Abstract
The purpose of this experiment was to study the effects of oregano essential oil (OEO) on the performance, intestinal morphology and intestinal flora of fattening Sewa sheep. Sixty 20-month-old Sewa sheep with similar body weights (BWs) and in good health were randomly divided into four groups. The groups were fed a diet containing 0 mg/kg OEO (group A), 150 mg/kg (group B), 300 mg/kg (group C) or 450 mg/kg (group D). Daily gain, slaughter rate and other production performance parameters were analysed in the test sheep (mean ± standard error). Intestinal morphology was analysed by tissue sectioning, and intestinal flora was analysed by 16S rRNA genome sequencing. The indexes, including average daily gain (ADG), slaughter rate, villus length of the small intestine and number of beneficial bacteria in the intestinal flora, in group C were significantly higher than those in the other groups. Supplementation with 300 mg/kg OEO concentrate in the diets can improve the growth performance of Sewa sheep by changing their intestinal morphology and modifying their intestinal flora structure. Conclusively, these encourage to further verifying in practice positive evidence in improving growth performance of OEO supplementation in the diets for Sewa sheep.
Oregano essential oil (OEO) can improve the performance of Sewa sheep by changing the intestinal morphology and regulating the intestinal flora structure.
Highlights
Introduction
Oregano essential oil (OEO) is globally recognised as a natural feed additive that can promote the growth of livestock and poultry, enhance host health (Zeng et al. Citation2015) and improve the structure of intestinal flora (Allen et al. Citation2011; Yan et al. Citation2017). It can also improve the quality of pork to a certain extent (Hector et al. Citation2019) and improve the performance and intestinal microflora structure of laying hens (Ri et al. Citation2017). The antioxidant and bactericidal effects of OEO have been well documented in experiments in various animals (Gopal and Asmita Citation2014). The main phenolic components in OEO can effectively treat bacterial diseases in the digestive tracts of livestock and poultry, especially those caused by intestinal Escherichia coli, the curative effect on diseases caused by Salmonella is greatest. OEO can also be used to prevent coccidiosis (Paulina et al. Citation2018). OEO is an antibacterial, growth-promoting additive approved by the Ministry of Agriculture of China. It is prepared as a 10% premix and used as a feed additive. It can promote the growth of animals, promote the digestion and absorption of nutrients, improve the utilisation rate of feed and have a good fattening effect on animals (Alagawany et al. Citation2018). OEO can be used as a nutritional feed additive for ruminants to improve the performance and feed efficiency of dairy cows and meat animals. OEO can improve the performance of chickens (Botsoglou et al. Citation2002; Amad et al. Citation2013; Peng et al. Citation2016; Ramirez et al. Citation2021; Zhang et al. Citation2021;Roofchaee et al., 2011), pigs (Forte et al. Citation2017; Liang et al. Citation2017; Cheng et al. Citation2018; Deng et al. Citation2018;Jugl-Chizzola et al., 2006), and rabbits (Botsoglou et al. Citation2004) to achieve the effect of fattening animals. On the Qinghai-Tibet Plateau, the oxygen content is low. This experiment was conducted to compare the effects of dietary OEO supplementation on performance, intestinal morphology and intestinal microorganism of Sewa sheep. An experiment was carried out in Naqu, Tibet, which has an average elevation of approximately 4800 m. The region experiences long-term hypoxic conditions, a large temperature difference between day and night, and a windy and dry climate that persists throughout the year. However, the effects of OEO as a concentrated supplement on the performance, intestinal flora and microflora structure of Sewa sheep are await further studies.
In this study, we hypothesised that dietary inclusion of OEO would positively alter the villi length and intestinal morphological structure of the ileum, jejunum and duodenum. Therefore, this study aimed to investigate the effects of dietary OEO supplementation on the growth performance.
The purpose of this experiment was to study the effects of OEO supplemented with 0 mg/kg (group A), 150 mg/kg (group B), 300 mg/kg (Group C) and 450 mg/kg (Group D) on the growth performance, slaughter performance, intestinal morphology and intestinal flora of Sewa sheep. Daily weight gain, slaughter rate and production performance of Sewa sheep were analysed. Additionally, the intestinal morphology of Sewa sheep was analysed, and the intestinal flora was analysed by 16S rRNA genome sequencing. Analyses were conducted to explore the effects of OEO on the growth performance, slaughter performance, intestinal morphology and intestinal flora of fattening Sewa sheep; to provide strong support for the fattening of Sewa sheep; and to promote the development of the Sewa sheep industry.
Materials and methods
Animal care
All of the methods used in this study comply with the standards of the institutional guidelines for ethics in animal experimentation (Rule number 86/609/EEC-24/11/86), and all experimental procedures were approved by the Institutional Animal Care and Use Committee of Tibet Agricultural and Animal Husbandry University (Linzhi, China). The institutional certification number is 12540000MB0P013721.
The OEO was purchased from Jiangxi Tianjia Biotechnology Co., LTD., Nanchang, Jiangxi province, China. OEO is the volatile oil component of oregano, a traditional medicine plant (Attia et al. Citation2017). The effective ingredients of OEO are carvacrol and thymol. The carrier materials include gelatine, sucrose, starch and dextrin. The concentration of carvacrol and thymol in OEO determined by high-performance liquid chromatography (HPLC) was ≥5.5% and ≥0.15%, respectively, in the final OEO purity of 5.65%.
The experiment was conducted at the Sewa Sheep Fattening Base Farm, in Duoerge Village, Pubao Town, Bange County, Naqu City, Tibet Autonomous Region (90°01 63.64 N,31°39 86.41 E), at an altitude of about 4800 m. In this study, compared with indoor feeding on the Tibetan Plateau, grazing sheep had to endure poor weather and walk long distances climb high mountains for various feedstuff under grazing circumstances. Sixty 20-month-old Sewa sheep with similar body weights (BWs) and in good health were randomly divided into four groups. Each group was fed a diet containing 0 mg/kg (OEO, group A), 150 (Group B), 300 (Group C) or 450 (Group D), with three replicates per group and five sheep per replicate. The sheep grazed in the morning and were supplemented with the fodder in the afternoon. Experimental animals were fed in indoor regimes twice a day at 9:00 and 17:00 h and grazed from 12:00 to 16:00 h, respectively, every day. Sheep were adaptive to the experiment diets for 10 d and then fed for 60 d. OEO was sprayed on a carrier and dilute to appropriate amount with carrier step by step, and then stir evenly in premix, then added to the diet. All the experimental diets were prepared every week, packed in covered containers and stored in a dry and well-ventilated storeroom. The basic diet was comprised of pellet feed, which was provided in a semi-open house. The basal diet composition and nutrient levels are shown in the Supplementary information (Table S1) which was formulated to meet the nutritional requirements for growing sheep for 100 g weight gain per day, and the fed amount was adjusted according to the sheep BW. The concentration of digestive energy was calculated from the ingredient values based on the feeding standard for meat-producing sheep and goats in accordance with the China Agricultural Standard (NY/T816, 2004).
Table 1. Effects of OEO on the growth performance of Sewa sheep.
Growth performance
All sheep remained in good health and medical intervention was not applied to any sheep during the whole feeding period. The experimental sheep were weighed with an empty stomach in the morning on the sixtieth day of the formal study period, during which the daily feed intake was measured. The growth performance data of each group, such as the average daily gain (ADG), dry matter intake (DMI), feed-to-gain ratio (F/G) were calculated.
Slaughter performance
At the end of the formal study period, five Sewa sheep were randomly selected from each of the four groups for slaughter. Prior to slaughtering, the sheep were fasted for 24 h, and stopped drinking water for 2 h by electrically stunning and exsanguinating were withheld for 24 and 2 h, respectively, before slaughter. The carcase weight, eye muscle area, and slaughter rate were measured immediately after slaughter. The specific determination method of slaughter performance was carried out according to Wang and Tao (Citation2008).
Determination of the morphology and structure of the small intestine in Sewa sheep
The jejunum, ileum and duodenum of three sheep in each experimental group were removed, and three samples from each intestinal segment were obtained. After removal, sections were prepared to observe the height of the villi, depth of the crypts and thickness of the muscle layer. Tissue blocks fixed with 10% formaldehyde were embedded in paraffin to prepare the sections. After haematoxylin-eosin (H.E.) staining, the villus height and crypt depth of the intestine were measured with Image-Pro Plus 6.0 software is (Media Cybernetics, Inc. 1700 Rockville Pike, Suite 240, Rockville, Maryland USA 20852.), and the ratio of the villus height to the crypt height (V/C) was calculated. The specific method with which to assess small intestinal morphology was carried out according to Lu et al. (Citation2020).
Analysis of intestinal flora in Sewa sheep
At the end of the experiment, sheep were slaughtered after 60 d of experimental feeding. Immediately after slaughter, the contents of the small intestine were removed with surgical scissors that had been disinfected with alcohol and were placed in marked tubes, which were then stored in liquid nitrogen and then preserved at −80 °C until intestinal flora analysis. Shanghai Personal Biotechnology Co., Ltd. (Shanghai, China) for the 16S rRNA genome sequencing of the intestinal flora. The specific method of intestinal microflora determination was carried out according to Cheng et al. (Citation2018) and Li et al. (Citation2021).
Statistical analysis
The test data were analysed and sorted in Excel, and one-way ANOVA was performed with SPSS version 19.0 (SPSS Institute Inc., Chicago, IL). ANOVA was used for the statistical analysis, and Tukey’s multiple comparison test was used to analyse the results. The test results are expressed as the means ± standard errors to determine the significance of differences. p < .05 was considered statistically significant.
Results
Effects of OEO on the growth performance of Sewa sheep
The effects of OEO on the growth performance of Sewa sheep are shown in Table . Table shows that the ADG values in the groups supplemented with OEO (groups C and D) were significantly higher than that of the group A (p < .05), and the ADG in group C was the highest. In the four experimental groups, the lower the F/G ratio was, the better the feed performance and the more effective the feed was in the experimental sheep. Among the four groups, the F/G ratios in the OEO supplementation groups (groups B–D) were significantly lower than those in group A (p < .05), and the F/G ratio in group C was significantly different from that of group A (p < .05). The total weight gain in groups B–D was significantly higher than that in group A (p < .05), the total weight gain in group C was the highest, but there was no significant difference between groups C and D (p > .05). Among the four groups, there was no significant difference in DMI between group A and group B (p > .05). The DMI of groups C and D was significantly different from that of groups A and B, but there was no significant difference in the DMI between groups C and D (p > .05). There was no significant difference between group C and group D (p > .05), considering economic benefit, group C had the best weight gain effect.
Effect of OEO on the slaughter performance of Sewa sheep
As shown in Table , the carcase weights of the OEO groups were significantly higher than those of group A (p < .05). The carcase weight and eye muscle area of groups B–D were significantly higher than those of group A (p < .05). Although the carcase weight and eye muscle area in group C were the highest, there were no significant differences among the three OEO groups (p > .05). The slaughter rate of OEO group was higher than that of group A, but the slaughter rate of group C was the highest. There was no significant difference in slaughter rate among groups B–D (p > .05). It can be concluded that adding OEO to the diet can improve the slaughter performance of Sewa sheep.
Table 2. Effects of OEO on the slaughter performance of Sewa sheep.
Effects of OEO on the morphology and structure of the small intestine of Sewa sheep
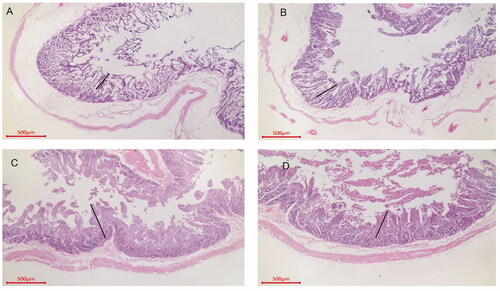
As shown in Table S2 (Supplementary material) and Figures , the villus lengths in the ileum, duodenum and jejunum in the group C were all higher than those in group A, and the differences were significant for the duodenum and jejunum (p < .05). The villus length in group C was the longest among those in the OEO groups, and the difference was significant (p < .05). The V/C ratios in the ileum, duodenum and jejunum were higher in the OEO groups than in group A, and those in group C were significantly different from those in the other three groups with respect to various intestinal segments (p < .05). There were significant differences in the V/C ratio in the ileum between group C and group A (p < .05) and in the ileum and duodenum between group C and group A (p < .05).
Figure 1. Ileum sections from each group of Sewa sheep. Ileum group A, H. E, 40×, Bar = 500 μm. Villus length (200.2), crypt depth (154.3) and muscle layer thickness (91.2). Ileum group B, H. E, 40×, Bar = 500 μm. Villus length (242.4), crypt depth (121.2) and muscle layer thickness (120.4). Ileum group C, H. E, 40×, Bar = 500 μm. Villus length (650.2), crypt depth (161.2) and muscle layer thickness (232.3). Ileum group D, H. E, 40×, Bar = 500 μm. Villus length (230.2), crypt depth (117.5) and muscle layer thickness (109.3).
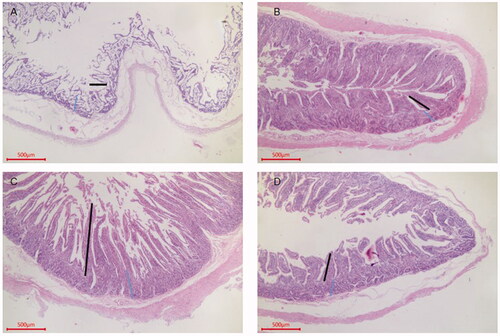
Figure 2. Intestinal sections of the jejuna of Sewa sheep. Jejunum from group A, H. E, 40×, Bar = 500 μm. Villus length (423.5), crypt depth (202.7) and muscle layer thickness (142.3). Jejunum from group B, H. E, 40×, Bar = 500 μm. Villus length (474.2), crypt depth (177.3) and muscle layer thickness (188.9). Jejunum from group C, H. E, 40×, Bar = 500 μm. Villus length (698.4), crypt depth (145.6) and muscle layer thickness (212.3). Jejunum from group D, H. E, 40×, Bar = 500 μm. Villus length (459.3), crypt depth (172.3) and muscle layer thickness (162.3).
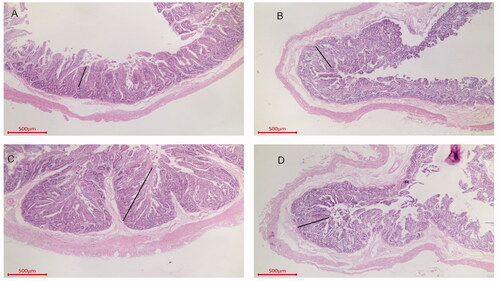
Figure 3. Duodenal sections from each group of Sewa sheep. Group A, H. E, 40×, Bar = 500 μm. Villus length (248.3), crypt depth (133.5) and muscle layer thickness (81.3). Group B, H. E, 40×, Bar = 500 μm. Villus length (322.3), crypt depth (240.3) and muscle layer thickness (87.3). Group C, H. E, 40×, Bar = 500 μm. Villus length (578.5), crypt depth (171.2) and muscle layer thickness (182.8). Group D, H. E, 40×, Bar = 500 μm. Villus length (562.3), crypt depth (252.3) and muscle layer thickness (142.3).
Effects of OEO on the intestinal flora of Sewa sheep
As shown in Table and Figure , the rarefaction curve gradually stabilises, indicating that under reasonable and effective sample volumes. At the phylum level, there was no significant difference between the OEO groups and group A (p > .05), and the number of bacteria in group C was the highest. At the class level, The value in OEO group B was higher than those in OEO groups C and D, and there was a significant difference between OEO groups A and B (p < .05); there was no significant difference between OEO groups C and D (p > .05). At the order level, the values in the OEO groups were higher than those in group A. The value in OEO group A was less than those in OEO groups B and C, and there was a significant difference between OEO groups C and D (p < .05). There was no significant difference between OEO groups D and A (p > .05). At the family level, the values in OEO groups B and D were significantly higher than those in group A (p < .05). There was a significant difference between OEO groups A and D (p < .05), and there was no significant difference between OEO groups A and C (p > .05). At the genus level, the values in each OEO group were higher than those in group A. There was a significant difference between the three OEO groups compared with group A (p < .05). At the species level, the value of the OEO group was lower than that of group A, and the value of OEO group B was higher than that of OEO groups C and D. There was a significant difference between OEO groups A and C (p < .05), and there was a significant difference between oregano oil groups C and D (p < .05).
Figure 4. Rarefaction curves related to intestinal flora. CD1, CD2 and CD4 represent group A (0 mg/kg OEO supplemented); CA2 represents group B (150 mg/kg OEO supplemented); CC7, CC9 and CC10 represent group C (300 mg/kg OEO supplemented); and CB5, CB6 and CB8 represent group D (450 mg/kg OEO supplemented).
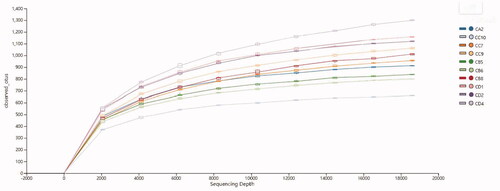
Table 3. Statistical table for the number of microbialbacteria at each level.
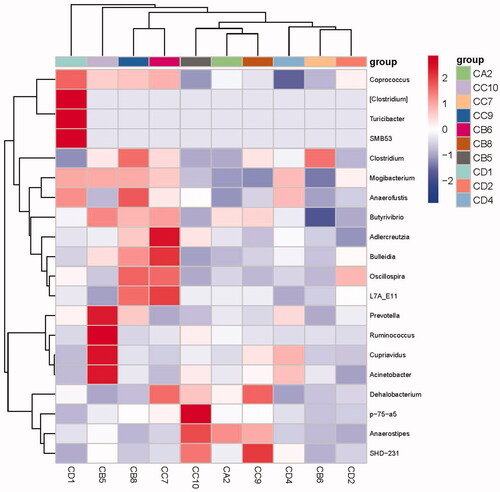
As shown in Figure , the top 20 genera in the figure were selected for analysis. Among the OEO groups, compared with group A, coprococcus, clostridium, anaerofustis, butyrivibrio, adlercreutzia, bulleidia, oscillospira, 17 A-E11, prevotella, ruminococcus, cupriavidus, acinetobacter, dehalobacterium, P-75-A5 and anaerostipes had higher abundance values. The abundances of prevotella, ruminococcus, cupriavidus and acinetobacter were significantly increased in group C. The abundances of P-75-A5, adlercreutzia, L7A-E11 and bulleidia were significantly increased in the OEO D group. The figure shows that OEO can improve the richness of intestinal flora and improve the intestinal flora structure.
Figure 5. Heat map of samples showing the species composition. In the figure, red blocks indicate that the richness of that genus was higher than that of other genera in the same sample, while blue blocks indicate lower genus richness in the sample. CD1, CD2 and CD4 = group A (0 mg/kg OEO supplemented); CA2 = group B (150 mg/kg OEO supplemented); CC7, CC9 and CC10 = group C (300 mg/kg OEO supplemented); and CB5, CB6 and CB8 = group D (450 mg/kg OEO supplemented).
Discussion
Effects of OEO on the growth performance of Sewa sheep
Daily gain is often used to express the growth rate of livestock and is an important index to reflect the growth performance and feed utilisation rate of livestock. Zhang Shanzhi and others found that adding OEO to the diet can reduce heat stress, improve feed intake in beef cattle, promote growth, prevent diarrhoea and increase the economic benefits of beef cattle fattening in summer (Zhang Citation2011). Halle found that adding plant extracts or OEO into diets can improve the production performance and meat quality of pigs (Halle et al. Citation2001). Dietary addition of OEO can also effectively delay microbial growth in rabbits during 7 d of cold storage of the carcase (Soultos et al. Citation2009). The main OEO compounds, such as carvacrol and thymol, have anticoccidial action against Eimeria tenella (Giannenas et al. Citation2018). Such as the phenolics can be absorbed in the intestine and enter the egg yolk through blood circulation of the hen, providing their superior antioxidant properties to the egg (Giannenas et al. Citation2018). In this experiment, The results showed that the ADG values in groups B, C and D were significantly higher than that in group A (p< .05), and the ADG in group C was the highest. The ADG in group C was significant, indicating that OEO can improve the daily gain in Sewa sheep; these results are similar to those obtained in sheep (Chu Citation2019), chickens (Botsoglou et al. Citation2002; Amad et al. Citation2013; Peng et al. Citation2016; [AQ2]; Ramirez et al. Citation2021; Zhang et al. Citation2021), pigs (Forte et al. Citation2017; Liang et al. Citation2017; Cheng et al. Citation2018; Deng et al. Citation2018) and rabbits (Botsoglou et al. Citation2004). The total weight gain of groups B, C and D was significantly higher than that of group A (p< .05), and group C had the greatest total weight gain. The ratio of feed to gain in group C was significantly different from that in group A (p< .05). In the four groups, there was no significant difference in DMI between group A and group B (p> .05), and there was no significant difference in DMI between group C and group D (p> .05). However, the DMI of group A was significantly different from that of group C and D. In summary, OEO can improve the growth performance of Sewa sheep in Tibet, and the effect of 300 mg/kg OEO in group C was the best.
Effect of OEO on the slaughter performance of Sewa sheep
Slaughter performance is an important reference for the slaughtering value of mutton sheep. The slaughter rate is an important index reflecting the slaughtering performance of animals. The eye muscle area reflects the development degree of animal carcases and has an important impact on the economic benefits of breeding (Cai Citation2006; Alvarez-Rodriguez et al. Citation2007). Jing Hui (Jiang et al., 2018) stated that dietary OEO can improve daily gain in and the carcase weight of Hexi goats and can significantly improve their fattening performance. In this study, we found: the carcase weight, eye muscle area, and slaughter rate, reflecting slaughter performance, were significantly higher in the OEO groups than in group A, but the differences among the OEO groups were not significant (p> .05). However, the values of these parameters in group C were the highest. Therefore, adding OEO to the diet can improve slaughter performance, and adding OEO at 300 mg/kg has the best fattening effect.
Effects of OEO on the morphology and structure of the small intestine of Sewa sheep
The phenolics can be absorbed in the intestine and enter the egg yolk through blood circulation of the hen, providing their superior antioxidant properties to the egg (Giannenas et al. Citation2018). The small intestine is the main site of nutrient absorption in animals and plays a major role in animal growth (Botsoglou et al. Citation2004). Studies have shown that the shortening of villi in the small intestine affects the number of absorbing cells per villus, reduces digestion and absorption capabilities, and reduces animal productivity (Xue Citation2011). In this experiment, OEO was added to feed as microcapsules with two layers of encapsulation structure coating for sustaining targeted release in the intestinal tract. The comprehensive ability of the small intestine to absorb nutrients in feed depends on the V/C ratio, and the ratio is directly correlated with the absorption of nutrients which OEO can enhance in the small intestine of Sewa sheep. In this experiment, in the ileum, duodenum, and jejunum, the V/C ratios were significantly higher in the OEO groups than in group A, which illustrates that OEO can improve the small intestinal structure of Sewa sheep and improve the ability of the small intestine to absorb nutrients. These results are similar to those found for chicken (Han Citation2013) and piglet diets supplemented with OEO (Li Citation2018). Clearly, adding OEO to the diet enhances the digestion and absorption of nutrients in the small intestine.
Effects of OEO on the intestinal flora of Sewa sheep
Dietary addition of OEO can also effectively delay microbial growth in rabbits during 7 d of cold storage of the carcase (Soultos et al. Citation2009). Experiments have shown that adding OEO to the diet can improve animal growth performance, reduce local oxidative stress, improve the production of natural antibodies and have a good regulatory effect on the composition of intestinal flora (Hall et al. Citation2021; Ruan et al. Citation2021). It can be seen from the test data that OEO increased the richness in terms of some bacterial genera, such as Coprococcus (faecal coccus), Clostridium and Acetobacter, all of which were significantly increased in this study. With an increase in richness, the digestive enzymes secreted by different microorganisms increase, which is conducive to absorption in the intestinal tract and the utilisation of nutrients (Zhou et al. Citation2019; Su et al. Citation2021). In this experiment, the richness of the intestinal flora was most significant in OEO group C, corresponding to the greatest improvement in digestion and absorption, which is in accordance with the production performance and other experimental data obtained in this study.
Conclusions
In conclusion, the results demonstrate that add of 300 mg/kg OEO (purity of OEO was 5.65%) in Sewa sheep have a positive effect on fattening performance which may be the result of the digestion and absorption of nutrients in the small intestine by regulating intestinal morphology and intestinal microorganisms. Thus, the finding of this study can provide reference information on feed additives improving the production application of OEO in the fattening sheep improving the production performance of Sewa sheep. This study provides a useful animal model for studying the growth-promoting effect of OEO in the Qinghai-Tibet Plateau.
Supplemental Material
Download MS Word (16 KB)Supplemental Material
Download MS Word (48.2 KB)Acknowledgements
The authors thank AJE (www.AJE.cn) for its linguistic assistance during the preparation of this manuscript.Ethics approval and consent to participate All the methods used in this study comply with the standards of the institutional guideline for ethics in animal experimentation (Rule number 86/609/EEC-24/11/86), and all experimental procedures were approved by the Institutional Animal Care and Use Committee of Tibet Agricultural and Animal Husbandry University. The institutional certification number is 12540000MB0P013721.
Disclosure statement
No potential conflict of interest was reported by the authors. Conceptualisation, J.S. and Z.R.; Data curation, Z.C.; Formal analysis, J.S.; Investigation, J.S., Z.C., Y.Z., Y.W., H.W. and Z.R.; Writing-original draft, J.S.; Writing-review and editing, J.S. and Z.R. All authors read and approved the final manuscript.
Data availability statement
All data generated or analysed during this study are included in this article and its supplementary information files.
Additional information
Funding
References
- Alagawany M, Abd El-Hack ME, Farag MR, Shaheen HM, Abdel-Latif MA, Noreldin AE, Patra AK. 2018. The usefulness of oregano and its derivatives in poultry nutrition. World’s Poult Sci J. 74(3):463–474.
- Allen HK, Looft T, Bayles DO, Humphrey S, Levine UY, David A, Stanton TB. 2011. Antibiotics in feed induce prophages in swine fecal microbiomes. mBio. 2(6):174–232.
- Alvarez-Rodriguez J, Sanz A, Delfa R, Revilla R, Joy M. 2007. Performance and grazing behaviour of Churra Tensina sheep stocked under different management systems during lactation on Spanish mountain pastures. Livest Sci. 107(2–3):152–161.
- Amad AA, Wendler KR, Zentek J. 2013. Effects of a phytogenic feed additive on growth performance, selected blood criteria and jejunal morphology in broiler chickens. Emir J Food Agric. 25(7):549–554.
- Attia YA, Bakhashwain AA, Bertu NK. 2017. Thyme oil (Thyme vulgaris L.) as a natural growth promoter for broiler chickens reared under hot climate. Ital J Anim Sci. 16(2):275–282.
- Botsoglou NA, Florou-Paneri P, Christaki E, Fletouris DJ, Spais AB. 2002. Effect of dietary oregano essential oil on performance of chickens and on iron-induced lipid oxidation of breast, thigh and abdominal fat tissues. Br Poult Sci. 43(2):223–230.
- Botsoglou NA, Florou-Paneri P, Christaki E, Giannenas I, Spais AB. 2004. Performance of rabbits and oxidative stability of muscle tissues as affected by dietary supplementation with oregano essential oil. Arch Anim Nutr. 58(3):209–218.
- Cai JS. 2006. Nutrient requirement of meat sheep. Chin Anim Husbandry Assoc. 5:272–276.
- Cheng CS, Xia M, Zhang XM, Wang C, Jiang SW, Peng J. 2018. Supplementing oregano essential oil in a reduced protein diet improves growth performance and nutrient digestibility by modulating intestinal bacteria, intestinal morphology, and antioxidative capacity of growing-finishing pigs. Animals. 8(9):159.
- Chu HP. 2019. Effects of oregano oil on growth performance, slaughter performance and meat quality of Jining green goats. Feed Ind. 40(3):15–18.
- Deng QF, Liu ZQ, Fan JX, Zhang Y, Xiao SH. 2018. Research progress of oregano oil and its application in pig production. China’s Pig Ind. 13(2):41–46.
- Forte C, Ranucci D, Beghelli D, Branciari R, Acuti G, Todini L, Cavallucci C, Trabalza M. 2017. Dietary integration with oregano (Origanum vulgare L.) essential oil improves growth rate and oxidative status in outdoor-reared, but not indoor-reared, pigs. J Anim Physiol Anim Nutr (Berl). 101(5):e352– 361.
- Giannenas I, Bonos E, Christaki E, Florou-Paneri P. 2018.
- Therapeutic Foods.Academic Press. Chapter 6, Oregano: A Feed Additive With Functional Properties;179-208.
- Gopal K, Asmita N. 2014. Use of essential oils in poultry nutrition: a new approach. J Adv Vet Anim Res. 1(4):156–162.
- Hall HN, Wilkinson DJ, Le BM. 2021. Oregano essential oil improves piglet health and performance through maternal feeding and is associated with changes in the gut microbiota. Anim Microbiome. 3(1):2–2.
- Halle I, Schubert R, Flachowsky G, Jahreis G, Bitsch R. 2001. Effects of essential oils and herbal mixtures on the growth of broiler chicks. Rims Kokyuroku. 1220(1220):63–77.
- Han X. 2013. Effects of oregano oil on intestinal digestion and absorption of laying hens [master’s thesis]. Daqing, China: Heilongjiang Bayi Agricultural University.
- Hector JV, Esmeralda PG, Alma D, Alarcon R, Juan OG, Nelson A. 2019. Determination of carcase yield, sensory and acceptance of meat from male and female pigs with dietary supplementation of oregano essential oils. Ital J Anim Sci. 18(1):668–678.
- Jiang H, Lei ZM, Jiao T. 2018. Effects of oregano oil on fending performance of Hexi Cashmere goats. J Grass Ind. 27(2):142–149.
- Jugl-Chizzola M, Ungerhofer E, Gabler C, Hagmüller W, Chizzola R, Zitterl-Eglseer K, Franz C. 2006. Testing of the palatability of thymus vulgaris l. and origanum vulgare l. as flavouring feed additive for weaner pigs on the basis of a choice experiment. Berl Munch Tierarztl Wochenschr. 119(5–6):238–243.
- Li CY, Niu JL, Liu YX, Li FC, Lei LH. 2021. The effects of oregano essential oil on production performance and intestinal barrier function in growing Hyla rabbits. Ital J Anim Sci. 20(1):2165–2173.
- Liang MM, Liu K, Huang WJ, Weng YM, Su QC. 2017. Effects of oregano oil, Acme and enteric antibiotic substitution on performance of nursery pigs. Feed Research. 40(1):21–23.
- Li LH. 2018. Effects of substitution of acidifier and oregano oil for Myxistin sulfate in weaned piglets [master’s thesis]. Nanchang, China: Jiangxi Agricultural University.
- Lu J, Zhu DX, Lu PF, Zhang DH, Xu XQ. 2020. Effects of probiotics on small intestinal morphology and digestive enzyme activities in puppies. Heilongjiang Animal Science and Veterinary Medicine. 63(2):132–134.
- Paulina D, Andrzej JK, Wiktor B, Michal M, Klaudiusz S, Scouarnec JL, Schmidová J, Krzysztof T, Grzybek M. 2018. Effect of dietary supplementation with preparation comprising the blend of essential oil from Origanumvulgare (lamiaceae) and Citrus spp. (citraceae) on coccidia invasion and lamb growth. Ital J Anim Sci. 17(1):57–65.
- Peng QY, Li JD, Li Z, Duan ZY, Wu YP. 2016. Effects of dietary supplementation with oregano essential oil on growth performance, carcass traits and jejunal morphology in 427 broiler chickens. Anim Feed Sci Technol. 214:148–153.
- Ramirez SY, Peñuela-Sierra LM, Ospina MA. 2021. Effects of oregano (Lippia origanoides) essential oil supplementation on the performance, egg quality, and intestinal morphometry of Isa Brown laying hens. Vet World. 14(3):595–602.
- Ri CS, Jiang XR, Kim MH, Wang J, Zhang HJ, Wu SG, Valentino B, Qi GH. 2017. Effects of dietary oregano powder supplementation on the growth performance antioxidant status and meat quality of broiler chicks. Ital J Anim Sci. 16(2):246–252.
- Roofchaee A, Irani M, Ebrahimzadeh MA, Akbari MR. 2011. Effect of dietary oregano (Origanum vulgare L.) essential oil on growth performance, cecal microflora and serum antioxidant activity of broiler chickens. Afr J Biotechnol. 10(32):6177–6183.
- Ruan D, Fan Q, Fouad AM, Sun Y, Huang S, Wu A, Lin C, Kuang Z, Zhang C, Jiang S. 2021. Effects of dietary oregano essential oil supplementation on growth performance, intestinal antioxidative capacity, immunity and intestinal microbiota in yellow-feathered chickens. J Anim Sci. 99(2):033.
- Soultos N, Tzikas Z, Christak E, Papageorgiou K, Steris V. 2009. The effect of dietary oregano essential oil on microbial growth of rabbit carcasses during refrigerated storage. Meat Sci. 81(3):474–478.
- Su GQ, Wang L, Zhou XW, Wu XY, Chen DW, Yu B, Huang ZQ, Luo YH, Mao XB, Zheng P, et al. 2021. Effects of essential oil on growth performance, digestibility, immunity and intestinal health in broilers. Poultr Sci. 100(8):101242–101242.
- Wang ML, Tao ST. 2008. Comparative study on meat Productivity and meat Quality of F1 lambs and Lambs of Small-tailed Han Sheep [master’s thesis]. Urumqi, China: Xinjiang Agricultural University.
- Xue Q. 2011. Developmental studies on mucosal Structure of small intestine and Mucosal immune-related cells of Datong yak [master’s thesis]. Xining, China: Qinghai University.
- Yan W, Sun CJ, Yuan JW, Yang N. 2017. Gut metagenomic analysis reveals prominent roles of Lactobacillus and cecal microbiota in chicken feed efficiency. Sci Rep. 7(1):591–602.
- Zeng ZK, Zhang S, Wang HL, Piao XS. 2015. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: a review. J Anim Sci Biotechnol. 6(1):7.
- Zhang SZ. 2011. Effects of oregano oil supplementation on growth performance of summer beef cattle. Anim Husb Vet Med. 43(7):39–41.
- Zhang LY, Peng QY, Liu YR, Ma QG, Zhang JY, Guo YP, Xue Z, Zhao LH. 2021. Effects of oregano essential oil as an antibiotic growth promoter alternative on growth performance, antioxidant status and intestinal health of broilers. Poultr Sci. 100(7):101163– 101163.
- Zhou R, Wu JP, Zhang LP, Liu LS, David PC, Jiao J, Liu T, Wang JF, Lang X, Song SZ, et al. 2019. Effects of oregano essential oil on the ruminal pH and microbial population of sheep. PLoS One. 14(5):e0217054.
