghai Yuanye Bio-Technology Co., Ltd (Shanghai, China). The supplemental dose of AFB1 and lycopene were based on the previous studies which reported that broiler chickens exposed to 100 μg/kg AFB1 in diet exhibited adverse effects (Sun et al. Citation2015; Zhang et al. Citation2016), and 200-400 mg/kg lycopene alleviated heat-stress induced adverse effects in broiler chickens (Sahin et al. Citation2016). Broilers were raised in environmentally controlled room with 32 °C–34 °C for the first 3 days, then gradually decreased to 22 ± 1 °C by 2 °C–3 °C per week and maintained until the end of the experiment (42 day of age). All broilers had free access to feed and water with a lighting cycle of 23 h light and 1 h dark. The data of growth performance showed that dietary lycopene supplementation to AFB1 contaminated diets increased average daily body weight gain and decreased feed/gain ratio compared to the AFB1 diet (Sarker et al. Citation2021).
Table 1. Composition and nutrient level of basal diet (as-fed basis)a.
Sample collection
At the end of the experiment, one bird per replicate with an averaged body weight of the replicate was selected after 12 h feed deprivation. Blood sample was aseptically obtained from the wing vein, then the serum was collected after centrifuged at 3 000 × g for 10 min at 4 °C, and stored at −20 °C for further analysis. After bleeding, the broilers were euthanized by cervical dislocation, the liver samples were excised and snap-frozen in liquid nitrogen, then stored at −70 °C for further analysis.
Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities
The activities of AST and ALT in serum were determined spectrophotometrically at wavelength of 510 nm using the commercial kits by Reitman-Frankel method (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The results were expressed as unit/liter (U/L).
Activities of hepatic microsomal CYP450 isozymes
According to the method of previous studies, the ultracentrifugation method was used to prepare hepatic microsomes, and the hepatic microsomal activities of CYP450 1A1 (CYP1A1), CYP450 1A2 (CYP1A2), CYP450 2A6 (CYP2A6), and CYP450 3A4 (CYP3A4) in broiler chickens were determined (Diaz et al. Citation2010). The bicinchoninic acid protein quantification method was used to determine the protein content of the aliquot (Bainor et al. Citation2011). The results were corrected for the protein concentrations in each sample and expressed as nanomole per milligram of protein per minute (nmol/mg protein/min).
Determination of hepatic AFBO-DNA adduct
Takara Universal Genomic DNA Extraction Kit was used to extract the liver genomic DNA according to the instruction of the kit (TaKaRa Biotechnology Co. Ltd., Dalian, China). The quality and quantity of the DNA was measured with a microspectrophotometer (NaroDrop 2000c, Thermo Scientific, Waltham, MA, USA). Genomic DNA was used to measure the AFBO-DNA adduct level with a competitive ELISA kit according to the manufacturer’s directions (Cell Biolabs, Inc., CA, USA). The results were expressed as nanogram AFBO-DNA/microgram total DNA (ng/μg).
Measurement of hepatic ROS concentration
As described by Zhang et al. (Citation2018), the hepatic ROS concentration was measured with a 2,7-dichlorofluorescein-diacetate (DCFH-DA) fluorescence probe. In brief, the freshly isolated hepatocytes were incubated with the DCFH-DA solution at 37 °C for 20 min. The excitation wavelength of 488 nm and emission wavelength of 525 nm were used to measure the fluorescence intensity of DCFH using a FACS Calibur flowcytometer (BD, Heidelberg, Germany). The results were expressed as the percentage of the control group.
Measurement of hepatic antioxidant status
The liver homogenate was prepared following the corresponding instructions of the kits listed below, and the protein concentration of liver homogenate was measured by bicinchoninic acid assay (Bainor et al. Citation2011). According to the directions of the corresponding detection kits of Nanjing Jiancheng Bioengineering Institute (Nanjing, China), the GSH concentration, the activities of GST, glutathione reductase (GR), glutamine-cysteine ligase (GCL), glutathione synthase (GS), total superoxide dismutase (T-SOD), catalase (CAT), and glutathione peroxidase (GPx) were measured by colorimetric method at wavelength of 420 nm, 412 nm, 340 nm, 660 nm, 340 nm, 550 nm, 405 nm, and 412 nm, respectively. The GSH concentration was expressed as nanogram per gram of protein (ng/g protein). The activities of GST, GR, GCL, GS, and GPx were expressed as units per gram of protein (U/g protein). The activities of T-SOD and CAT were expressed as units per milligram of protein (U/mg protein).
The concentrations of malondialdehyde (MDA), 4-hydroxynonenal (4-HNE), protein carbonyl (PC), and 8-hydroxydeoxyguanosine (8-OHdG) were determined by commercial ELISA kits of Shanghai YuBo Biotech Co., Ltd (Shanghai, China). The concentrations of MDA and PC were expressed as nanomoles per gram of protein (nmol/g protein). The concentration of 4-HNE was expressed as nanogram per milligram of protein (ng/mg protein). The concentration of 8-OHdG was expressed as nanogram per gram of protein (ng/g protein).
Statistical analysis
Statistical analysis was carried out in unit of replicate and performed by SPSS software package (version 22 for Windows, SPSS Inc, Chicago, USA). Data in the completely randomised design experiment were analysed by one-way ANOVA. Duncan’s multiple range test was used to evaluate the differences among groups, and P < .05 was considered significant. Data were shown as mean ± SEM.
Results
Serum AST and ALT activities
The effects of lycopene on serum AST and ALT activities of AFB1-exposed broiler chickens are presented in Figure . Broiler chickens exposed to AFB1 had higher activities of serum AST and ALT than that in the control group (P < .05). Lycopene treatment in AL1, AL2, and AL3 groups ameliorated the increase of AST and ALT activities in comparison to the AFB1 exposure (P < .05).
Figure 1. Effects of lycopene on serum aspartate aminotransferase (AST) (A) and alanine aminotransferase (ALT) (B) in AFB1-exposed broiler chickens. Data are represented as mean ± SEM. Different letters above bars are significantly different (P < .05). AFB1, aflatoxin B1; Control, basal diet; AFB1, basal diet with 100 μg/kg AFB1; AL1, basal diet with 100 μg/kg AFB1 and 100 mg/kg lycopene; AL2, basal diet with 100 μg/kg AFB1 and 200 mg/kg lycopene; AL3, basal diet with 100 μg/kg AFB1 and 400 mg/kg lycopene.
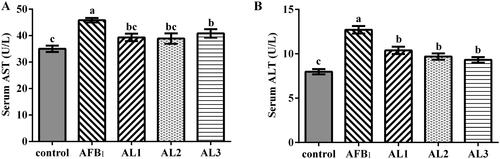
Activities of hepatic CYP450 isozymes
Figure shows the effects of lycopene on activities of hepatic CYP450 isozymes in AFB1-exposed broiler chickens. AFB1 exposure led to increased activities of CYP1A1 and CYP2A6 in hepatic microsomes of broiler chickens compared to the control group (P < .05), whereas the increased activities of CYP1A1 and CYP2A6 in the AFB1 group were reduced by 100–400 mg/kg lycopene supplementation (P < .05).
Figure 2. Effects of lycopene on hepatic cytochrome P450 isozymes activities in AFB1-exposed broiler chickens. Data are represented as mean ± SEM. Different letters above bars are significantly different (P < .05). AFB1, aflatoxin B1; Control, basal diet; AFB1, basal diet with 100 μg/kg AFB1; AL1, basal diet with 100 μg/kg AFB1 and 100 mg/kg lycopene; AL2, basal diet with 100 μg/kg AFB1 and 200 mg/kg lycopene; AL3, basal diet with 100 μg/kg AFB1 and 400 mg/kg lycopene. CYP1A1, cytochrome P450 1A1; CYP1A2, cytochrome P450 1A2; CYP2A6, cytochrome P450 2A6; CYP3A4, cytochrome P450 3A4.
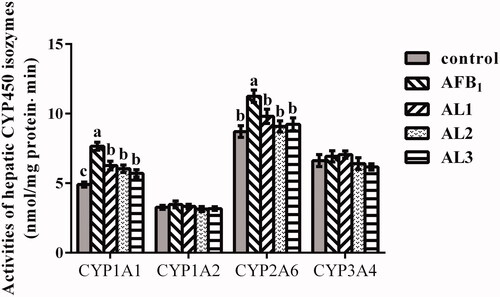
Hepatic AFBO-DNA adduct concentrations
As shown in Figure , the hepatic AFBO-DNA adduct concentration was strikingly affected by different dietary treatment. Broiler chickens exposed to AFB1 had increased AFBO-DNA adduct concentration compared to the control (P < .05). In the AL1, AL2, and AL3 groups, AFBO-DNA adduct concentration decreased compared to the AFB1 group (P < .05).
Figure 3. Effects of lycopene on hepatic AFBO-DNA adducts concentration in AFB1-exposed broiler chickens. Data are represented as mean ± SEM. Different letters above bars are significantly different (P < .05). AFB1, aflatoxin B1;AFBO, AFB1-8,9-epoxide. Control, basal diet; AFB1, basal diet with 100 μg/kg AFB1; AL1, basal diet with 100 μg/kg AFB1 and 100 mg/kg lycopene; AL2, basal diet with 100 μg/kg AFB1 and 200 mg/kg lycopene; AL3, basal diet with 100 μg/kg AFB1 and 400 mg/kg lycopene.
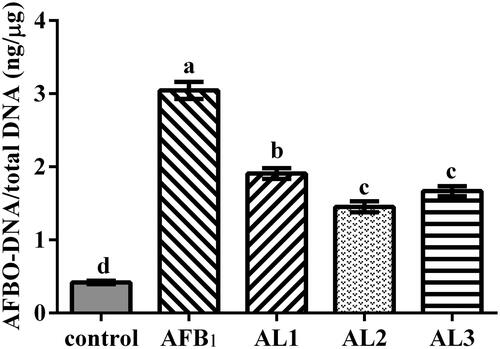
Hepatic ROS concentration
The hepatic ROS concentration was higher in broiler chickens in the AFB1 group than those in the control group (P < .05, Figure ). Dietary 100–400 mg/kg lycopene supplementation reduced the ROS concentration in comparison with the AFB1 group (P < .05).
Figure 4. Effects of lycopene on hepatic reactive oxygen species (ROS) concentration in AFB1-exposed broiler chickens. Data are represented as mean ± SEM. Different letters above bars are significantly different (P < .05). AFB1, aflatoxin B1; Control, basal diet; AFB1, basal diet with 100 μg/kg AFB1; AL1, basal diet with 100 μg/kg AFB1 and 100 mg/kg lycopene; AL2, basal diet with 100 μg/kg AFB1 and 200 mg/kg lycopene; AL3, basal diet with 100 μg/kg AFB1 and 400 mg/kg lycopene.
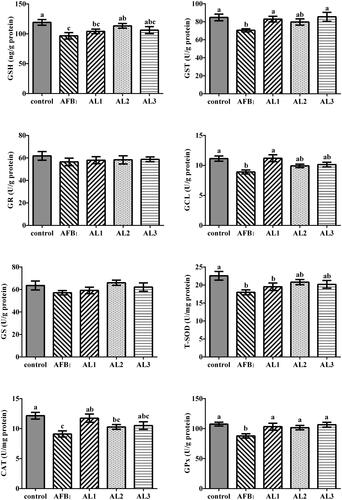
Hepatic antioxidant status
Compared to the control group, birds in the AFB1 group had reduced GSH concentration and decreased activities of GST, GCL, T-SOD, CAT, and GPx (P < .05, Figure ). The GSH concentration in AL2 group, the GST activity in AL1 and AL3 groups, the GCL and CAT activities in AL1 group, and GPx activity in AL1, AL2, and AL3 groups were higher than those in the AFB1 group (P < .05).
Figure 5. Effects of lycopene on hepatic antioxidant capacity in AFB1-exposed broiler chickens. Data are represented as mean ± SEM. Different letters above bars are significantly different (P < .05). AFB1, aflatoxin B1; Control, basal diet; AFB1, basal diet with 100 μg/kg AFB1; AL1, basal diet with 100 μg/kg AFB1 and 100 mg/kg lycopene; AL2, basal diet with 100 μg/kg AFB1 and 200 mg/kg lycopene; AL3, basal diet with 100 μg/kg AFB1 and 400 mg/kg lycopene. GSH, reduced glutathione; GST, glutathione-s-transferase; GR, glutathione reductase; GCL, glutamine-cysteine ligase; GS, glutathione synthase; T-SOD, total superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase.
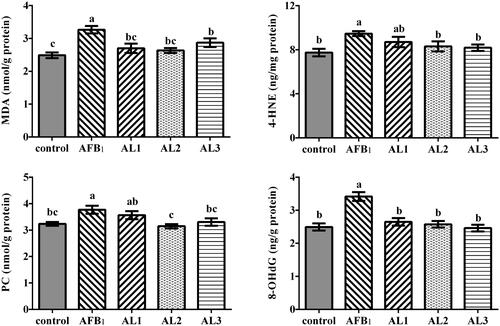
The concentrations of MDA, 4-HNE, PC, and 8-OHdG were significantly increased in AFB1 group compared to the control group (P < .05, Figure ). However, the MDA and 8-OHdG concentrations in AL1, AL2, and AL3 groups, and the 4-HNE and PC concentrations in AL2 and AL3 groups were lower than those in the AFB1 group (P < .05).
Figure 6. Effects of lycopene on hepatic oxidative damage products concentrations in AFB1-exposed broiler chickens. Data are represented as mean ± SEM. Different letters above bars are significantly different (P < .05). AFB1, aflatoxin B1; Control, basal diet; AFB1, basal diet with 100 μg/kg AFB1; AL1, basal diet with 100 μg/kg AFB1 and 100 mg/kg lycopene; AL2, basal diet with 100 μg/kg AFB1 and 200 mg/kg lycopene; AL3, basal diet with 100 μg/kg AFB1 and 400 mg/kg lycopene. MDA, malondialdehyde; 4-HNE, 4-hydroxynonenal; PC, protein carbonyl; 8-OHdG, 8-hydroxydeoxyguanosine.
Discussion
The adverse effects of AFB1 in poultry are generally recognised, and growth depression is accepted as a common phenomenon of AFB1 exposure (Murugesanet al. Citation2015; Magnoli et al. Citation2017). Agreed with previous reports, broiler chickens fed diet supplemented with 100 μg/kg AFB1 had lower growth performance than the control group in the current study. The applied dietary lycopene supplementation neutralised the detrimental effects of AFB1 on growth performance of broiler chickens in this study. The increased AST and ALT activities in serum of broiler chickens exposed to AFB1 compared to the control group provided evidences that liver damage occurred. As expected, broiler chickens exposed to AFB1 and simultaneously fed diets supplemented with lycopene mitigated the alterations of AFB1 induced serum AST and ALT activities. These results are consistent with prior studies, which indicated that lycopene could play protective effects against liver damage induced by toxic substances including AFB1, ochratoxin A, atrazine in rats and mice (Tang et al. Citation2007; Aydin et al. Citation2013; Xia et al. Citation2016).
The CYP450 isozymes are phase I metabolic enzymes which play a crucial part in drug metabolism and clearance of xenobiotics (Anzenbacher and Anzenbacherová Citation2001; Ioannides Citation2008; Zanger and Schwab Citation2013). AFB1 is primarily bioactivated in liver, CYP1A1 and CYP2A6 are crucial enzymes of the CYP450 isozymes that bioactivate AFB1 into AFBO in poultry hepatic microsomes (Klein et al. Citation2000; Diaz et al. Citation2010). The AFBO-DNA adducts are major toxic products due to AFB1 poisoning (Williams et al. Citation2011). The hepatic activities of CYP1A1 and CYP2A6, as well as hepatic AFBO-DNA adducts concentration were elevated by AFB1, and dietary lycopene addition prevented these changes in the present study. Similarly, dietary selenium and curcumin prevented AFB1 induced elevation of CYP450 isozymes (Sun et al. Citation2015;; Zhang et al. Citation2016). Thus, it is reasonable that the reduced AFBO-DNA adducts could be ascribed to the decreased AFBO generation in AFB1 bioactivation with the inhibition of CYP450 isozymes. Previous studies revealed that lycopene blocked phase I metabolic enzymes of AFB1 such as CYP1A2, CYP2A6 and CYP3A4 (Yilmaz et al. Citation2017), protected against atrazine induced hepatic CYP450 system disorder in mice (Xia et al. Citation2016), inhibited the activity of CYP2E1 in rats (Louisa et al. Citation2009), and reduced phase I metabolites in urine and AFBO-DNA adducts in liver of AFB1-exposed rats and mice (Tang et al. Citation2007; Xu et al. Citation2017). Given that lycopene is a regulator of CYP450 isozymes, it could be used as a potentially efficient nutritional and chemical preventive agent for hepatoxicity induced by toxic substances. Together with the abovementioned studies, it is convincible that the protective effects of lycopene against AFB1-exposed broiler chickens might through inhibiting the activities of crucial CYP450 isozymes, thereby reducing the production of AFBO.
The concentration of AFBO in liver not only depends on the generation but also on the elimination. In phase II detoxification reaction, AFBO conjugates with GSH to form non-toxic adduct with the catalysis of GST, and this is the main detoxification pathway (Rawal et al. Citation2010). Thus, GST plays a pivotal role in the detoxification of AFB1. Yates et al. (Citation2006) and Gao et al. (Citation2012) showed that triterpenoid analogues and phloretin had chemopreventive effects against AFB1 by increasing GST activity. Hence, the hepatic AFB1 detoxification effect could be enhanced by the elevation of GST activity. Furthermore, the GST catalysed detoxification reaction accompanied by GSH consumption. The synthesis of GSH is catalysed by GCL and GS (Wu et al. Citation2004; Forman et al. Citation2009). In the present study, broiler chickens exposed to AFB1 had reduced hepatic GSH concentration and decreased activities of GST and GCL, compared to that fed basal diet. Whereas the applied dietary lycopene supplementation prevented these changes to some extent. Similar to the present results, pre-treatment with lycopene in mycotoxins intoxicated mice performed hepatoprotective effect by inducing GST isoenzymes and GSH synthesis (Boeira et al. Citation2014; Xu et al. Citation2017). These evidenced the opinion that lycopene is an inducer of GST and can improve GSH concentration (Yilmaz et al. Citation2017). Therefore, the hepatoprotective effect of lycopene relies on its stimulation on GSH-related detoxification system.
Moreover, GSH is an important member of non-enzymatic antioxidants in redox system, which can counteract ROS thereby resulting in the formation of oxidised glutathione (GSSG) during oxidative stress, and GSH can be regenarated by the reduction of GSSG with the catalysis of GR (Wu et al. Citation2004; Forman et al. Citation2009). Mycotoxin-related reduction of GSH production can lead to oxidative stress (Guilford and Hope Citation2014). Additionally, it has been reviewed that AFB1 exposure can result in overproduction of ROS during bioactivation with the catalysis of CYP450 enzymes, thereby inducing oxidative stress (Marin and Taranu Citation2012). Indeed, the increased ROS concentration was observed in AFB1 challenged broilers in the present study. SOD, CAT and GPx are members of enzymatic antioxidants in redox system. MDA and 4-HNE concentrations are biomarkers of lipid peroxidation. The concentration of PC and 8-OHdG can be used to evaluate the oxidative damage of proteins and DNA, respectively. Marin and Taranu (Citation2012) reviewed that AFB1 was able to increase concentrations of oxidative damage products including MDA, 4-HNE, PC, and 8-OHdG in tissues. The decreased GSH concentration and activities of GST, SOD, CAT, and GPx, along with increased concentrations of MDA and 8-OHdG were observed in AFB1-exposed broilers in previous studies (Sun et al. Citation2015). ; Zhang et al. Citation2016; Similarly, in this study, the decreased GSH concentration and activities of different antioxidant enzymes, concurrently elevated ROS concentration, as well as increased concentrations of oxidative damage products in liver of AFB1-exposed broiler chickens, confirming the occurrence of oxidative stress. Whereas dietary lycopene supplementation exhibited mitigative effects. As a famous antioxidant, lycopene has excellent free radical scavenging ability. Previous studies exhibited that lycopene increased GSH concentration and improved activities of antioxidases in AFB1 and zearalenone challenged rats and mice (Boeira et al. Citation2015; Xu et al. Citation2017; Yilmaz et al. Citation2018), and increased antioxidant capacity in growing rabbits and heat-stressed broilers (Sahin et al. Citation2016; Casamassima et al. Citation2017). The oxidative stress induced by mycotoxins and anti-stress ability of plant-derived additives in animals have been recognised (El-Agamy Citation2010; Jiang et al. Citation2011, Citation2014; Sridhar et al. Citation2015; Zhang et al. Citation2016). Therefore, it is convincible that lycopene can effectively relieve the oxidative damage in broilers exposed to mycotoxins not only through improving enzymatic antioxidant system, but also through ameliorating non-enzymatic antioxidant system.
Conclusion
The results observed in our study indicated that lycopene protected broiler chickens from AFB1 induced liver damage by inhibiting crucial cytochrome 450 isozymes to reduce the bioactivation of AFB1, and improving the GSH-dependent detoxification system, as well as prompting the enzymatic and non-enzymatic antioxidant systems. Therefore, lycopene and lycopene-enriched materials could be taken into consideration as effective hepatoprotective and antioxidant additives in poultry.
Ethical approval
All animal experiments were approved by Ethical Committee and conducted in accordance with the Institutional Animal Care and Use Committee of Yangzhou University.
Disclosure statement
All authors report no conflicts of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [Z. Y. W.], upon reasonable request.
Additional information
Funding
References
- An BK, Won-Don C, Kang CW, Jienny L, Kyung-Woo L. 2019. Effects of dietary lycopene or tomato paste on laying performance and serum lipids in laying hens and on malondialdehyde content in egg yolk upon storage. Jpn Poult Sci. 56(1):52–57.
- Anzenbacher P, Anzenbacherová E. 2001. Cytochromes P450 and metabolism of xenobiotics. Cell Mol Life Sci. 58(5–6):737–747.
- Aydin S, Palabiyik ŞS, Erkekoglu P, Sahin G, Başaran N, Giray BK. 2013. The carotenoid lycopene protects rats against DNA damage induced by ochratoxin A. Toxicon. 73:96–103.
- Bainor A, Chang L, McQuade TJ, Webb B, Gestwicki JE. 2011. Bicinchoninic acid (BCA) assay in low volume. Anal Biochem. 410(2):310–312.
- Boeira SP, Funck VR, Borges Filho C, Del'Fabbro L, de Gomes MG, Donato F, Royes LFF, Oliveira MS, Jesse CR, Furian AF. 2015. Lycopene protects against acute zearalenone-induced oxidative, endocrine, inflammatory and reproductive damages in male mice. Chem Biol Interact. 230:50–57.
- Boeira SP, Filho CB, Del'Fabbro L, Roman SS, Royes LFF, Fighera MR, Jessé CR, Oliveira MS, Furian AF. 2014. Lycopene treatment prevents hematological, reproductive and histopathological damage induced by acute zearalenone administration in male Swiss mice. Exp Toxicol Pathol. 66(4):179–185.
- Casamassima D, Palazzo M, Vizzarri F, Costagliola C, Corino C, Di Costanzo A. 2017. Dietary effects of plant extracts, based on verbascoside, lycopene and horseradish on several blood variables and plasma oxidative status in growing rabbits. Livest Sci. 206:148–153.
- Diaz GJ, Murcia HW, Cepeda SM. 2010. Cytochrome P450 enzymes involved in the metabolism of aflatoxin B1 in chickens and quail. Poult Sci. 89(11):2461–2469.
- El-Agamy DS. 2010. Comparative effects of curcumin and resveratrol on aflatoxin B1-induced liver injury in rats. Arch Toxicol. 84(5):389–396.
- Fallah R, Kiani A, Khaldari M. 2021. Supplementing lycopene combined with corn improves circulating IgG concentration in pregnant ewes and their lambs. Trop Anim Health Prod. 53(3):360.
- Forman HJ, Zhang H, Rinna A. 2009. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 30(1–2):1–12.
- Gao SS, Chen XY, Zhu RZ, Choi BM, Kim SJ, Kim BR. 2012. Dual effects of phloretin on aflatoxin B1 metabolism: activation and detoxification of aflatoxin B1. Biofactors. 38(1):34–43.
- Gonzalvez AG, Martin D, Slowing K, Gonzalez Ureña A. 2014. Insights into the β-carotene distribution in carrot roots. Food Struct. 2(1–2):61–65.
- Guilford FT, Hope J. 2014. Deficient glutathione in the pathophysiology of mycotoxin-related illness. Toxins (Basel)). 6(2):608–623.
- Ioannides C. (Eds). 2008. Cytochromes P450: Role in the metabolism and toxicity of drugs and other xenobiotics. Guildford, UK: University of Surrey.
- Jiang SZ, Li Z, Wang GY, Yang ZB, Yang WR, Zhang GG, Wu YB. 2014. Effects of feed-borne fusarium mycotoxins with or without yeast cell wall absorbent on hematology, serum biochemistry and oxidative stress in broiler chickens. J Appl Poult Res. 23(2):165–173.
- Jiang SZ, Yang ZB, Yang WR, Gao J, Liu FX, Broomhead J, Chi F. 2011. Effects of purified zearalenone on growth performance, organ size, serum metabolites, and oxidative stress in postweaning gilts. J Anim Sci. 89(10):3008–3015.
- Kang H-G, Lee S, Jeong P-S, Kim MJ, Park S-H, Joo YE, Park SH, Song B-S, Kim S-U, Kim MK, et al. 2021. Lycopene improves in vitro development of porcine embryos by reducing oxidative stress and apoptosis. Antioxidants. 10(2):230.
- Klein PJ, Buckner R, Kelly J Jr, Coulombe RA. 2000. Biochemical basis for the extreme sensitivity of turkeys to aflatoxin B(1). Toxicol Appl Pharmacol. 165(1):45–52.
- Lee SE, Campbell BC, Molyneux RJ, Hasegawa S, Lee HS. 2001. Inhibitory effects of naturally occurring compounds on aflatoxin B(1) biotransformation. J Agric Food Chem. 49(11):5171–5177.
- Liang X, Ma C, Yan X, Liu X, Liu F. 2019. Advances in research on bioactivity, metabolism, stability and delivery systems of lycopene. Trends Food Sci. Tech. 93:185–196.
- Louisa M, Suyatna FD, Setiawati A, Jusma SWA. 2009. The effect of lycopene on the total cytochrome P450, CYP1A2 and CYP2E1. Med J Indones. 18:233–238.
- Magnoli AP, Rodriguez MC, González Pereyra ML, Poloni VL, Peralta MF, Nilson AJ, Miazzo RD, Bagnis G, Chiacchiera SM, Cavaglieri LR. 2017. Use of yeast (pichia kudriavzevii) as a novel feed additive to ameliorate the effects of aflatoxin B1 on broiler chicken performance. Mycotoxin Res. 33(4):273–283.
- Marin DE, Taranu I. 2012. Overview on aflatoxins and oxidative stress. Toxin Reviews. 31(3–4):32–43.
- Murugesan GR, Ledoux DR, Naehrer K, Berthiller F, Applegate TJ, Grenier B, Phillips TD, Schatzmayr G. 2015. Prevalence and effects of mycotoxins on poultry health and performance, and recent development in mycotoxin counteracting strategies. Poult Sci. 94(6):1298–1315.
- Nobre BP, Palavra AF, Pessoa FLP, Mendes RL. 2009. Supercritical CO2 extraction of trans-lycopene from Portuguese tomato industrial waste. Food Chem. 116(3):680–685.
- NRC. 1994. Nutrient Requirements of Poultry, 9th Rev. ed. Washington (DC): National Research Council, National Academy of Science.
- Peng W-X, Marchal JLM, van der Poel AFB. 2018. Strategies to prevent and reduce mycotoxins for compound feed manufacturing. Anim Feed Sci Technol. 237:129–153.
- Rawal S, Kim JE, Coulombe R. 2010. Aflatoxin B1 in poultry: toxicology, metabolism and prevention. Res Vet Sci. 89(3):325–331.
- Residiwati G, Azari-Dolatabad N, Tuska HSA, Sidi S, Van Damme P, Benedetti C, Montoro AF, Luceno NL, Budiono , Pavani KC. 2021. Effect of lycopene supplementation to bovine oocytes exposed to heat shock during in vitro maturation. Theriogenology. 173:48–55.
- Sahin K, Orhan C, Tuzcu M, Sahin N, Hayirli A, Bilgili S, Kucuk O. 2016. Lycopene activates antioxidant enzymes and nuclear transcription factor systems in heat-stressed broilers. Poult Sci. 95(5):1088–1095.
- Saini SS, Kaur A. 2012. Aflatoxin B1: toxicity, characteristics and analysis: mini review. Glo Adv Res J Chem Mat Sci. 1:63–70.
- Sarker MT, Wang ZY, Yang H, Wan X, Emmanuel A. 2021. Evaluation of the protective effect of lycopene on growth performance, intestinal morphology, and digestive enzyme activities of aflatoxinB1 challenged broilers. Anim Sci J. 92(1):e13540.
- Sridhar M, Suganthi RU, Thammiaha V. 2015. Effect of dietary resveratrol in ameliorating aflatoxin B1-induced changes in broiler birds. J Anim Physiol Anim Nutr. 99(6):1094–1104.
- Sun LH, Zhang NY, Zhu MK, Zhao L, Zhou JC, Qi DS. 2015. Prevention of aflatoxin B1 hepatoxicity by dietary selenium is associated with inhibition of cytochrome P450 isozymes and up-regulation of 6 selenoprotein genes in chick liver. J Nutr. 146(4):655–661.
- Takeshima M, Ono M, Higuchi T, Chen C, Hara T, Nakano S. 2014. Anti-proliferative and apoptosis-inducing activity of lycopene against three subtypes of human breast cancer cell lines. Cancer Sci. 105(3):252–257.
- Tang L, Guan H, Ding X, Wang JS. 2007. Modulation of aflatoxin toxicity and biomarkers by lycopene in F344 rats. Toxicol Appl Pharmacol. 219(1):10–17.
- Wan X, Yang Z, Ji H, Li N, Yang Z, Xu L, Yang H, Wang Z. 2021. Effects of lycopene on abdominal fat deposition, serum lipids levels and hepatic lipid metabolism-related enzymes in broiler chickens. Anim Biosci. 34(3):385–392.
- Williams JG, Deschl U, Williams GM. 2011. DNA damage in fetal liver cells of turkey and chicken eggs dosed with aflatoxin B1. Arch Toxicol. 85(9):1167–1172.
- Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. 2004. Glutathione metabolism and its implications for health. J Nutr. 134(3):489–492.
- Xia J, Lin J, Zhu SY, Du ZH, Guo JA, Han ZX, Li JL, Zhang Y. 2016. Lycopene protects against atrazine-induced hepatotoxicity through modifications of cytochrome P450 enzyme system in microsomes. Exp Toxicol Pathol. 68(4):223–231.
- Xu F, Yu K, Yu H, Wang P, Song M, Xiu C, Li Y. 2017. Lycopene relieves AFB1-induced liver injury through enhancing hepatic antioxidation and detoxification potential with Nrf2 activation. J Funct Foods. 39:215–224.
- Yates MS, Kwak M-K, Egner PA, Groopman JD, Bodreddigari S, Sutter TR, Baumgartner KJ, Roebuck BD, Liby KT, Yore MM, et al. 2006. Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole. Cancer Res. 66(4):2488–2494.
- Yilmaz S, Kaya E, Karaca A, Karatas O. 2018. Aflatoxin B1 induced renal and cardiac damage in rats: protective effect of lycopene. Res Vet Sci. 119:268–275.
- Yilmaz S, Kaya E, Kisacam MA. 2017. The Effect on oxidative stress of aflatoxin and protective effect of lycopene on aflatoxin damage. Chapter 4 in Aflatoxin-Control, Analysis, Detection and Health Risks. Published by InTech.
- Zanger UM, Schwab M. 2013. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 138(1):103–141.
- Zhang J, Bai K, He J, Niu Y, Lu Y, Zhang L, Wang T. 2018. Curcumin attenuates hepatic mitochondrial dysfunction through the maintenance of thiol pool, inhibition of mtDNA damage, and stimulation of the mitochondrial thioredoxin system in heat-stressed broilers. J Anim Sci. 96(3):867–879.
- Zhang N-Y, Qi M, Zhao L, Zhu M-K, Guo J, Liu J, Gu C-Q, Rajput S, Krumm C, Qi D-S, et al. 2016. Curcumin prevents aflatoxin B1 hepatoxicity by inhibition of cytochrome P450 isozymes in chick liver. Toxins. 8(11):327–336.
