 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Rumex nervosus leaves (RNL) and Cinnamomum verum bark (CNB), phytogenic herbs, have received much attention in recent years for their antimicrobial properties; however, there is limited knowledge about their potential anticoccidial functions. The prophylactic effects of RNL and CNB were compared with salinomycin, a standard synthetic anticoccidial agent, in broilers experimentally infected with Eimeria tenella (E. tenella). One-day-old broiler chicks (n = 225) were randomly divided into nine groups. Birds were either fed a basal diet containing 1, 3, or 5 g RNL or 2, 4, or 6 g CNB/kg feed, or treated with salinomycin within the basal diet, or the infected (IUT) or non-infected (UUT) groups were fed a basal diet only. Birds infected (n = 25 bird/group) with 40,000 sporulated E. tenella oocysts/bird at d 21 except UUT. Bodweight gain (BWG) and feed conversion ratio (FCR) were significantly (p < .05) lower in IUT compared to UUT. On day 7 post-infection (DPI), birds treated with RNL, CNB, or salinomycin had fewer lesions in the caeca and a lower oocyst value, and a higher oocyst reduction rate in the faeces than birds in the IUT. Although RNL was not able to reduce weight loss caused by coccidiosis, CNB at 6 g improved FCR and production efficiency index (PEI) at 7 DPI compared to the infected groups. In conclusion, RNL at 5 g and CNB at 6 g have moderate anti-coccidial activity and could be used to treat poultry coccidiosis in the field. However, more research is needed to identify active ingredients that make it effective compared to commercially available drugs.
Phytogenic feed additives prevented weight loss and caecum pathology in broiler chickens at risk of coccidiosis.
Rumex nervosus leaves and Cinnamomum verum bark had similar effects to the coccidiostat salinomycin at high doses.
Traditional medicinal plants are potential alternatives to pharmaceutical coccidiostats to promote the health and growth of broiler chickens.
HIGHLIGHTS
Introduction
Coccidiosis is a protozoan infection that affects the health and performance of most vertebrates and is caused by coccidia (Eimeria spp. in birds and livestock or Isospora in cats and dogs) (Jones and Garcia Citation2021). In Riyadh, Saudi Arabia, the overall prevalence of coccidiosis in home-reared chicks in one year was 80% (Al-Quraishy et al. Citation2009). Dubey (Citation2019) reported that Eimeria acervulina, Eimeria maxima, Eimeria tenella, and Eimeria praecox are often found in broiler chickens.
Various chemical anticoccidials are used in the poultry industry to control avian coccidiosis (Felici et al. Citation2021). Salinomycin (Sacox®) is a monovalent ionophore and is used in conjunction with food as a preventive agent or coccidiostat rather than as a coccidicidal drug. Because it acts earlier in the parasite's life cycle by disrupting ion gradients across the parasite's cell membrane (Noack et al. Citation2019). However, the problematic emergence of resistance to antiparasitic agents has led to higher treatment doses and residues in poultry products, and consumer desire for residue-free meat has led several researchers to propose and develop alternative agents (Upadhaya et al. Citation2019).
Some phytogenic supplements have been shown in recent decades to be effective in protecting chickens from coccidia-related damage without having a positive effect on performance (Galli et al. Citation2021). In addition, the organic acid mixtures inhibited in vitro and in vivo pathogens, including E. tenella and Eimeria bovis parasites through reduced E. tenella oocysts, reduced inflammatory oxidative stress, the enhanced immune response through reduced reactive oxygen species, increased manganese superoxide dismutase, and short-chain fatty acid levels (Balta et al. Citation2021). Mixtures of thymol eugenol, piperine, and vitamin D3 could be used as an anticoccidial feed additive in broilers with coccidial infection (Upadhaya et al. Citation2019). Although Upadhaya et al. (Citation2019) found that cumulative BWG was increased and FCR was decreased, studies on the synergistic effects of natural herbs in reducing coccidial infection while improving growth performance are limited. Barbour et al. (Citation2015) have investigated the interest of medicinal herbs and their derivatives as substitutes for coccidiostats in the poultry sector. These herbal compounds produce bioactive phytochemicals that are effective in controlling poultry coccidiosis while being safe, nutritious, and therapeutic for poultry meat consumers (Alhotan and Abudabos Citation2019; El-Shall et al. Citation2022).
Othrob (Rumex nervosus) is an ethnobotanical shrub traditionally used for centuries in a variety of human cases, as the parts of RN and its metabolites contain bioactive constituents that have diverse biological, medicinal, and pharmacological activities (Shankar and Balasubramanium Citation2014; Al-Sunafi Citation2016; Desta et al. Citation2016; Quradha et al. Citation2018). Countries where RN is indigenous, such as some countries in East Africa and the Arabian Peninsula, need alternative anti-coccidiosis drugs capable of controlling avian coccidiosis without compromising the efficiency of production or human health, whether among poultry company customers or end consumers (Al-Asmari et al. Citation2015; Al-Naqeb Citation2015; Al-Sunafi Citation2016).
The use of RNL powder as a dietary supplement in coccidiosis therapy remains limited. Moreover, Cinnamomum verum is a natural product of the laurel family (Lauraceae) and has many biological and traditional activities as it contains many secondary plant compounds, such as cinnamaldehyde, carvacrol, eugenol, camphor and proanthocya, and other important macro and micronutrients (Adarsh et al. Citation2020). Moreover, we hypothesised that these natural feed additives of herb sources can be used as a substitute to synthetic coccidiosis drugs because of the potent compounds they contain. Consequently, this experiment compared the efficacy of different amounts of CNB or RNL powder as a means of controlling coccidia and promoting growth in broilers experimentally infected with E. tenella with a synthetic drug.
Materials and methods
Ethical approval
Appropriate measures were taken to minimise the birds' pain or discomfort. The study was approved by the Ethics, Methodology and Animal Welfare Studies Committee at King Saud University, Saudi Arabia (KSU) (Ethics Committee Approval No. KSU-SE-20-44).
Housing and experimental design with broiler chickens
The local hatchery of Al-Khumasia Company, Riyadh, Saudi Arabia, supplied 225 one-day-old hatched broilers (Ross 308). The birds were then randomly distributed into nine treatment groups. Each treatment consisted of five replicates, each replicate have five birds (n = 25 birds/group). Birds in groups 1, 2, and 3 were fed a control diet containing 1, 3, and 5 g RNL/kg diet, respectively, challenged with E. tenella. Birds in groups 4, 5, and 6 were fed a control diet containing 2, 4, and 6 g CNB/kg feed, respectively, challenged with E. tenella. Birds in group 7 received the control diet plus 66 mg salinomycin (SAL), challenged with E. tenella. Group 8: positive control (IUT): basal diet, untreated, challenged with E. tenella. Group 9: negative control (UUT): basal diet, untreated, unchallenged. The product used in this study were: ionophore anticoccidial salinomcyin, (Sacox60; Huvepharma NV, Belgium) added at 66 mg/kg to feed based on Abdelrahman et al. (Citation2014) and Tonda et al. (Citation2018).
Birds were housed in an environmentally controlled room in a cage 58 cm long, 50 cm wide, and 35 cm high for a total of 28 days. At 1 day of age, the temperature was set at 35 °C and gradually decreased by 1 °C every 2 days until a stable temperature of 22 °C was reached. Then it was maintained until the end of the experiment. The relative humidity was between 65 and 85%. The photoperiod ‘23 h on and 1 h off’ was used. Feed and water were provided ad libitum. Two diets were administered, 1 to 21 d (starter) and 22 to 34 d (finisher), based on Ross 308 guidelines (Supplementary Table S1).
Inoculation of Eimeria oocysts
Oocysts of E. tenella were cleaned by flotation on 2.5% sodium dichromate and washed three times using distilled water. E. tenella sporulated oocysts were provided kindly by Professor Saleh Al-Quraishy and Dr. Mahmood Qasem at parasitology laboratory, Zoology Department at King Saud University in Saudi Arabia. On day 21 of age (day 0 pre-infection), the experimental chickens were infected orally by inculcating 1 mL suspension of distilled water containing an oral infectious dose of E. tenella 4*104 sporulated oocysts/bird directly to pharynx using a long nozzle pipette (Lee et al. Citation2012; Al-Shaibani et al. Citation2020). The control chickens (IUT) (n = 25) received distilled water through the same route.
Preparation of RNL leaves and CNB and analysis of its nutrient composition
RN was harvested from the mountains and valleys around the village of Bait Al-Aqra in the Yemeni province of Ibb. The harvested RNL was air-dried every 5 h for 15 days under sunlight. A taxonomist at King Saud University, Department of Botany validated the plant botanical identity. Moreover, CNBs were purchased in Riyadh, Saudi Arabia, from a local store. Then, the dried leaves and bark were ground into a fine powder (particle size; 0.25–0.30 mm) using a blender in our laboratory. Then, the ground leaves of RN were mixed with broiler diet at levels of 1, 3, and 5 g RNL/kg diet. In addition, ground cinnamon bark was added to broiler diets at concentrations of 2, 4, and 6 g CNB/kg feed. According to Melesse et al. (Citation2018), the nutritional values of RNL and CNB were investigated using nutritional proximate analysis. The phytochemical compounds in the RNL and CNB extract mixture were identified, separated, and dosed using high-performance liquid chromatography (HPLC). Gas chromatography-mass spectrometry (GC-MS) assay was done to detect the phytochemicals as stated by Adaszyńska-Skwirzyńska and Szczerbińska (Citation2019).
Anticoccidia assessment
Anticoccidial index (ACI) or anticoccidial efficacy is a measure for assessing the prophylactic effects of RNL and CNB powders against coccidia. Anticoccidial parameters, such as bloody diarrhoea, oocyst value ‘OV’, relative weight gain (RWG), lesion value (LV), and survival rate (SR) were measured to calculate ACI using the formula below (Cheng et al. Citation2020).
(1)
(1)
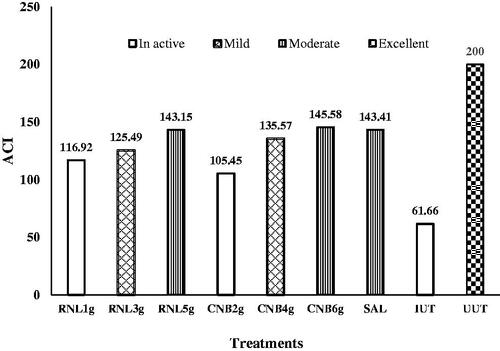
Briefly, ACI values below 120 were considered to have an inactive anticoccidial effect, ACI values usually range from 0 to 200 (Shetshak et al. Citation2021). Therefore, ACI scores that were either 120–140, 140–160, 160–180, or above 180 were classified as mild, moderate, marked, or excellent.
Bloody diarrhoea in birds was recorded in the morning by daily veterinary monitoring and counting the number of blood pieces in the faeces during the period of 4 to 7 days after infection. Every day the faeces were removed after each observation to expose the fresh bloody faeces. The numbers 0, 1, 2, 3, and 4 correspond to the numbers 0, 1, 2, 3, and 4 of the bloody pieces in the faeces of each group (Lan et al. Citation2016).
(2)
(2)
On the seventh day of infection, faecal samples were collected from each pen (n = 5 replicate). Each sample was thoroughly mixed and dispersed in 10 mL of water. The number of oocysts in a 10 μL suspension was then counted using a glass microscope slide and cleaned square microscope coverslips, 18 × 18 mm, under an Olympus optical microscope (Olympus Corporation, Tokyo, Japan) using a microscope digital camera (Al-Quraishy et al. Citation2020). The outcomes were expressed as the number of oocysts per gram (OPG). The reduction rate of oocyst production and oocyst value was calculated as indicated by Chauhan et al. (Citation2017).
(3)
(3)
(4)
(4)
Clinical symptoms and mortality were assessed and recorded each day after infection. EquationEquation 5(5)
(5) was then used to calculate the survival rate.
(5)
(5)
The RWG of the UUT was set to 100% and the RWG of the other groups was calculated using equation.
(6)
(6)
When broilers were 28 days old, the experiment is over ‘at 7th DPI’, a 30 chickens (samples size 1 bird/replicate, n = 5 birds/group) was subjected to 10 h of feed deprivation, with drinking water available ad libitum. The birds were then slaughtered and dissected in accordance with the Animal Welfare Act. After slaughter by severing the jugular vein, the fowls were completely bled and then de-feathered and eviscerated. The caecum of each bird was removed and examined. Lesion values were graded rely on the gross lesions observed at necropsy on a scale of 0–4, based on the severity of the lesion and atrophy of the caecum (fluid accumulation, epithelial colour, and overall caeca appearance). Where zero stands for no lesions ‘normal condition’, one stands for mild lesions: small-scattered petechiae, normal thickness of the caecal walls. Two stands for moderate lesions: numerous petechiae, small thickness of caecal walls. Three stands for severe lesions: large bleeding, swollen caecal walls, morphological changes of the caecum, and four stands for more severe lesions: Darkening, blood clots and atrophy in the caecum, severe hypertrophy of the caecal walls, including deaths from coccidiosis. Furthermore, the caecum lengths were recorded as a mean ± SEM (in cm) to assess the grade of atrophy.
Efficiency performance indices
To assess the performance of the birds, each animal was weighed separately at 0 and 7 DPI. The added feed and the remaining feed were weighed. Then the BWG, feed intake (FI), and FCR were calculated using the following equations.
(7)
(7)
(8)
(8)
(9)
(9)
(10)
(10)
Mortality was recorded when it occurred.
Statistical analysis
The obtained anticoccidial and performance data were subjected to one-way analysis of variance (ANOVA) in a general linear model (GLM) using Statistical Analysis System package (SAS) version 9.1 software (SAS Institute Inc., Cary, NC, USA) (SAS Citation2012). The following models were used:
where: Yij = the observed j variable in the ith treatment; μ = overall mean of the variables; Ti = the effect the ith treatment; and eij = random residual error. To compare bloody diarrhoea and lesions score values in each experimental group, the non-parametric Kruskal-Wallis H test was used. The means of the data were expressed as mean ± SEM and separated using Duncan’s multiple range test. Comparison with p-values <.05 was considered significantly different.
Results
Nutrient analysis and phytochemical screening of Rumex nervosus leaves and cinnamon bark
Table shows the chemical compositions of RNL and CNB. The crude protein, ether extract, crude fibre, ash, neutral detergent fibre, acid detergent fibre, and energy of RNL were determined to be 14.45, 1.63, 8.73, 19.40, 21.43, 16.41, and 3469.89 kcal/kg, respectively, and those of CNB were determined to be 4.43, 4.03, 23.84, 3.12, 65.34, 45.75, and 4974.77 kcal/kg, respectively, indicating its potential as a food supplement.
Table 1. Proximate analysis of cinnamon bark (CNB) and dried leaves of Rumex nervosus (RNL) (% on dry matter basis).
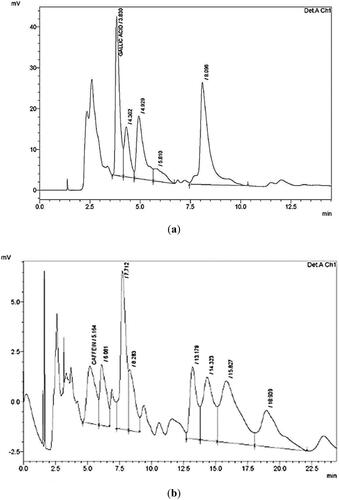
Gallic acid was the component with the highest composition (700 g/g) in the RNL extracts, as shown by the HPLC results (Figure ). Although caffeine was identified in high concentrations, the HPLC outcomes showed another unidentified component that reached the peaks in the CNB extracts could be Cinnamaldehyde (Figure ). Consequently, GC-MS was used to identify the major volatile mixtures in the CNB and RNL extracts.
Figure 1. HPLC chromatogram of phenolic and flavonoids compounds standards at 280 nm of (a) Rumex nervosus; (b) cinnamon.
The leaf extracts of RN and the bark extracts of CN were analysed by GC-MS. RNL has 13 phytochemicals (Table and Figure ), while CNB has 25 phytochemicals (Table , Figure ). Methyl ester of Octadecadienoic acid, Hexadecanoic acid, Pentadecanoic acid were the highest quantity compounds detected in RNL extract by GC-MS (Table ). Hexadecanoic acid-2-methyl ester, pentadecanoic acid, 14-methyl-ester, Cinnamaldehyde, (E)-2-propenal-3-phenyl, methoxyphenyl, and 2-methylbenzofuran were the highest quantity compounds detected in CNB extract by GC-MS (Table ).
Figure 2. GC-MS tracing of methanolic extracts from Rumex nervosus leaves (a) and cinnamon bark (b).
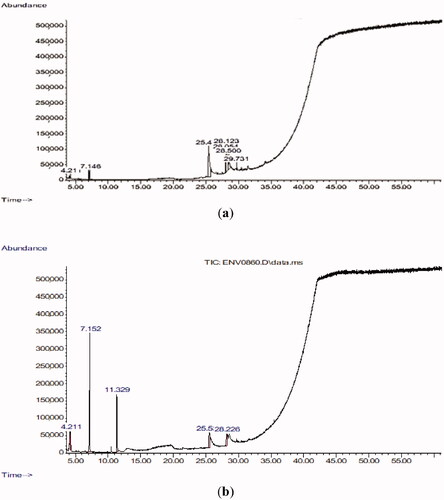
Table 2. Volatile compounds of Rumex nervosus leaves identified by GC/MS.
Table 3. Volatile compounds of cinnamon bark identified by GC/MS.
Anticoccidial activity
Behaviour changes and bloody diarrhoea
Figure shows the clinical signs seen in broilers and their frequency. The severity of clinical signs varied among the experimental chickens. The most common symptom was diarrhoea. Signs of coccidiosis, like crouching, inappetence, depression, and ruffled feathers, were noted obviously in IUT but not in uninfected or salinomycin-treated chickens. Table demonstrated the influence of supplements RNL or CNB with on mean OPG output, oocyst score, inhibition rate, bloody diarrhoea, lesion score, and caecal lengths of each treatment at 7 DPI.
Figure 3. Clinical symptom and their frequency observed in broilers unmedicated infected (IUT) experimentally with E. tenella.
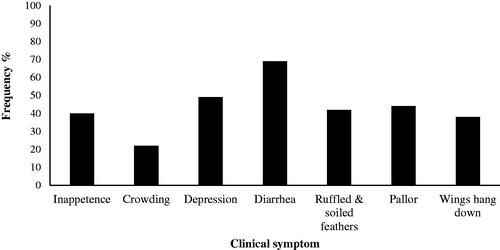
Table 4. Effect of experimental groups on oocyst per gram (OPG) output, lesion score, oocyst value, reduction rate and caecal length at 27th day of birds age (7th DPI).
a–cDifferent lowercase letters within the columns indicated significant differences.
There was no mortality in any of the experimental groups, except for one bird in IUT. Thus, the mortality rate was 4% in the IUT group and 0.04% in all experimental groups. However, throughout the study, no diarrhoea was noticed in the salinomycin-treated groups or UUT. There was no bloody diarrhoea in the faeces during the first four days after infection. From day 4 to 7 post-infection with E. tenella, bloody diarrhoea was observed in all infected groups except in the salinomycin treated group.
Lesion scores
Table , shows the length of the caecum and the scores of the pathological lesions in the groups graded from normal to severe. The severe pathological lesions found in the caeca of IUT were shrinkage of caecal length (atrophy), wall thickening, erosion, and dark blood clots (Supplementary Figure S1). Caecal morphology and shrinking length were significantly (p < .05) improved in the drug-treated group (group 2) compared with IUT. The bird that received 4 g CNB/kg feed had the longest caecum. The caeca in both NC and salinomycin groups exhibited typical morphology.
When all pathological characteristics were combined, lesion scores showed that the herb-treated groups had significantly (p < .05) decreased adverse caecal lesions caused by coccidiosis with increasing amounts of RNL or CNB powder (Table ). Each herb had the least pathological features, including the lowest lesion scores, when the dosage was increased. All groups treated with RNL or CNB had significantly (p < .05) lower lesion scores than the IUT.
Faecal oocyst excretion
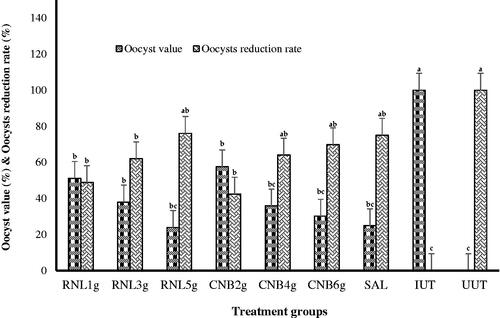
The number of oocysts per g of faeces was absent during the first 4 DPI. When we collected the excreta of all replicates of each group in the period of 5–7 DPI, we observed under the microscope that the excretion of E. tenella oocysts starts around the 5th DPI and peaks around the 6th-7th DPI and decreases thereafter. Faecal examination on 7th DPI revealed the highest number (mean*10^6) of oocyst excretions, which reached 12.54 in the infected non-medicated group and significantly decreased (p < .05) with increasing dose in the infected medicated groups. Birds treated with the higher herbal dosage of RNL and CNB were significantly similar to those treated with salinomycin (Table ). The oocysts reduction rates of RNL medicated groups 1–3, CNB medicated groups 4–6, and salinomycin medicated group was 48.85, 61.99, 76.07, 42.45, 64.09, 69.81, and 75.06%, respectively (Figure ). It was found that the production of oocysts decreased with the increase in herbal doses.
Figure 4. Effect of experimental treatments and challenge with E. tenella on oocyst value and oocysts reduction rate at 7 d post-inoculation. RNL 1g, RNL 3g, and RNL 5g represent 1, 3, and 5 g Rumex nervosus leaf powder/kg diet, respectively; CNB 2g, CNB 4g, and CNB 6g represent 2, 4, and 6 g cinnamon/kg diet, respectively; SAL: 66 mg of salinomycin/kg diet; IUT and UUT: basal diet with and without coccidiosis challenge, respectively. a–cDifferent lowercase letters indicate significant differences.
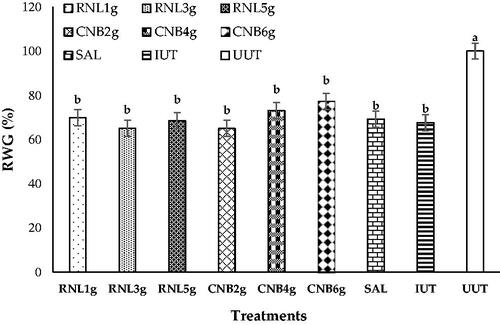
Relative weight gain ratio
The mean RWG of each group is shown in Figure . The initial weights between the chicken groups were not significant (data not shown). The BWG of the groups supplemented with RNL or CNB was not significantly different when compared to each other, to the IUT (p > .05), or even to the salinomycin-treated group. Thus, our data indicate that RNL powder or even CNB powder does not effectively increase BWG at the dose currently used. Moreover, the decrease in BWG of all infected groups compared to the uninfected groups clearly shows the negative effects of Emeria infection.
Figure 5. Effect of experimental treatments and infection with E. tenella on the weight gain ratio (RWG) of birds at 7 DPI. RNL 1g, RNL 3g, and RNL 5g represent 1, 3, and 5 g Rumex nervosus leaf powder/kg diet, respectively; CNB 2g, CNB 4g, and CNB 6g represent 2, 4, and 6 g cinnamon/kg diet, respectively; SAL: 66 mg of salinomycin/kg diet; IUT and UUT: basal diet with and without coccidiosis challenge, respectively. Different lowercase letters indicate significant differences.
Anticoccidial indices
As shown in Figure , the group IUT, the group with the lowest RNL dose (1 g RNL/kg feed) and the group with the lowest cinnamon dose (2 g CNB/kg feed) had no anticoccidial effect, and the lowest ACI value. In addition, the group with the highest doses of RNL, CNB, and salinomycin treated group showed moderate anticoccidial effect with ACI indices of 143.2, 145.6, and 143.4, respectively. The ACI index of the groups with 3 g RNL/kg diet and 4 g CNB/kg diet were 125.5 and 135.6, respectively, representing mild anticoccidial effect. Herb-treated groups showed no significant anticoccidial effects among themselves, while they were significantly higher than the IUT and lower than the UUT. The ACI score of the UUT (ACI = 200) was higher than that of the infected groups, including the salinomycin supplementation group.
Figure 6. Effect of experimental treatments and coccidiosis on anti-coccidial index (ACI) of each group at 7 days after inoculation. RNL 1g, RNL 3g, and RNL 5g represent 1, 3, and 5 g Rumex nervosus leaf powder/kg diet, respectively; CNB 2g, CNB 4g, and CNB 6g represent 2, 4, and 6 g cinnamon/kg diet, respectively; SAL: 66 mg of salinomycin/kg diet; IUT and UUT: basal diet with and without coccidiosis challenge, respectively. ACI scores that were either 120–140, 140–160, 160–180, or above 180 were classified as mild, moderate, marked, or excellent.
Efficiency performance
Table shows the effects of herbal feed supplements on the feed efficiency of birds. BWG, FCR, and PEI were significantly (p < .05) affected by the experimental treatments. On the other hand, the experimentally treated groups had no effect (p > .05) on body weight (BW) or FI. Here, the BWG of the medicated groups did not differ significantly from that of the IUT but was significantly (p < .05) lower than that of the UUT (Table ; Figure ).
Table 5. Mean values of body weight (BW), weight gain (BWG), feed intake (FI), feed conversion ratio (FCR) and production efficiency index (PEI) of birds fed experimental diets at 27th day of birds’ age (7th DPI).
Although birds receiving RNL at a dose of 1 g/kg had improved broiler FCR on 7 DPI, there were no significant differences (p > .05) in bird performance between RNL-treated groups or even compared with salinomycin- or IUT-treated groups at the dose currently used. Although birds receiving CNB at a dose of 6 g/kg improved broiler FCR and PEI on 7 DPI, there were no significant differences in broiler performance between the cinnamon treated groups at the current dose (p > .05).
Compared to the IUT, broilers in the UUT gained excessive BW, consumed too much feed, had the best feed conversion ratio, resulting in increased PEI. In addition, the performance of broilers in RNL treated groups and CNB treated groups was considerably lower (p < .05) than UUT. Cinnamon treated birds, especially at 6 g CNB/kg basal diet, improved significantly in BWG, FCR and PEI when compared to the IUT birds and salinomycin treated birds. Finally, our data presented that the CNB groups performed better than the IUT. However, this effect was not significant at the levels currently used.
Discussion
Salinomycin as anticoccidials ionophores that act on chicken coccidia by disrupting ion gradients across the membrane of Eimeria cell. Salinomycin producer Streptomyces albus ATCC 21838, which produces the pseudodisaccharide ‘salbostatin’ and is often used to prevent coccidiosis (Noack et al. Citation2019). For poultry researchers, the search for synthetic anticoccidial alternatives is a critical area of research (Lan et al. Citation2016; Al-Shaibani et al. Citation2020; Balta et al. Citation2021). Here, we tried to mimic a synthetic anticoccidial by focussing on finding a traditional product with anticoccidial activities that has a unique, safe anticoccidial drug with no tissue residues or even drug resistance. After looking at a variety of herbs, we focussed on RNL and CNB supplementation.
The various phytochemicals of selected herbs confer various bioactive properties to this shrub and spice (Rao and Gan Citation2014; Al-Sunafi Citation2016). Our data confirm that RNL and CNB powders have significant anticoccidial properties. Clinical signs of coccidiosis observed in the birds in this study included pallor, diarrhoea, anorexia, depression, and ruffled feathers that were consistent with previous studies (Dubey Citation2019; Al-Shaibani et al. Citation2020). Bloody diarrhoea in the infected treated groups was numerically reduced in this experiment compared to the IUT. Therefore, the reduced haemorrhagic diarrhoea may protect the infected birds from secondary infections, inflammatory reactions, and ingestion of toxic substances (Shetshak et al. Citation2021). The antidiarrheal effect of RNL might be related to its therapeutic activity, such as lowering intestinal motility and direct anticoccidial activity and direct anticoccidial activity. This could be because RN contains high levels of flavonoids, such as quercetin, which inhibits the release of acetylcholine in the digestive system, a suggestion supported by similar statements made by Asad et al. (Citation2004). The remarkable antidiarrheal effect of cinnamon leaf extracts in a castor oil-induced diarrhoea model proves their efficacy in a variety of diarrheal disorders (Kayande et al. Citation2014). Moreover, cinnamon contains active components, especially cinnamaldehyde, which stimulates digestion and leads to the release of digestive enzymes (Kumar et al. Citation2014).
Synthetic salinomycin, a standard drug, exhibited moderate anticoccidial activity in this study. Salinomycin treated group had significantly fewer coccidiosis symptoms. According to Behnamifar et al. (Citation2019), the coccidial inhibitory effect could be partly due to the fact that salinomycin is a pure compound. Since the studied herbs comprise a rich group of phytochemicals that may have a synergistic effect on infected birds. The pure constituents of RNL and CNB should be isolated and identified to further evaluate their anticoccidial activity. This will contribute to better drug development.
In agreement with previous reports (Lan et al. Citation2016; Abudabos et al. Citation2017; Alhotan and Abudabos Citation2019), the natural product effectively reduced the number of faecal oocysts in coccidia-infected birds. The herb-treated groups drastically reduced OPG output in the RNL and cinnamon-treated groups, implying that RNL and CNB powders could be useful in controlling large-scale coccidiosis outbreaks in chicken farms. One of the most crucial digestive organs in birds is the caecum. Chickens suffer malabsorption and diarrhoea when Eimeria destroys the epithelial cells of the caecum, resulting in low BWG (Lan et al. Citation2016; Abdisa et al. Citation2019). The severity of the gross lesion and caecal atrophy in the herb-treated groups ranged from mild atrophy and scattered petechiae to severe atrophy and profuse bleeding in the IUT. Compared with IUT, the data of caecal lesions and oocyst reduction rate presented that RNL, CNB, and salinomycin could significantly reduce the disturbances of caecal lesions.
This confirms the existence of biologically active compounds in RNL or CNB powders that inhibit or delay the survival of E. tenella in vivo, resulting in inhibition of lesions and oocyst shedding. Consequently, cinnamon (Yang et al. Citation2020) and RNL (Akanbi and Taiwo Citation2020), in addition to direct control of coccidian parasites, could play a key role in improving the situation of diseased birds through their organ-protective properties. Coccidiosis had an adverse effect on the affected group, which was alleviated by cure with the natural product. The positive effects of RNL and cinnamon on anti-coccidiosis criterion may be attributed to the existence of abundant alkaloids, flavonoids, saponins, and tannins, which may have a combined influence on anti-parasitic, anti-inflammatory, antioxidant, and antidiarrheal actions in broiler chickens (Rao and Gan Citation2014; Adarsh et al. Citation2020; El-Hack et al. Citation2020; Khater et al. Citation2020).
The phytochemicals gallic acid and 13 compounds for RNL and caffeine, cinnamaldehyde, and other 23 phytochemicals for CNB were detected in RNL and CNB by HPLC and GC-MS analysis. These demonstrated phytochemicals should be further investigated in vivo or in vitro to determine whether the anticoccidial activity and mode of action if any, alone or in mixtures, are directly or indirectly related to the reduction of faecal oocyst counts in the birds.
Gallic acid, found in the RNL plant, has antioxidant, anti-inflammatory, antibacterial, antiallergic, anticarcinogenic, and antimutagenic properties. Therefore, antioxidants are extensively used in the poultry industries as dietary supplements to alleviate the oxidative stress that is produced by high oxidative free radicals produced during the immune response of host cells to Eimeria infestation (El-Shall et al. Citation2022). This could be useful in defending against parasitic infections and in eliminating cytotoxicity and tissue damage. Natural plant and their extracts, such as Myrobalan, Rhus chinensis, and Quercus infectoria containing gallotannins, gallic acid, etc. play a role in controlling Eimeria infestation in birds by, for example, reducing lesion number, oocyst excretion, and mortality (Thangavel et al. Citation2020).
As expected, performance was most impaired in the coccidiosis-exposed group not treated with natural or synthetic drugs. These findings are consistent with those of Abudabos et al. (Citation2018), who observed that Eimeria-infected groups had a substantial decrease in BW and FI compared to uninfected groups due to coccidian challenge, leading to bad nutrient intake and reduced immune response, and then gut tissue injury (Adhikari et al. Citation2020). Fortunately, the performance indicators of the birds recovered closer to those of the UUT and drug-treated groups. The therapeutic properties of herbal anticoccidia supplements are attributed to the presence of some bioactive compounds in cinnamon that improve immunity and antioxidant status, reduce intestinal inflammation, modulate gut microflora, and reduce parasitosis (Gende et al. Citation2008; Khaki et al. Citation2014; El-Shall et al. Citation2022). Natural anticoccidial agents promote bird growth by reducing the destructive effects of coccidiosis.
Here, the performance and PEI of RNL, CNB and salinomycin treated groups were negatively affected by the Eimeria challenge compared to UUT and positively affected compared to IUT resulting in less BWG due to E. tenella infection while herb treated groups, especially 6 g CNB/kg mitigated the effects. Birds had higher BW in the salinomycin groups than in the IUT; however, BWG did not differ. In agreement with Alhotan and Abudabos (Citation2019), performance indicators improved in the herbal groups compared to the IUT. The non-significant results detected in the herbal groups compared to the salinomycin groups in this study contradict the findings of Alhotan and Abudabos (Citation2019) who found that higher BW, BWG, and PEI were achieved in the Elancoban treated group. In the present study, the performance was not dose-dependent. In contrast to Tanweer et al. (Citation2014), who found that performance increased linearly with increasing dose, FI was not considered. The synthetic drug salinomycin, used here as a control, had intermediate anticoccidial activity. Birds in the salinomycin-treated group consumed more feed than birds in the other infected groups, resulting in poorer FCR, PEI, as the birds gained less weight and consumed more feed. Our results differ from those of (Abdelrahman et al. Citation2014; Tonda et al. Citation2018) who found that salinomycin-treated groups performed best.
Eimeria infection in animals results in significant loss of BW and decreased FI (Mehlhorn Citation2014) due to bad nutrient absorption and diminished immune response leading to intestinal damage (Adhikari et al. Citation2020). Our results are consistent with weight loss in birds infected with E. tenella compared to UUT. However, there were no significant differences in FI between the experimental groups.
Although this study confirms and supports the above important findings on the decreasing number of oocysts and lesions in the caecum, it has some limitations. Although the 6 g CNB/kg diet improved performance compared to the infected groups, neither herbs were able to improve performance to meet or exceed the UUT. It is possible that an overdose of coccidial oocysts or a subtherapeutic dose could be harmful to the chickens.
Although our data show that RNL and CNB have intermediate anti-coccidial activity at high doses, most of the performance parameters for CNB (6 g CNB) were significantly higher at higher doses and numerically higher at lower doses for RNL (1 g RNL) simultaneously. In the situation of RNL, anticoccidial indicators are in conflict with performance indicators, while in the situation of CNB they agree. Therefore, future prescriptions in both cases should take into account the anticoccidial and ameliorative effects of RN. According to these results, the dose could be increased by 5 g RNL or 6 g CNB by adding it to chicken feed or using it as an extract in the case of coccidiosis to improve health. To relive the negative impacts on performance, more researches need to be carefully conducted using a lower dose of E. tenella oocysts or a higher drug dose. In addition, more studies are needed, either alone or in a mixture with other herbal products, to see if it can improve FCR, increase BWG, and reduce coccidial oocysts.
To evaluate the in vitro anti-coccidial activity of the efficacy of RNL and/or CNB or their isolated phytochemicals on the sporulation of oocysts, it is also needed to check the acute toxicity or lethal dose 50 (LD50) of selected herb extract and to recognise the relationship between the major constituents and their anti-coccidial effects to develop new drugs. In the present study, our data confirmed that the powder of both herbs exhibited moderate anticoccidial activity. In this study, the nutrient content analysis revealed that RNL had moderate crude protein content (14.45%) and high carbohydrate content (55.79%) resulting in higher nutritional value (346.99 Kcal/100 g). CNB, on the other hand, had low crude protein content (4.43%) and high carbohydrate content (64.58%), resulting in a higher nutritional value (4974.77 Kcal/100 g). The pharmacognostic study of RN, conducted in Yemen by Al-Sunafi (Citation2016), found that the RN extract was non-toxic up to 7.1 g/kg BW and exhibited cytotoxic activity against human cancer cell lines. We suggest that RN could be considered non-toxic in the dose range administered.
Conclusions
Feeding RNL and CNB powders to birds suggests that RNL at a dosage of 5 g RNL/kg feed and CNB at a dosage of 6 g RNL/kg feed have an intermediate anti-coccidial effect. By reducing the lesions in the caecum and suppressing oocysts in the faeces of the birds, and thus can be used to treat poultry coccidiosis in the field. Compared with IUT, RNL at a level of 1 g improved FCR, and CNB at 6 g improved FCR, and PEI during the 7 days post-infection. The wide distribution, economic nature, and ease of use of RN shrub or cinnamon bark spice also suggest that both herbs at high doses could also serve as moderate alternatives to anti-coccidial agents. This study would ultimately support a new strategy to control broiler coccidiosis under field conditions.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abdelrahman W, Mohnl M, Teichmann K, Doupovec B, Schatzmayr G, Lumpkins B, Mathis G. 2014. Comparative evaluation of probiotic and salinomycin effects on performance and coccidiosis control in broiler chickens. Poult Sci. 93(12):3002–3008.
- Abdisa T, Hasen R, Tagesu T, Regea G, Tadese G. 2019. Poultry coccidiosis and its prevention. Control J Vet Ani Res. 2:103.
- Abudabos AM, Alyemni AH, Hussein EO, Al-Ghadi MQ. 2018. Anticoccidial effect of some natural products in experimentally induced Eimeria spp. infection on carcass quality, intestinal lesion and ileal histology in broilers. JAPS. 28(1):73–79.
- Abudabos AM, Alyemni AH, Swilam EO, Al-Ghadi MQ. 2017. Comparative anticoccidial effect of some natural products against Eimeria spp. infection on performance traits, intestinal lesion and occyte number in broiler. Pak J Zool. 49(6):989–1995.
- Adarsh A, Chettiyar B, Kanthesh B, Raghu N. 2020. Phytochemical screening and antimicrobial activity of “Cinnamon zeylanicum”. Int J Pharm Res Innov. 13:22–33.
- Adaszyńska-Skwirzyńska M, Szczerbińska D. 2019. The effect of lavender (Lavandula angustifolia) essential oil as a drinking water supplement on the production performance, blood biochemical parameters, and ileal microflora in broiler chickens. Poult Sci. 98(1):358–365.
- Adhikari P, Kiess A, Adhikari R, Jha R. 2020. An approach to alternative strategies to control avian coccidiosis and necrotic enteritis. J Appl Poult Res. 29(2):515–534.
- Akanbi OB, Taiwo VO. 2020. The effect of a Local isolate and Houghton strain of Eimeria tenella on clinical and growth parameters following challenge in chickens vaccinated with Immucox® and Livacox® vaccines. J Parasit Dis. 44(2):395–402.
- Al-Asmari ARK, Siddiqui YM, Athar MT, Al-Buraidi A, Al-Eid A, Horaib GB. 2015. Antimicrobial activity of aqueous and organic extracts of a Saudi medicinal plant: Rumex nervosus. J Pharm Bioallied Sci. 7(4):300–303.
- Alhotan RA, Abudabos A. 2019. Anticoccidial and antioxidant effects of plants derived polyphenol in broilers exposed to induced coccidiosis. Environ Sci Pollut Res Int. 26(14):14194–14199.
- Al-Naqeb G. 2015. Antioxidant and antibacterial activities of some Yemeni medicinal plants. Int J Herb Med. 3(3 Part A):6–11.
- Al-Quraishy S, Abdel-Baki A, Dkhil M. 2009. Eimeria tenella infection among broiler chicks Gallus domesticus in Riyadh city, Saudi Arabia. J King Saud Univ Sci. 21(3):191–193.
- Al-Quraishy S, Qasem MA, Al-Shaebi EM, Murshed M, Mares MM, Dkhil MA. 2020. Rumex nervosus changed the oxidative status of chicken caecum infected with Eimeria tenella. J King Saud Univ Sci. 32(3):2207–2211.
- Al-Shaibani I, Al-Khadher A, AlHibah A. 2020. Anticoccidial activity of Allium sativum and Punica granatum against experimentally induced Eimeria tenella infection in broiler chickens. Asian J Anim Vet Sci. 5(4):20–29.
- Al-Sunafi SMY. 2016. Pharmacognostical study of Rumex nervosus Vahl. family (Polygonaceae) growing in Yemen [Master theses]. Cairo University.
- Asad M, Getachew A, Ahmad M. 2004. Antidiarrheal activity of methanolic extract of Rumex nervosus. J Pharm Res. 3(4):67–69.
- Balta I, Marcu A, Linton M, Kelly C, Stef L, Pet I, Ward P, Pircalabioru GG, Chifiriuc C, Gundogdu O. 2021. The in vitro and in vivo anti-virulent effect of organic acid mixtures against Eimeria tenella and Eimeria bovis. Sci Rep. 11(1):1–11.
- Barbour E, Ayyash D, Iyer A, Harakeh S, Kumosani T. 2015. A review of approaches targeting the replacement of coccidiostat application in poultry production. Rev Bras Cienc Avic. 17(4):405–418.
- Behnamifar A, Rahimi S, Kiaei M, Fayazi H. 2019. Comparison of the effect of probiotic, prebiotic, salinomycin and vaccine in control of coccidiosis in broiler chickens. Iran J Vet Res. 20(1):51–54.
- Chauhan S, Singh V, Thakur V. 2017. Effect of Calotropis procera (madar) and amprolium supplementation on parasitological parameters of broilers during mixed Eimeria species infection. Vet World. 10(8):864–868.
- Cheng H, Zhang H, Xu G, Peng J, Wang Z, Sun B, Aouameur D, Fan Z, Jiang W, Zhou J. 2020. A combinative assembly strategy inspired reversibly borate-bridged polymeric micelles for lesion-specific rapid release of anti-coccidial drugs. Micro Nano Lett. 12(1):1–19.
- Desta KT, Lee WS, Lee SJ, Kim Y-H, Kim G-S, Lee SJ, Kim ST, Abd El-Aty AM, Warda M, Shin H-C, et al. 2016. Antioxidant activities and liquid chromatography with electrospray ionization tandem mass spectrometry characterization and quantification of the polyphenolic contents of Rumex nervosus Vahl leaves and stems. J Sep Science. 39(8):1433–1441.
- Dubey JP. 2019. Coccidiosis in livestock. In: Poultry, companion animals and humans. CRC Press. 1st ed. Taylor & Francis Group. Boca Raton, London New York. FL 33487- 274. 1-394.
- El-Hack MEA, Alagawany M, Abdel-Moneim A-ME, Mohammed NG, Khafaga AF, Bin-Jumah M, Othman SI, Allam AA, Elnesr SS. 2020. Cinnamon (Cinnamomum zeylanicum) oil as a potential alternative to antibiotics in poultry. Antibiotics. 9(5):210.
- El-Shall NA, Abd El-Hack ME, Albaqami NM, Khafaga AF, Taha AE, Swelum AA, El-Saadony MT, Salem HM, El-Tahan AM, AbuQamar SF, et al. 2022. Phytochemical control of poultry coccidiosis: a review. Poult Sci. 101(1):101542.
- Felici M, Tugnoli B, Piva A, Grilli E. 2021. In vitro assessment of anticoccidials: methods and molecules. Animals. 11(7):1962.
- Galli GM, Petrolli TG, Aniecevski E, Santo AD, Leite F, Griss LG, Dazuk V, Boiago MM, dos Santos HV, Simões CADP, et al. 2021. Phytogenic blend protective effects against microbes but affects health and production in broilers. Microb Pathog. 152:104590.
- Gende LB, Floris I, Fritz R, Eguaras MJ. 2008. Antimicrobial activity of cinnamon (Cinnamomum zeylanicum) essential oil and its main components against Paenibacillus larvae from Argentine. Bull Insectol. 61(1):1.
- Jones K, Garcia G. 2021. Teaching of veterinary parasitology to university students in Trinidad, West Indies; are our local wildlife species neglected? Braz J Biol. 83:1–10.
- Kayande N, Kushwah P, Vir D. 2014. Evaluation of anti-diarrheal potential of cinnamon leaves. PharmaTutor. 2(5):124–127.
- Khaki A, Khaki AA, Hajhosseini L, Golzar FS, Ainehchi N. 2014. The anti-oxidant effects of ginger and cinnamon on spermatogenesis dys-function of diabetes rats. Afr J Trad Compl Alt Med. 11(4):1–8.
- Khater HF, Ziam H, Abbas A, Abbas RZ, Raza MA, Selim A. 2020. Avian coccidiosis: recent advances in alternative control strategies and vaccine development. Agrobiol Rec. 1:11–25.
- Kumar M, Kumar V, Roy D, Kushwaha R, Vaswani S. 2014. Application of herbal feed additives in animal nutrition–a review. Int J Livest Res. 4(9):1–8.
- Lan L, Zuo B, Ding H, Huang Y, Chen X, Du A. 2016. Anticoccidial evaluation of a traditional chinese medicine—Brucea javanica—in broilers. Poult Sci. 95(4):811–818.
- Lee H-A, Hong S, Chung Y-H, Song K-D, Kim O. 2012. Anticoccidial effects of Galla rhois extract on Eimeria tenella-infected chicken. Lab Anim Res. 28(3):193–197.
- Mehlhorn H. 2014. Encyclopedic reference of parasitology. 4th ed. Berlin: Springer.
- Melesse A, Masebo M, Abebe A. 2018. The substitution effect of Noug seed (Guizotia abyssinica) cake with cassava leaf (Manihot Escutulata C.) meal on feed intake, growth performance, and carcass traits in broiler chickens. Res J Anim Husb Dairy Sci. 2(2):1–9.
- Noack S, Chapman HD, Selzer PM. 2019. Anticoccidial drugs of the livestock industry. Parasitol Res. 118(7):2009–2026.
- Quradha MM, Khan R, Rehman M, Abohajeb A. 2018. Chemical composition and in vitro anticancer, antimicrobial and antioxidant activities of essential oil and methanol extract from Rumex nervosus. Nat Prod Res. 33(17):1–6.
- Rao PV, Gan SH. 2014. Cinnamon: a multifaceted medicinal plant. Evid Based Complement Alternat Med. 2014:1–12.
- SAS. 2012. SAS Institute/OR 9.3 user's guide: mathematical programming examples. SAS Institute.
- Shankar PR, Balasubramanium R. 2014. Antimicrobial resistance: global report on surveillance 2014. Australas Med J. 7(5):237–238.
- Shetshak M, Suleiman M, Jatau I, Ameh M, Akefe I. 2021. Anticoccidial efficacy of Garcinia kola (Heckel H.) against experimental Eimeria tenella infection in chicks. J Parasit Dis. 45(4):1034–1048.
- Tanweer AJ, Chand N, Saddique U, Bailey CA, Khan RU. 2014. Antiparasitic effect of wild rue (Peganum harmala L.) against experimentally induced coccidiosis in broiler chicks. Parasitol Res. 113(8):2951–2960.
- Thangavel G, Mukkalil R, Chirakkal H. 2020. Plant parts and extracts having anticoccidial activity. Google Patents.
- Tonda R, Rubach J, Lumpkins B, Mathis G, Poss M. 2018. Effects of tannic acid extract on performance and intestinal health of broiler chickens following coccidiosis vaccination and/or a mixed-species Eimeria challenge. Poult Sci. 97(9):3031–3042.
- Upadhaya SD, Cho SH, Chung TK, Kim IH. 2019. Anti-coccidial effect of essential oil blends and vitamin D on broiler chickens vaccinated with purified mixture of coccidian oocyst from Eimeria tenella and Eimeria maxima. Poult Sci. 98(7):2919–2926.
- Yang C, Kennes YM, Lepp D, Yin X, Wang Q, Yu H, Yang C, Gong J, Diarra MS. 2020. Effects of encapsulated cinnamaldehyde and citral on the performance and cecal microbiota of broilers vaccinated or not vaccinated against coccidiosis. Poult Sci. 99(2):936–948.
