Abstract
In beef cows, cycle is expected to resume within 30–35 days postpartum. Uterine diseases may delay these processes, extending the partum to conception to 50 days. Biomarkers for the diagnosis of Purulent vaginal discharge (PVD) in beef cows remain undefined. Creatine kinase (CK) has been investigated in dairy cows as a marker for clinical endometritis but not in beef cows. Mucus score and blood sampling were performed at 30 ± 5 in 264 non-pregnant Piedmontese beef cows and 28 of them were diagnosed with PVD. Cows that showed no successful conception after more than three AI with no apparent clinical disease were defined as repeat breeders (N = 33). Kruskal-Wallis test was used to detect difference in CK between cows with PVD and heathy ones and RB (p = <.001 and p = .048 respectively). No difference was found between healthy cows and RB (p > .05), cows PVD showed lower reproductive performances (PC and n°/IA) than healthy ones. Parity and farm didn’t show differences between healthy and PVD cows. ROC curve was created to define a CK cut-off value for PVD detection (241 U/L, Sp 69%, Se 92%, AUC 0.81, Younden Index (J) 0.61) and to determine CK accuracy in predicting infertility at 120- and 150-days postpartum (Sp 77%, Se 42%, AUC 0.57, J 0.19 and Sp 82%, Se 34%, AUC 0.59, J 0.16 respectively). This study underlines the potential of CK as a marker of PVD in beef cows.
Creatine kinase seems to be a useful on field marker of PVD in beef cows
Creatine kinase cut-off for PVD is 241 U/L
Creatine kinase has low accuracy in prevision of infertility
HIGHLIGHTS
Introduction
Beef cattle breeding is less standardised than dairy cattle because of the large number of different breeds and crossbreed and farming systems, ranging from intensive to extensive (Diskin and Kenny Citation2016). Moreover, the characteristics of some breeds are little investigated, thus low reproductive performances are often caused by failure of information about nutritional requirements, breeding, and farming management. Piedmontese beef cow is a high-quality double-muscled breed, due to a mutation of the myostatin gene (Kleiser and Fürll Citation1998) causing muscular hypertrophy. Piedmontese cows, as all double muscle breeds, are affected by a high incidence of dystocia and subsequent lower fertility and develop reproductive inflammatory conditions (Zaborski et al. Citation2009). In general, a complete uterine involution requires about 30 days postpartum (dpp) and a total resumed oestrus cycle is expected to happen within 30–35 dpp in beef cows depending on breeds, nutritional status and on general managerial conditions (Diskin and Kenny Citation2016), but uterine conditions such as purulent vaginal discharge (PVD) can delay this process, causing economic damage to the farm (Williams et al. Citation2005; Bel and Roberts Citation2007; Dubuc et al. Citation2011). PVD is an inflammatory condition of the uterus associated with bacterial infection with purulent or muco-purulent uterine discharge with no systemic signs from 21 days after calving (Dubuc et al. Citation2010a, Ernstberger et al. Citation2019). However, not all the cows affected by uterine contamination postpartum will develop uterine disease (LeBlanc Citation2014), in fact the presence of PVD is a result of endometritis, cervicitis/vaginitis or the combination of both (Dubuc et al. Citation2011; Deguillaume et al. Citation2012) and the detrimental effects of endometritis and cervicitis/vaginitis on reproductive performances are additive (Sheldon et al. Citation2006). PVD incidence ranges from 15% to 60% in cows at 4–6 weeks postpartum due to differences in time of diagnosis, disease categorisation, and method used for the diagnosis and it has severe effects on fertility in both dairy and beef cows (LeBlanc et al. Citation2002; Ricci et al. Citation2017; Ryan et al. Citation2020). In general, cows affected by PVD need about 30 days more to become pregnant than unaffected cows (Dubuc et al. Citation2010b, Citation2011; Ricci et al. Citation2017). Early and non-invasive diagnosis of PVD is a key point to reduce partum to conception days (PC), in order to decrease the number of inseminations per pregnancy and improve reproductive performances (Dubuc et al. Citation2010a, Citation2010b). Assessment of PVD is normally performed through vaginoscopy, manual examination of the vagina, and Metricheck (LeBlanc Citation2008), whereas transrectal palpation of the uterus has lower predictive value for reproductive performances (Biswal et al. Citation2014; Ernstberger et al. Citation2019). Animals experiencing poor human-animal interaction, as it happens for Piedmontese cows, can show reactive behaviour and poor adaptation to handling and restrain, experiencing high levels of stress (Ceballos et al. Citation2018). The exam of the vaginal mucus requires the cleaning of the external genitalia and the manual collection of the mucus from the vaginal lumen. This process prolongs the clinical examination and could cause stress to the animals, making the restrain harder (LeBlanc Citation2014; Adnane et al. Citation2017). Therefore, blood sample could be an alternative diagnostic tool to evaluate uterine disease in cows.
Markers such as scute phase proteins (APPs) have been considered as indicators for general acute response, such as inflammation, tissue damage, and infection (Baumann and Gauldie Citation1994; Petersen et al. Citation2004) and stress (Hicks et al. Citation1998; Arthington et al. Citation2003; Hickey et al. Citation2003). Among APPs, haptoglobin has been suggested to serve as an indicator of PVD (Dubuc et al. 2010; Yasui et al. Citation2014), especially in the first two weeks postpartum (Pascottini and LeBlanc Citation2020). However, the use APPs as diagnostic biomarker is still debated for metritis and endometritis (Humblet et al. Citation2006; Azawi et al. Citation2008; Chang et al., Citation2010).
Creatine kinase (CK) serum concentrations have been investigated as a marker for PVD, showing different values in healthy and diseased cows (Sattler and Fürll Citation2004; McDougall et al. Citation2007). Creatine kinase is an intracellular cytosolic enzyme that catalyses the reaction of creatine and adenosine triphosphate (ATP) to phosphocreatine and adenosine diphosphate (ADP) (Aujla and Patel Citation2020). It is a dimeric molecule composed of two subunits (M and B) and combinations of these subunits form the isoenzymes CK–MM, CK–MB, and CK–BB. CK is abundant in tissues with elevated energy transfer such as skeletal muscle, myocardium, and brain. In other visceral tissues (Cabaniss Citation1990), noticeable CK concentrations can be found in the uterine tissue and in every inner organ (Sattler and Fürll Citation2004). The serum of healthy cows contains almost entirely CK-MM, whereas inner organs contain mostly CK-BB. Mechanical and metabolic stress of the uterine tissue is known to cause elevated CK activities before and after normal parturition in cows (Abramov et al. Citation1996). Furthermore, serum concentrations of CK three days after parturition are lower in healthy Holstein cows (median of 121 U/L) than in cows with retained placenta (median 175 U/L), dystocia (median 310 U/L), milk fever (median of 385 U/L) (Kleiser and Fürll Citation1998), and abomasal displacement (Sattler and Fürll Citation2004). As for PVD, CK has been assessed in dairy cows (Sattler and Fürll Citation2004) and in Iraqi buffalo cows (Azawi et al. Citation2008) between 3 to 6 weeks pp. Results showed that animals with PVD had higher CK activity than healthy ones. To the best of our knowledge, CK has never been investigated as a diagnostic tool for PVD in beef cows.
The main objective of the present study was to evaluate the accuracy of CK serum concentrations in detecting PVD and in predicting infertility at 120 and 150-days postpartum. Moreover, we aimed to investigate the CK serum concentrations in repeat RB, that require three or more artificial inseminations (AI) without conception in the absence of clinical signs, representing a major reproductive issue in cows.
Materials and methods
The present study obtained the approval of the Ethical Committee of the Dipartimento di Scienze Veterinarie of the Università di Torino. All the included procedures did not interfere with the clinical management of the included animals and were performed in compliance with EU Directive 2010/63/CE. Treatment was always provided according to the clinical evaluation of the animals. Proper informed consent was obtained by the owners of the farms.
The present study was carried out in two farms of similar size (200 and 220 animals) with free stall barns and delivery parlour. All animals were feed with ad libitum feed (hay, bent grass, and corn flour and soya) enriched with vitamins (A and E) and mineral supplementation (Ca, P and Mg). All animals were vaccinated for bovine viral diarrhoea (BVD) and infectious bovine rhinotracheitis (IBR). Both farms were officially free from tuberculosis, brucellosis and pneumonia.
A group of 264 non-pregnant Piedmontese cows was used to assess CK performances as a diagnostic tool for PVD. All cows underwent a first clinical examination at 30 ± 5 pp., including an investigation for PVD presence, in order to detect postpartum disease. Animals that presented other conditions such as lameness, pneumonia, and trauma were excluded from the study.
PVD was analysed by the gloved hand technique as described by Williams et al. (Citation2005), using a 4-point classification system: 0 = no or clear mucus, 1 = mucus containing few flecks, 2 = discharge containing less than 50% pus, 3 = discharge containing more than 50% pus. Cows with a score of 0 or 1 were grouped as HEALTHY. Cows were considered diseased when scores where equal or higher than 2 (cut-off score = 2) (Williams et al. Citation2005) and they were grouped as ‘PVD’ and treated with one intrauterine infusion of 500 mg cephapirin benzathine (RCL) (Metricure, MSD Animal Health, Roma, ITALY) as proposed by Tison et al. Citation2017, and rechecked 7 days later. All cows underwent a pregnancy check at 30 ± 5 days after AI by ultrasound examination (Ibex® EVO II®, E.I. Medical Imaging, Loveland, CO). Fertility data (PC and number of AI) were recorded retrospectively. Cows that showed more than three subsequent AI with regular cycles with no apparent clinical reproductive disease that did not show successful conception were defined as repeat breeder cows (RB).
Blood samples were collected at 30 ± 5 days pp. by venipuncture from the coccygeal vein using an 8 mL evacuated serum collection tube and a 20 G needle (Vacutainer® Venoject, Terumo, Leueven, Belgium). Blood samples were immediately refrigerated and transported to the laboratory within 4 hours. Blood was centrifuged at 2000 rpm for 10 min and the serum was separate and stored at −20 °C in 1 mL SafeLock tubes (Eppendorf®, Hamburg, Germany). Creatine kinase was measured with a clinical chemistry analyser KUADRO® BPC (Biosed s.r.l, Rimini, Italy) that uses Creatine Kinase immunologic kinetic UV-test (MTD Diagnostics, Caserta, Italy), in accordance with International Federation of Clinical Chemistry (IFCC) guidelines.
Statistical analysis
Individual animal data were manually collected from the computerised herd systems and recorded on Microsoft Excel (Microsoft Corp., Redmond, WA) work file. Statistical analyses were performed using R statistical software (ver. 2.15.2, R Core Team, Vienna, Austria). p values ≤ .05 were considered significant, and trends were considered at p values between .06 and .08.
Sample size calculation was performed (for two independent means) based on limited information available in the literature (Azawi et al. Citation2008, Sattler and Fürll Citation2004). R software was used (Package ‘pwr’) and means ± DS, alpha of 0.05 and Power of 0.8 were used for the calculation.
Descriptive statistical analysis was performed to calculate the CK among all three experimental groups (HEALTHY, PVD, and RB). CK serum concentration was analysed using Kruskal-Wallis test considering the three animal groups (HEALTHY, RB, and PVD).
Furthermore, Kruskal-Wallis test was used to evaluate reproductive performances such as PC and number of AI per pregnancy among groups (HEALTHY, RB, and PVD). Bonferroni pot-hoc test was used for pairwise comparisons.
Receiver operating characteristic (ROC) curves (package pROC; Robin et al., Citation2011) was created and areas under the curve (AUC, package cvAUC) and Youden Index (J), calculated as (Se(c) + Sp (c) −1), were calculated to set the optimal serum CK concentration cut-off point to score PVD at 30 ± 5 days pp and to assess infertility in terms of PC at 120 and 150 days and number of AI.
Results and discussion
The aims of the present study were to investigate the serum CK concentrations as a marker for PVD during the postpartum and to assess differences in CK serum concentrations in RB cows.
From descriptive statistic 236 (89.4%, 236/264) cows were diagnosed as negative for PVD at the first clinical examination. However, 33 animals (13.9%, 33/236) were subsequently classified as RB when data were analysed retrospectively (RB group). Therefore, only 203 (76.9%, 203/264) animals were considered as healthy. Finally, twenty-eight (10.6%, 28/264) cows were diagnosed with PVD.
It is noticeable that, although Piedmontese cow is a double muscle breed and CK is abundant in muscular tissue, basal serum CK concentrations did not differ from the values reported in literature for dairy cows and Iraqui buffaloes (Sattler and Fürll Citation2004; Azawi et al. Citation2008). were higher in PVD positive Piedmontese beef cows than in Furthermore, in dairy cows the possible interference of the postpartum period diseases and the influence of the oestrus, could be associated with higher mean CK serum concentrations (Sattler and Fürll Citation2004; Crane et al. Citation2016). In this study, cows diagnosed with PVD showed an increase in serum CK concentrations when compared to HEALTHY ones (p < .001, Table ). Furthermore, some of the animals that were negative to PVD were subsequently classified as repeat breeder cows (RB) because of the higher number of required AI. According to Salasel et al. (Citation2010), incidence of RB ranges from 10% to 24% and many risk factors for repeat breeding have been described including parity, peri-parturient disease, uterine diseases, season, herd size, milk yield, and poor fertility (Perez-Marin et al. Citation2012). Furthermore, conditions such as subclinical endometritis (SCE), could increase the incidence of repeat breeder syndrome, being 52.7% of RB cows positive to SCE (Salasel et al. Citation2010; Ricci et al. Citation2015). In this study, intrauterine cytology has not been performed to investigate the presence of SCE in RB cows. Although, no data about CK values for SCE are available in the literature and because all RB cows in this study showed that serum CK concentration that did not differ from those of healthy cows, (Figure ) then we can speculate that SCE does not influence the CK concentration in blood (p > .05). In this study differences for PC and AI were analysed among all groups (HEALTHY, RB, and PVD) with significant differences mainly in PVD cows (85 ± 35, 191 ± 65, 144 ± 27, p < .001, Table ). Parity and farms effects didn’t’ show any difference (p > .05)
Figure 1. Creatine kinase (CK) concentration (mean ± SD, U/L) in healthy, RB and PVD cows. Healthy: not diseased cows; RB (repeat breeder cows): cows without clinical uterine disease with >3 AI after parturition; PVD (purulent vaginal discharge): cows positive for PVD using a 4-point classification system: 0 = no or clear mucus, 1 = mucus containing few flecks, 2 = discharge containing less than 50% pus, 3 = discharge containing more than 50% pus.
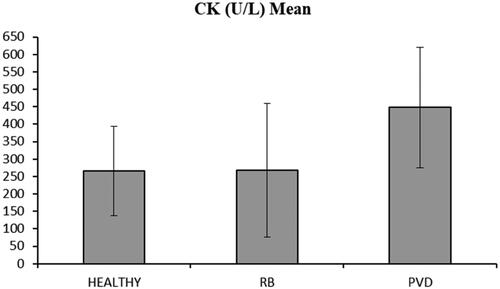
Table 1. Serum Creatine kinase (CK) concentration, for healthy (HEALTHY), repeat breeder (RB), and diseased (PVD) cows.
Table 2. Partum-to-conception interval (PC), and number of artificial insemination per pregnancy (n AI/preg) for healthy (HEALTHY), repeat breeder (RB), and diseased (PVD) cows.
It is known that cows affected by PVD have a delay in conception compared to healthy ones (Ricci et al. Citation2015). Specifically, PVD has been indicated as detrimental on the reproductive performances of dairy cows and as previously mentioned, its incidence ranges from 15% to 60% at 4–6 weeks postpartum in dairy cows depending on time of diagnosis, housing method, and it has severe effects on fertility in both dairy and beef cows (Deguillaume et al. Citation2012; Ricci et al. Citation2017; Ryan et al. Citation2020). It is commonly known that beef cows are not affected by remarkable metabolic imbalance and immunosuppression during the first and late postpartum, therefore, beef cows are expected to show a significantly lower incidence of PVD than dairy cows. In the present study 11% (28/264) of cows showed PVD, which is slightly lower than the one reported for dairy cows (10% to 35%) (Runciman et al. Citation2008; Deguillaume et al. Citation2012; de Boer et al. Citation2015). Nevertheless, no precise data about clinical uterine disease in beef cows is available in the literature.
PVD is commonly associated to current bacterial uterine infection, and it is a more practical routine cow-side method than cytological investigation (LeBlanc Citation2014).
Adnane et al. (Citation2017) analysed cervico-vaginal mucus (CVM) as biomarker for clinical endometritis (CE), by performing cytology and assessing total protein and inflammatory biomarkers on CVM. Although, the collection trough uterine washings by lavage expose to the risk of an unknown dilution factor. Nevertheless, Adnane et al. Citation2017 measured high levels of cytokines and other inflammatory biomarkers are successfully measured in CVM, suggesting that CVM may provide a more reliable sample for measuring inflammatory markers specific for the uterus. Both blood CK concentration measurement and CVM assessment require the collection of a sample on field and to have a laboratory to process them. Moreover, we think that using a simple blood marker with a specific cut-off is more feasible and easier on field than a more precise but more complex CVM assessment. Also, a blood sample require less material and the processing is cheaper than the analysis of CVM by cytology, total protein, and immunological pattern. Various acute phase proteins and blood metabolites have already been investigated in dairy and beef cows as inflammatory and stress response markers (Yasui et al. Citation2014; Pascottini and LeBlanc Citation2020). In accordance with other authors (Sattler and Fürll Citation2004; Azawi et al. Citation2008), in our study serum CK concentrations increase more in cows with PVD than in healthy and repeat breeding cows.
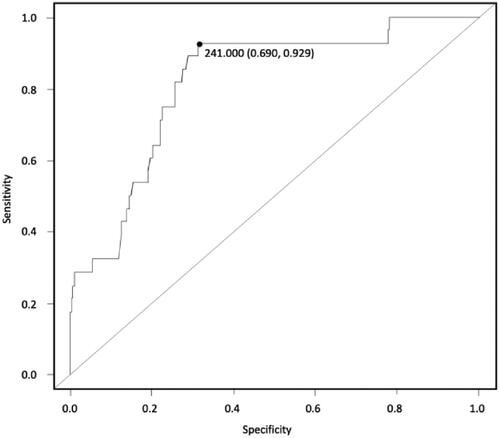
As already mentioned, no data about serum CK concentrations in beef cows are available in the literature, therefore a ROC curve was created with the aim of defining a cut-off value for the diagnosis of PVD in postpartum. As showed in Figure , the ROC curve for a precise diagnosis of PVD indicates a cut-off of 241 U/L for CK to predict PVD, showing good accuracy (Se 92%, Sp 69%, AUC 0.81, J 0.61%). The sensitivity of a test (true positive rate) is defined as the proportion of individuals with the disease who will have a positive result. Therefore, a highly sensitive test can be useful for ruling out a disease if an individual has a negative result (Petrie and Watson Citation2013). A highly specific test can be useful for ruling in patients who have a certain disease.
Figure 2. Creatine kinase (CK) blood concentration cut off for for PVD prediction. ROC curve (cut off indicates a cut-off of 241 U/L Se 92%, Sp 69%, AUC 0.81, J 61%).
We have also found that serum CK at 30 ± 5 days postpartum is not a good predictor for infertility at 120 and 150-days postpartum (Table ). The reason might be that infertility is not always strictly associated with inflammatory conditions or tissue damage (Weber et al. Citation2019; Moorey and Biase Citation2020).
Table 3. Receiver Operating Characteristics (ROC) curve results for serum Creatine kinase (CK) concentrations for detection of PVD and for prediction of infertility at 120- and 150-days postpartum.
In conclusion, the results of this study underline the potential of CK as a cow-side marker for uterine disease in beef breeds, with the final goal to use serum CK as a good and fast method for the diagnosis of PVD mainly in conditions were manual on-field techniques are not easy to perform. Although at least 24 hours are needed for the results to be available due to the lack of portable readers, this could serve as a screening to determine which animals need more thorough investigations for PVD. Future studies should focus on investigating CK to assess its accuracy as a predictor of PVD at different postpartum times. Furthermore, the association between serum CK and SCE should be specifically investigated, performing cytology or CVM. Finally, a future goal could be the development of a quick tool for the assessment of blood CK on field, leading to a preventive and not invasive on-field diagnostic method, which could be implemented in the health check routine of postpartum cows.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All relevant data are within the manuscript and its Supporting Information files.
References
- Abramov Y, Abramov D, Abrahamov A, Durst R, Schenker J. 1996. Elevation of serum creatine phosphokinase and its MB isoenzyme during normal labor and early puerperium. Acta Obstet Gynecol Scand. 75(3):255–260.
- Adnane M, Chapwanya A, Kaidi R, Meade KG, O'Farrelly C. 2017. Profiling inflammatory biomarkers in cervico-vaginal mucus (CVM) postpartum: potential early indicators of bovine clinical endometritis? Theriogenology. 103:117–122.
- Arthington JD, Eicher SD, Kunkle WE, Martin FG. 2003. Effect of transportation and commingling on the acute-phase protein response, growth, and feed intake of newly weaned beef calves. J Anim Sci. 81(5):1120–1125.
- Aujla RS, Patel R. 2020. Creatine phosphokinase. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. [accessed 2020 April 26]. https://www.ncbi.nlm.nih.gov/books/NBK546624/.
- Azawi OI, Omran SN, Hadad JJ. 2008. A study of endometritis causing repeat breeding of cycling iraqi buffalo cows. Reprod Dom Anim. 43(6):735–743.
- Baumann H, Gauldie J. 1994. The acute phase response. Immunol Today. 15(2):74–80.
- Bel MJ, Roberts DJ. 2007. The impact of uterine infection on a dairy cow’s performance. Theriogenology. 68(7):1074–1079.
- Biswal SS, Das S, Balasubramanian S, Mohanty DN, Sethy K, Dasgupta M. 2014. Serum amyloid A and haptoglobin levels in crossbred cows with endometritis following different therapy. Vet World. 7(12):1066–1070.
- Cabaniss CD. 1990. Creatine kinase. In: Walker HK, Hall WD, Hurst JW, editors. Clinical methods: the history, physical, and laboratory examinations. 3rd ed. Boston (MA): Butterworths.
- Ceballos MC, Sant’Anna AC, Boivin X, Costa FDO, Carvalhal MVDL, Paranhos da Costa MJR. 2018. Impact of good practices of handling training on beef cattle welfare and stock people attitudes and behaviors. Livest Sci. 216(1):24–31.
- Chan JPW, Chang CC, Chin C, Hsu WL, Liu WB, Chen TH. 2010. Association of increased serum acute-phase protein concentrations with reproductive performance in dairy cows with postpartum metritis. Vet Clin Pathol. 39(1):72–78.
- Crane EM, Munro JC, Bourgon SL, Diel de Amorim M, Ventura R, Fredeen AH, Montanholi YR. 2016. Metabolic blood profile of beef heifers during oestrous and non-oestrous states. Reprod Dom Anim. 51(5):819–826.
- de Boer M, Buddle BM, Heuer C, Hussein H, Zheng T, LeBlanc SJ, McDougall S. 2015. Associations between intrauterine bacterial infection, reproductive tract inflammation, and reproductive performance in pasture-based dairy cows. Theriogenology. 83(9):1514–1524.
- Deguillaume L, Geffré A, Desquilbet L, Dizien A, Thoumire S, Vornière C, Constant F, Fournier R, Chastant-Maillard S. 2012. Effect of endocervical inflammation on days to conception in dairy cows. J Dairy Sci. 95(4):1776–1783.
- Diskin MG, Kenny DA. 2016. Managing the reproductive performance of beef cows. Theriogenology. 86(1):379–387.
- Dubuc J, Duffield TF, Leslie KE, Walton JS, Leblanc SJ. 2010a. Definitions and diagnosis of postpartum endometritis in dairy cows. J Dairy Sci. 93(11):5225–5233. e
- Dubuc J, Duffield TF, Leslie KE, Walton JS, LeBlanc SJ. 2010b. Risk factors for postpartum uterine diseases in dairy cows. J Dairy Sci. 93(12):5764–5771.
- Dubuc J, Duffield TF, Leslie KE, Walton JS, Leblanc SJ. 2011. Randomized clinical trial of antibiotic and prostaglandin treatments for uterine health and reproductive performance in dairy cows. J Dairy Sci. 94(3):1325–1338.
- Ernstberger M, Oehl H, Haessig M, Hartnack S, Bollwein H. 2019. Predicting the probability of conception in dairy cows with clinical endometritis based on a combination of anamnestic information and examination results. Theriogenology. 138:127–136.
- Hickey MC, Drennan M, Earley B. 2003. The effect of abrupt weaning of suckler calves on the plasma concentrations of cortisol, catecholamines, leukocytes, acute-phase proteins and in vitro interferon-gamma production. J Anim Sci. 81(11):2847–2855.
- Hicks TA, McGlone JJ, Whisnant CS, Kattesh HG, Norman RL. 1998. Behavioral, endocrine, immune, and performance measures for pigs exposed to acute stress. J Anim Sci. 76(2):474–483.
- Humblet MF, Guyot H, Boudry B, Mbayahi F, Hanzen C, Rollin F, Godeau JM. 2006. Relationship between haptoglobin, serum amyloid A, and clinical status in a survey of dairy herds during a 6-month period. Vet Clin Pathol. 35(2):188–193.
- Kleiser L, Fürll M. 1998. Screening zur Früherkennung einer Disposition für die Dislocatio abomasi bei Kühen [Screening for early detection of a disposition for abomasal dislocation in cows]. In: Fürll M, editor. Stoffwechselbelastung, -dia stabilisierung beim Rind. Leipziger Samstagsakademie, Leipz - 248. Kirst E., Jacobi U.. p. 95–104.
- LeBlanc SJ, Duffield TF, Leslie KE, Bateman KG, Keefe GP, Walton JS, Johnson WH. 2002. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J Dairy Sci. 85(9):2223–2236.
- LeBlanc SJ. 2008. Postpartum uterine disease and dairy herd reproductive performance: a review. Vet J. 176(1):102–114.
- LeBlanc SJ. 2014. Reproductive tract inflammatory disease in postpartum dairy cows. Animal. 8(1):54–63.
- McDougall S, Macaulay R, Compton C. 2007. Association between endometritis diagnosis using a novel intravaginal device and reproductive performance in dairy cattle. Anim Reprod Sci. 99(1–2):9–23.
- Moorey SE, Biase FH. 2020. Beef heifer fertility: importance of management practices and technological advancements. J Anim Sci Biotechnol. 11:97.
- Pascottini BO, LeBlanc SJ. 2020. Metabolic markers for purulent vaginal discharge and subclinical endometritis in dairy cows. Theriogenology. 155(155):43–48.
- Perez-Marin CC, Moreno LM, Calero GV. (February 22nd 2012). Clinical Approach to the Repeat Breeder Cow Syndrome, A Bird's-Eye View of Veterinary Medicine, Carlos C. Perez-Marin, IntechOpen, DOI: 10.5772/31374. Available from: https://www.intechopen.com/chapters/28681.
- Petersen HH, Nielsen JP, Heegaard PM. 2004. Application of acute phase protein measurements in veterinary clinical chemistry. Vet Res. 35(2):163–187.
- Petrie A, Watson P. 2013. Statistics for veterinary and animal science. 3rd ed. Chichester (UK): Blackwell Pub.
- Ricci A, Bonizzi G, Sarasso G, Gallo S, Dondo A, Zoppi S, Vincenti L. 2017. Subclinical endometritis in beef cattle in early and late postpartum: cytology, bacteriology, haptoglobin and test strip efficiency to evaluate the evolution of the disease. Theriogenology. 94:86–93.
- Ricci A, Gallo S, Molinaro F, Dondo A, Zoppi S, Vincenti L. 2015. Evaluation of subclinical endometritis and consequences on fertility in piedmontese beef cows. Reprod Dom Anim. 50(1):142–148.
- Robin, X., Turck, N., Hainard, A. et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 12, 77 (2011). https://doi.org/10.1186/1471-2105-12-77
- Runciman DJ, Anderson GA, Malmo J, Davis GM. 2008. Use of postpartum vaginoscopic (visual vaginal) examination of dairy cows for the diagnosis of endometritis and the association of endrometritis with reduced reproductive performance. Aust Vet J. 86(6):205–213.
- Ryan NJ, Meade KG, Williams EJ, O’Farrelly C, Grant J, Evans ACO, Beltman ME. 2020. Purulent vaginal discharge diagnosed in pasture-based Holstein-Friesian cows at 21 days postpartum is influenced by previous lactation milk yield and results in diminished fertility. J Dairy Sci. 103(1):666–675.
- Salasel B, Mokhtari A, Taktaz T. 2010. Prevalence, risk factors for and impact of subclinical endometritis in repeat breeder dairy cows. Theriogenology. 74(7):1271–1278.
- Sattler T, Fürll M. 2004. Creatine kinase and aspartate aminotransferase in cows as indicators for endometritis. J Vet Med A Physiol Pathol Clin Med. 51(3):132–137.
- Sheldon IM, Lewis GS, LeBlanc S, Gilbert RO. 2006. Defining postpartum uterine disease in cattle. Theriogenology. 65(8):1516–1530.
- Tison N, Bouchard E, DesCôteaux L, Lefebvre RC. 2017. Effectiveness of intrauterine treatment with cephapirin in dairy cows with purulent vaginal discharge. Theriogenology. 89:305–317.
- Weber J, Zenker M, Köller G, Fürll M, Freick M. 2019. Clinical chemistry investigations in recumbent and healthy German Holstein cows after the fifth day in milk. J Vet Res. 63(3):383–390.
- Williams EJ, Fischer DP, Pfeiffer DU, England GCW, Noakes DE, Dobson H, Sheldon IM. 2005. Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle. Theriogenology. 63(1):102–117.
- Yasui T, McCann K, Gilbert RO, Nydam DV, Overton TR. 2014. Associations of cytological endometritis with energy metabolism and inflammation during the periparturient period and early lactation in dairy cows. J Dairy Sci. 97(5):2763–2770.
- Zaborski D, Grzesiak W, Szatkowska I, Dybus A, Muszynska M, Jedrzejczak M. 2009. Factors affecting dystocia in cattle. Reprod Domest Anim. 44(3):540–545.
