Abstract
Oxidative stress causes several pathological conditions in farm animals by inducing a reduction in animal welfare and production with major economic losses for livestock. The standardised phytoextract obtained by red orange and lemon processing has antioxidant and anti-inflammatory activities. In this study we have investigated whether treating lambs for 40 days with red orange and lemon extract (RLE) was able to prevent the antioxidant and anti-inflammatory mechanisms, typically in newborn period. For this purpose, at the end of treatment, redox status was assessed in plasma using oxidative stress markers: malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase (CAT) activities. Moreover, 8-hydroxy-2’-deoxyguanosine (8-OHdG) in urine was measured as a biomarker of DNA damage and finally, the markers of inflammatory status, interleukin (IL) 1β and 6, were measured in plasma by using an enzyme-linked immunosorbent assay (ELISA). Results showed that RLE-treated lambs improved antioxidant mechanisms as demonstrated by the increase in SOD, CAT and GPx activities and, furthermore, a significant reduction in oxidative stress-induced damage was demonstrated through MDA and 8-OHdG measurements. Moreover, RLE was also able to prevent the increase of pro-inflammatory cytokines concentration, such as IL- 1 β and IL 6. Results showed in the present paper demonstrate the potential beneficial effects of the RLE in lambs.
Oxidative stress causes economic loss and welfare impairment in livestock.
Several oxidative markers can be used to assess the redox status in plasma.
The activity of some antioxidant enzymes increased in RLE treated lambs.
A reduction of pro-inflammatory cytokines was recorded in RLE treated lambs.
HIGHLIGHTS
Introduction
Free radicals induce oxidative damage, with effects on lipids, proteins, carbohydrates and nucleic acids (Chaudhary et al. Citation1994) and decrease the performances of animal farm (Salzano et al. Citation2021). To reduce the free radical increase and the economic losses in animal productions, antioxidant protective systems are developed because they have an important role in modulating oxidative stress-induced cell injuries by scavenging free radicals (Carocho and Ferreira Citation2013). Synthetic antioxidants, such as tertiary butylhydroquinone, butylated hydroxytoluene and hydroxyanisole are usually used in the food industry but health risks associated with consuming synthetic antioxi-dants, such as teratogenic and carcinogenic effects in laboratory animals and primates (Hathway Citation1966), have led to a growing interest in the use of natural alternatives and their use as additives for livestock is well accepted by consumers because considered safe and healthy natural additives (Kahl and Kappus Citation1993). Polyphenols are the most extensive groups of chemicals in the fruits, vegetables, green and black teas, herbs, red wine, and they are extensively researched for their potential beneficial effects, such as anticancer, anti-inflammatory and degenerative diseases (Egert and Rimbach Citation2011). However, positive effects of polyphenols on intake and antioxidant in vivo defense were not clearly demonstrated (Jin et al. Citation2012). Flavonoids, the most important and the largest group of polyphenols, can be divided into several groups, including anthocyanins, flavones and chalcons (Surai Citation2014). Direct scavenging of reactive oxygen species (ROS), activation of antioxidant enzymes and mitigation of oxidative stress caused by nitric oxide are well demonstrated to be the mechanism of actions of flavonoids to prevent free radicals’ injury (Procházková et al. Citation2011; Damiano et al. Citation2020b). However, emerging research suggest that the potential mechanism of action in preventing disease, is not only linked to their antioxidant activity (Hodgson and Croft Citation2010). Several data in literature show that oxidative stress is directly linked with inflammation (Hiscott et al. Citation1993). If the oxidants production cannot be broken, the inflammatory process becomes chronic and the body’s cells and tissue face damage (De Felice et al. Citation2021). Free radical diseases and inflammatory processes occur not only in humans’ disorders (Ciz et al. Citation2012) but also in farm animals (Lykkesfeldt and Svendsen Citation2007; Gessner et al. Citation2017). Moreover, the effects of systemic inflammation on performance of several farm animal species were investigated resulting in a significant reduction of food intake and body weight and in an increase of ROS and cytokines productions (Gessner et al. Citation2017). However, a low number of studies were performed with farm animals, especially in lambs, to understand the exact mechanism of action of plant polyphenols and more studies are necessary in future.
The present study aimed to assess the efficacy of a red orange and lemon extract (RLE), rich in polyphenols, to combat oxidative stress processes in lambs where it is well demonstrated, by several authors, a significant increase of oxidative stress in newborn period, with a reduction of antioxidant defense systems (Frank and Groseclose Citation1984; Buonocore et al. Citation1998; Perez et al. Citation2019). In particular, the most present flavonoid in red orange is demonstrated to be anthocyanins, as such as cyanidin-3-glucoside, with beneficial effect on fat accumulation and diabetes and it is well demonstrated in our previously paper the RLE activity to counteract the renal toxicity induced by mycotoxin in in vivo experiments (Caruso et al. Citation2020). Therefore, we hypothesised that treatment of lambs with RLE extract could improve the animal performance, the antioxidant and anti-inflammatory status.
Material and methods
Ethics statement
The trial was authorised by the Animal Welfare Body of the University of Naples Federico II (PG/2019/0028161 of 03/19/2019) and carried out in accordance with the associated guidelines EU Directive 2010/63/EU.
Animals, experimental design and sampling collection
The experimental procedures (40 days) were carried out in the Council for Agricultural Research and Economics, Research Centre for Animal Production and Aquaculture (CREA-ZA), located in Bella (PZ, Italy). The trial was carried out on 120 lambs. After colostrum administration from the mothers within 2 h from birth, all lambs were randomly assigned and subsequetly (4 days ± 12 h of age and 3.7 ± 0.2 kg of weight) divided into two group as described by Maggiolino et al. (Citation2021): the RLE Group (treated group; n = 60) that received, daily, 90 mg/kg of RLE extract by gavage and the control Group (untreated group; n = 60) received, daily, saline solution by gavage. Animals were maintained in single boxes, where they received ad libitum lambs starter (20.5% crude protein DM, 1.8% fat DM, 25% crude fibres DM) and alfalfa hay (18.8% crude protein DM, 32.2% crude fibres DM) as reported in previous paper (Ferrara et al. Citation2021). The RLE dosage was calculated using the model of the US Food and Drug Administration (USFDA) (Wojcikowski and Gobe Citation2014) and according to our previous papers (Damiano et al. Citation2020a Damiano et al. Citation2020b,). At the start of the present trial, all animals were in good health; no disease was diagnosed during and at the end of the treatments. The lambs were transported approximately 15 km to the abattoir (groups separated) and the journey time was less than 30 min as described in previous papers (Maggiolino et al. Citation2021), and they were slaughtered on the same day at a European Community-approved abattoir in compliance with European Community laws on Animal Welfare in transport (1/2005EC) and the European Community regulation on Animal Welfare for slaughter of commercial animals (1099/2009EC). Before slaughtering, blood samples were collected, and after slaughtering the bladder was removed and an individual urine sample was aspirated by using a 18 G needle. Blood samples were aseptically collected via jugular vein puncture using disposable needles (23 G) as described by De Palo et al. (Citation2018), with a negative pressure system for plasma (4 mL tubes with 15 USP U/mL of heparin) (Becton, Dickinson Canada Inc, Vacutainer 1, Oakville, Canada). Heparinised tubes were stored on ice and centrifuged (1500 × g for 10 min) within 1 h. All plasma samples were stored at −80 °C until processing. Samples (2 mg) of the liver, kidney, and small intestine were removed from each animal and were put immediately at −20 °C until oxidative assay determination.
The standardised phytoextract rich in flavanones, anthocyanins and other polyphenols, created and obtained from red orange and lemon processing wastes (Red orange and Lemon Extract, here named RLE) (Caruso et al. Citation2020), was obtained by CREA - Research Centre for Olive, Fruit and Citrus Crops (CREA-OFA, Acireale, Italy) for research purposes only (Italian Patent No. 102017000057761). The chemical composition of RLE is shown in Table .
Table 1. Chemical composition of red orange and lemon extract (RLE) used in this study.
Growth performance
Animals were weighed weekly before the morning feed. Body weight (BW) and feed intake were also measured, initially and at the end of treatment. Cold and warm carcase weight of each lamb was recorded and expressed in kilograms (Kg).
Oxidative stress markers assay
MDA assay (Sigma‐Aldrich, Milan, Italy; Item No. MAK085) used to measure the lipid peroxidation was performed to evaluate the effect of RLE on the oxidative status on both plasma and organs of lambs at the end of treatment period.
In particular, to perform the MDA assay in plasma, it was homogenised with MDA Lysis Buffer and TBA solution was added to each sample and incubated at 95 °C for 60 minutes (Ciarcia et al. Citation2015; Alipour et al. Citation2019). Then, each reaction mixture was placed into a 96 well plate to measure the absorbance at 532 nm using Glomax Multi Detection System (Promega, Madison, WI, USA). Results were expressed as nanomoles/dL.
Lipid peroxidation in organs was determined by assaying the MDA levels according to Ohkawa et al. (Citation1979). It was determined by the reaction of MDA with thiobarbituric acid (TBA) to form a colorimetric (532 nm) product, proportional to the MDA present. Kidney, liver, and intestine were homogenised on ice with MDA Lysis Buffer. A TBA solution was added to each sample to form the MDA-TBA adduct and then it was incubated al 95 °C for 60 min. Then, each reaction mixture was placed into a 96 well plate to measure the absorbance at 532 nm. Results were expressed as nanomoles/mg of protein.
Nitrite and nitrate assay
The nitrite (NO2) and nitrate (NO3) production, stable metabolites of nitrous oxide (NO) production, was determined in the supernatant obtained before of the kidney, liver, and intestine by Griess reagent as described by Ciarcia et al. (Citation2010) and data were expressed as picomoles of nitrite for mg of proteins.
Antioxidant status assays
The activity of SOD (Sigma‐Aldrich, Milan, Italy, Item No.19160), CAT(Sigma‐Aldrich, Milan, Italy, Item No. CAT100) and GPx (Sigma‐Aldrich, Milan, Italy, Item No. 38185) in plasma, to evaluate the antioxidant status in treated and untreated group were determined by a spectrophotometer (Glomax Multi detection system, Promega, Milano, Italy) at 450 nm, 520 nm, and 412 nm, respectively (Alipour et al. Citation2019). The antioxidant enzymes activity was expressed as units per litre (U/L).
The same parameters were calculated on supernatant described before of of the kidney, liver, and intestine. Superoxide dismutase (SOD, EC 1.15.1.1) was evaluated according to the method described by Maggiolino et al. (Citation2020). The activity was determined considering its ability to inhibit the epinephrine autoxidation. Stimulation of epinephrine autoxidation by traces of heavy metals present as contaminants in the reagents or by the other metals under investigation was prevented by adding 10–4 M ethylenediaminetetraacetic acid (EDTA) in the buffer to chelate these ions. One unit of SOD is defined as the amount of enzyme required to inhibit the rate of epinephrine autoxidation by 50%. The enzyme activity was expressed as U/mg protein. Catalase (CAT, EC 1.11.1.6) activity was measured by the method described by Tateo et al. (Citation2020), following the decrease in absorbance of H2O2 at 240 nm (e = 40 M−1 cm−1). One unit of enzyme activity is defined as the amount of enzyme required to degrade 1 micromole of H2O2 in 1 min and is expressed as U/mg protein. Glutathione peroxidase (GPx, EC1.11.1.9.) activity was measured by method described by Dinardo et al (Citation2021). The reaction measured the rate of reduced glutathione (GSH) oxidation by tert-Butyl hydroperoxide, catalysed by GPx. GSH was maintained at constant concentration by the addition of exogenous glutathione reductase (GR) and NADPH, which converted the oxidised glutathione (GSSG) to GSH. The rate of GSSG formation was then measured by the change in the absorbance of nicotinamide adenine dinucleotide phosphate reductase (NADPH) at 340 nm. (e = 6.2 mM−1 cm−1). Its activity was expressed as nanomoles of NADPH oxidised/min/mg protein.
DNA damage assay
DNA damage was measured by the 8-OHdG ELISA kit from Stress Marq (Biosciences INC, Victoria, BC, Canada) in urine samples at the end of treatment (Varatharajan et al. Citation2013). The urine samples colour developed after dilution with phosphate-buffered saline (pH range to 6–8) was measured at 450 nm, spectrophotometrically. Urinary 8-OHdG was expressed as ug/day.
Plasma IL-1β and IL-6 levels
Plasma IL-1β and IL-6 protein concentrations were measured using a sandwich ELISA assay (Song et al. Citation2013). Plasma samples were incubated for 2 hours at room temperature in the 96 well microplate previously treated with 100 μL of capture antibodies from SeroTec (5 μg/mL; MCA1658 for IL-1β and MCA1659 for IL-6, East Brisbane, Australia) in 0.1 M carbonate buffer (pH 9.6) at 4 °C overnight and blocked with 3% skim-milk solution. After washing with PBST, the detection antibodies were added into the wells and incubated for 2 hours at room temperature (2 μg/mL; AHP423 for IL-1β and AHP424 for IL-6, East Brisbane, Australia) and then the antigen was detected with goat anti-rabbit IgG-HRP (1:2000; 7074S Cell Signalling Technology, Carlsbad CA, USA). Colour development was initiated by adding 3,3′,5,5′-tetramethyl-benzidine liquid substrate (Sigma, Castle Hill, Australia) and 0.5 M sulphuric acid was added to stop the reaction after 15 min. The optical density (OD) was measured at 450 nm, spectrophotometrically, and express as protein content.
Statistical analysis
The GraphPad InStat Version 3.00 for Windows 95 (GraphPad Software, San Diego, CA, USA) was used for statistical analysis. Statistically significant differences were evaluated by one-way analysis of variance (ANOVA), followed by Turkey’s post-test. The experiments were performed at least in triplicates. *p < .05 was considered statistically significant.
Results
Growth performances
The effects of the experimental treatments on the grow performance and hot and cold carcase weights on the lambs are shown in Table and Figure . There were no differences among RLE treatment and control one about the initial and final weight and hot and cold carcase of lambs (p > .05).
Figure 1. Effects of the red orange and lemon extract (RLE) on body weight growth in control group and RLE group during 40 days of treatment.
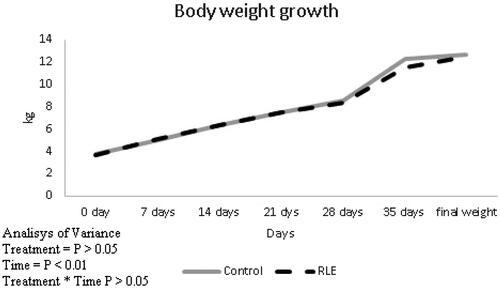
Table 2. Effects of red orange and lemon extract (RLE) on growth performances and carcase weight of kid lamb in control group and RLE group after 40 days of treatment.
Oxidative stress and antioxidant status
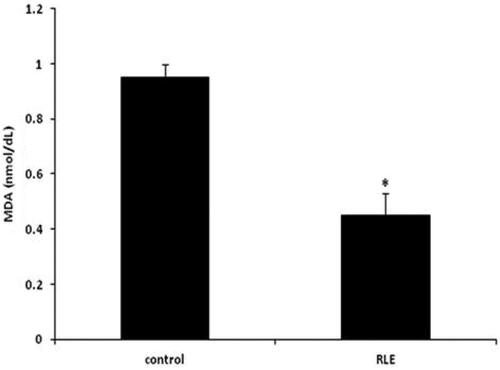
The effects of the RLE treatment on lambs on MDA levels are presented in Figure . There were differences among RLE treated lambs respect to control group, in fact the MDA level in RLE group was lower respect to the control one (p > .05).
Figure 2. Effects of the red orange and lemon extract (RLE on malondialdehyde (MDA), expressed in nmol/dL in control group and RLE group after 40 days of treatment. Data are shown as mean ± standard deviation (DS) and were compared by ANOVA (*p < .05 versus control).
Regarding antioxidant enzyme activities, significant differences of SOD, CAT and GPx were observed in the control group and the group that received RLE extract (*p < .05, Table ). It was observed that RLE treatment restore the antioxidant status. Differently, no effect on enzymes activity was observed in kidney, liver and intestine (p > .05)
Table 3. Effects of red orange and lemon extract (RLE) on antioxidant status of lamb in control group and RLE group at the end of treatment.
Tabel 4. Effects of orange and lemon extract (RLE) on the antioxidant status of lamb in the control group and in the RLE group in kidney, liver and intestine.
Moreover, the urinary 8-OHdG excretion was higher in control group than in RLE group (*p < .05, Figure ) demonstrating a beneficial effect of the new extract rich in anthocyanins to prevent the DNA damage.
Figure 3. Effects of the red orange and lemon extract (RLE on urinary 8-hydroxy-2’-deoxyguanosine [8-OHdG] concentration, expressed in ug/day in control group and RLE group after 40 days of treatment. Data are shown as mean ± standard deviation (DS) and were compared by ANOVA (*p < .05 versus control).
![Figure 3. Effects of the red orange and lemon extract (RLE on urinary 8-hydroxy-2’-deoxyguanosine [8-OHdG] concentration, expressed in ug/day in control group and RLE group after 40 days of treatment. Data are shown as mean ± standard deviation (DS) and were compared by ANOVA (*p < .05 versus control).](/cms/asset/a84ae28e-1bb1-475b-a259-e4f51fb3ddfb/tjas_a_2056527_f0003_b.jpg)
Plasma IL-1β and IL-6 levels
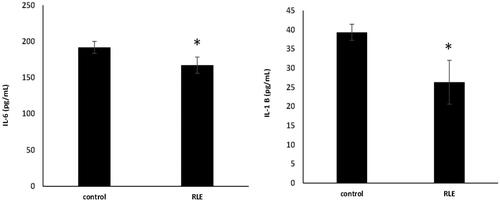
The effects of systemic inflammation on lambs’ performances were investigated by the measurement of pro-inflammatory cytokines concentration, such as IL- 1 β and IL- 6 by ELISA assay. The significant increase of IL-1 β and IL- 6 was observed in plasma of control lamb respect to the RLE group, where the extract, rich in polyphenols, appears to prevent these events (*p < .05, Figure ).
Figure 4. Effects of the red orange and lemon extract (RLE on interleuchin-6 (IL-6) (a) and interleuchin-1 β (IL -1 β) (b) levels, expressed as pg/mL, in control group and RLE group after 40 days of treatment. Data are shown as mean ± standard deviation (DS) and were compared by ANOVA (*p < .05 versus control).
Discussion
Natural compounds produced by plants might be good candidates for their use as feed additives in farm animals to maintain or improve animal health and performance. Among the group of metabolites produced by plants, polyphenols might be the most promising due to their well demonstrated antioxidant and anti-inflammatory effects, both in vitro and in vivo in mice (Baur et al. Citation2006; Chuang and McIntosh Citation2011) and rats (Damiano et al. Citation2021). Several studies have demonstrated that flavonoids improve feed efficiency and growth rate in animal farm, with positive effects on antioxidant systems (Cushnie and Lamb Citation2011). In this study we evaluated the effect of the new extract rich in polyphenols such as anthocyanins and flavanones, also on growth performance. Based on the results obtained in our previous work (Ferrara et al. Citation2021) where we demonstrated that lambs treated with RLE induced an increase in favourable bacteria and a reduction of potentially pathogenic ones, in this work we decided to estimate the effects of RLE extract also on inflammatory and oxidative process. However, we did not find any significant effects on lambs’ performances. Maybe the not significant effect of RLE on growth performances on treated lambs respect to the untreated one could be due to short trial duration and/or to dose administered. This let us suppose that differences could be observed in older animals with a longer treatment (due also to longer production life) and different development of digestive system in which probably 90 mg/kg used could be not the correct dose. According with our results, several researches have shown that the use of some plants rich in flavonoid had no effect on growth rate in lambs (Mesgaran, Citation2013; Zhao et al. Citation2013; Omidi et al. Citation2014). Moreover, carcase weight (both hot and cold) were not affected by RLE treatment, in fact we did not observe difference in these parameters between RLE treated lambs respect to control one and these data appears in agreement with the literature (Maiorano et al. Citation2016). Anyway, although in our previous work we have shown that there has been no improvement in the performance of lambs, significant results have been shown in the juiciness and colour detection that make the product more attractive to the consumer (Salzano et al. Citation2021).
Lipid peroxidation, measured by MDA levels, is a marker of oxidative stress and reflects the oxygen free radicals levels. 90 mg/kg live weight of RLE are able to prevent the MDA increase, typical in the newborn period and open new consideration in a new approach designed to prevent oxidation injury in small ruminant animal farm. Probably, this data is related to the RLE capacity to prevent the imbalance between ROS formation and antioxidant defences. In fact, the result of the present study showed that RLE have antioxidant activity in treated lambs, in particular SOD, CAT and GPx activity in plasma were significantly higher in RLE group, respect to the control, although no differences in tissues as kidney, liver and intestine was observed. These data seem linked to the high concentration of polyphenolic compounds, in particular, flavonoids that, it is well shown, act as primary antioxidant or free radical terminator (Huang and Frankel Citation1997). According to us, Alipour et al. (Citation2019) found the activities of GPx and SOD of plasma higher in Baluchi male lambs treated orally with a natural extract rich in polyphenols respect to the control. Moreover, the addition of RLE extract reduced the urinary 8-OHdG excretion respect to the control. The oxidative damage of DNA is caused by ROS that, it is well known, hydroxylates the base in DNA. Guanine is most prone, among the bases in the nucleic acids, to oxidative damage and, the repair of 8-hydroxylated guanine lesions, appears to be a good mechanism to repair the DNA lesion. The urinary 8-OHdG is excreted during DNA repair and it is considered not only an indicator of DNA repair capacity, but also a biomarker of cellular oxidative stress (Pantano et al. Citation2006). Similar results were observed in kids by Salzano et al. (Citation2021). Probably, considering the short-time trial (only 40 days) and the age of the animals, plasma enzymes resulted faster in reaction to anthocyanins effects compared to organs. However, this aspect must be deeply investigated.
Finally, in the present work we investigated the inflammatory status of lambs and the potential beneficial effects of the extract because it is known that oxidative stress is directly linked with inflammation (Ishida et al. Citation2015). Thus, typical proteins produced during the proinflammatory status are cytokines that stimulate the production of oxidants and, thereby, promoting oxidative stress (Zhang and An Citation2007). These events trigger a vicious cycle where the sustained production of oxidants induces a chronic inflammatory process and tissues are damaged (Ciz et al. Citation2012). These mechanisms, typical of many chronic inflammatory human disorders, also occur in farm animals (Lykkesfeldt and Svendsen Citation2007). In particular, the effects of inflammation on performance of several farm animal have been investigated, such as in pig, cattle and poultry (Gessner et al. Citation2017). Regarding to potential effects of RLE on inflammation in lambs, the present study investigated the effect of the oral administration of the new extract on the expression of IL-6 and IL-1B proinflammatory cytokines demonstrating that both were down reduced in RLE treated animals respect to control ones, according to published data where it is shown the anti-inflammatory, antioxidant and cytoprotective activities of polyphenols, both in vitro and in vivo in humans and experimental animal models (Rahman et al. Citation2006; Scapagnini et al. Citation2011; Tangney and Rasmussen Citation2013). In contrast, in lambs, the effects of plant polyphenols with respect to their anti-inflammatory and antioxidative and cytoprotective effects have been less investigated. Results showed in the present paper clearly demonstrated the beneficial power of the RLE extract rich in polyphenols in lambs, in particular its effect on inflammation and oxidative stress, both of which are linked to each other.
Conclusions
In conclusion, the addition of 90 mg/kg of RLE to lambs’ diet regimen did not affect in vivo growth performance and on meat chemical composition. However, RLE inclusion improved the oxidative and inflammatory status in lambs. Essential information emerges from these results concerning potential beneficial effects on lambs’ oxidative status and welfare, which should be taken into account before considering studies on older productive categories. In fact, although no beneficial effect has been elucidated about growth performance, this aspect should be studied in animals with a longer life and so with a longer administration period. So, RLE could be considered a potential feed supplement able to improve the health and welfare status of animals and potentially reduce the use of antimicrobial substances.
Ethical approval
The research was approved by Animal Wealfare Body of the University of Naples Federico II (PG/2019/0028161 of 03/19/2019).
| Abbreviations | ||
| RLE | = | red orange and lemon extract |
| MDA | = | malondialdehyde |
| SOD | = | superoxide dismutase |
| GPx | = | glutathione peroxidase |
| CAT | = | catalase |
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Alipour F, Vakili A, Danesh Mesgaran M, Ebrahimi H. 2019. The effect of adding ethanolic saffron petal extract and vitamin E on growth performance, blood metabolites and antioxidant status in Baluchi male lambs. Asian-Australas J Anim Sci. 32(11):1–1704.
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al. 2006. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 444(7117):337–342.
- Buonocore G, Zani S, Sargentini I, Gioia D, Signorini C, Bracci R. 1998. Hypoxia-induced free iron release in the red cells of newborn infants. Acta Paediatr Int. J Paediatr. 87(1):77–81.
- Carocho M, Ferreira ICFR. 2013. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol. 51:15–25.
- Caruso M, Fabroni S, Emma R, Ballistreri G, Amenta M, Currenti W, Rinzivillo C, Rapisarda P. 2020. A new standardized phytoextract from red orange and lemon wastes (red orange and lemon extract) reduces basophil degranulation and activation. Nat Prod Res. 35(23):5354–5359.
- Chaudhary AK, Nokubo M, Reddy GR, Yeola SN, Morrow JD, Blair IA, Marnett LJ. 1994. Detection of endogenous malondialdehyde-deoxyguanosine adducts in human liver. Science. 265(5178):1580–1582.
- Chuang C-C, McIntosh MK. 2011. Potential mechanisms by which polyphenol-rich grapes prevent obesity-mediated inflammation and metabolic diseases. Annu Rev Nutr. 31:155–176.
- Ciarcia R, d'Angelo D, Pacilio C, Pagnini D, Galdiero M, Fiorito F, Damiano S, Mattioli E, Lucchetti C, Florio S, et al. 2010. Dysregulated calcium homeostasis and oxidative stress in chronic myeloid leukemia (CML) cells. J Cell Physiol. 224(2):443–453.
- Ciarcia R, Damiano S, Florio A, Spagnuolo M, Zacchia E, Squillacioti C, Mirabella N, Florio S, Pagnini U, Garofano T, et al. 2015. The protective effect of apocynin on cyclosporine a-induced hypertension and nephrotoxicity in rats. J Cell Biochem. 116(9):1848–1856.
- Ciz M, Denev P, Kratchanova M, Vasicek O, Ambrozova G, Lojek A. 2012. Flavonoids inhibit the respiratory burst of neutrophils in mammals. Oxid Med Cell Longev. 2012:181295–181296.
- Cushnie TPT, Lamb AJ. 2011. Recent advances in understanding the antibacterial properties of flavonoids. Int J Antimicrob Agents. 38(2):99–107. https://doi.org/10.1016/j.ijantimicag.2011.02.014
- Damiano S, Iovane V, Squillacioti C, Mirabella N, Prisco F, Ariano A, Amenta M, Giordano A, Florio S, Ciarcia R. 2020a. Red orange and lemon extract prevents the renal toxicity induced by ochratoxin A in rats. J Cell Physiol. 235(6):5386–5393.
- Damiano S, Lauritano C, Longobardi C, Andretta E, Elagoz AM, Rapisarda P, Di Iorio M, Florio S, Ciarcia R. 2020b. Effects of a red orange and lemon extract in obese diabetic zucker rats: role of nicotinamide adenine dinucleotide phosphate oxidase. JCM. 9(5):1600.
- Damiano S, Longobardi C, Andretta E, Prisco F, Piegari G, Squillacioti C, Montagnaro S, Pagnini F, Badino P, Florio S, et al. 2021. Antioxidative effects of curcumin on the hepatotoxicity induced by ochratoxin a in rats. Antioxidants. 10(1):125.
- De Felice E, Giaquinto D, Damiano S, Salzano A, Fabroni S, Ciarcia R, Scocco P, de Girolamo P, D’angelo L. 2021. Distinct pattern of npy in gastro–entero–pancreatic system of goat kids fed with a new standardized red orange and lemon extract (Rle). Animals. 11(2):449.
- De Palo P, Maggiolino A, Albenzio M, Casalino E, Neglia G, Centoducati G, Tateo A. 2018. Survey of biochemical and oxidative profile in donkey foals suckled with one natural and one semi-artificial technique. Plos One. 13(6):e0198774.
- Dinardo FR, Maggiolino A, Casalino E, Deflorio M, Centoducati G. 2021. A Multi-Biomarker approach in european sea bass exposed to dynamic temperature changes under dietary supplementation with origanum vulgare essential oil. Animals. 11(4):982.
- Egert S, Rimbach G. 2011. Which sources of flavonoids: complex diets or dietary supplements? Adv Nutr. 2(1):8–14.
- Ferrara M, Sgarro MF, Maggiolino A, Damiano S, Iannaccone F, Mulè G, De Palo P. 2021. Effect of red orange and lemon extract-enriched diet in suckling lambs. Fecal Microbiota Agric. 11(7):572.
- Frank L, Groseclose EE. 1984. Preparation for Birth into an O2-rich environment: the antioxidant enzymes in the developing rabbit lung. Pediatr Res. 18(3):240–244.
- Gessner DK, Ringseis R, Eder K. 2017. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J Anim Physiol Anim Nutr. 101(4):605–628.
- Hathway DE. 1966. Metabolic fate in animals of hindered phenolic antioxidants in relation to their safety evaluation and antioxidant function. Adv Food Res. 15:1–56.
- Hiscott J, Marois J, Garoufalis J, D'Addario M, Roulston A, Kwan I, Pepin N, Lacoste J, Nguyen H, Bensi G. 1993. Characterization of a functional NF-kappa B site in the human interleukin 1 beta promoter: evidence for a positive autoregulatory loop. Mol Cell Biol. 13(10):6231–6240.
- Hodgson JM, Croft KD. 2010. Tea flavonoids and cardiovascular health. Mol Aspects Med. 31(6):495–502.
- Huang S-W, Frankel EN. 1997. Antioxidant activity of tea catechins in different lipid systems. J Agric Food Chem. 45(8):3033–3038.
- Ishida K, Kishi Y, Oishi K, Hirooka H, Kumagai H. 2015. Effects of feeding polyphenol-rich winery wastes on digestibility, nitrogen utilization, ruminal fermentation, antioxidant status and oxidative stress in wethers. Anim Sci J. 86(3):260–269.
- Jin H, Leng Q, Li C. 2012. Dietary flavonoid for preventing colorectal neoplasms. Cochrane Database Syst. Rev. 8:CD009350.
- Kahl R, Kappus H. 1993. [Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E]. Z Lebensm Unters Forsch. 196(4):329–338.
- Lykkesfeldt J, Svendsen O. 2007. Oxidants and antioxidants in disease: oxidative stress in farm animals. Vet J. 173(3):502–511.
- Maggiolino A, Lorenzo JM, Centoducati G, Domínguez R, Dinardo FR, Marino R, della Malva A, Bragaglio A, De Palo P. 2020. How volatile compounds, oxidative profile and sensory evaluation can change with vacuum aging in donkey meat. Animals. 10(11):2126.
- Maggiolino A, Bragaglio A, Salzano A, Rufrano D, Claps S, Sepe L, Damiano S, Ciarcia R, Dinardo FR, Hopkins DL, et al. 2021. Dietary supplementation of suckling lambs with anthocyanins: Effects on growth, carcass, oxidative and meat quality traits. Anim Feed Sci Technol. 276:114925.
- Maiorano G, Angwech H, Memmo DD, Wilkanowska A, Mucci R, Abiuso C, Tavaniello S. 2016. Effects of intramuscular vitamin E multiple injection on quality, oxidative stability and consumer acceptability of meat from Laticauda lambs fed under natural rearing conditions. Small Rumin Res. 139:52–59.
- Mesgaran DM. 2013. Effects of Crocus sativus petals extract on biochemical blood parameters in male rats, Arak Medical University Journal (AMUJ) Original Article.
- Ohkawa H, Ohishi N, Yagi K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 95(2):351–358.
- Omidi A, Rahdari S, Hassanpour Fard M. 2014. A preliminary study on antioxidant activities of saffron petal extracts in lambs. Vet Sci Dev. 4(1):5161.
- Pantano C, Reynaert NL, Vliet A, Van Der Janssen–Heininger YMW. 2006. Redox-sensitive kinases of the nuclear factor-kappaB signaling pathway. Antioxid Redox Signal. 8(9-10):1791–1806.
- Perez M, Robbins ME, Revhaug C, Saugstad OD. 2019. Oxygen radical disease in the newborn, revisited: Oxidative stress and disease in the newborn period. Free Radic Biol Med. 142:61–72.
- Procházková D, Boušová I, Wilhelmová N. 2011. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 82(4):513–523.
- Rahman I, Biswas SK, Kirkham PA. 2006. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 72(11):1439–1452.
- Salzano A, Damiano S, D’Angelo L, Ballistreri G, Claps S, Rufrano D, Maggiolino A, Neglia G, De Palo P, Ciarcia R. 2021. Productive performance and meat characteristics of kids fed a red orange and lemon extract. Animals. 11(3):809.
- Scapagnini G, Vasto S, Sonya V, Abraham NG, Nader AG, Caruso C, Calogero C, Zella D, Fabio G. 2011. Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol Neurobiol. 44(2):192–201.
- Song Y, Karisnan K, Noble PB, Berry CA, Lavin T, Moss TJM, Bakker AJ, Pinniger GJ, Pillow JJ. 2013. In Utero LPS exposure impairs preterm diaphragm contractility. Am J Respir Cell Mol Biol. 49(5):866–874.
- Surai PF. 2014. Polyphenol compounds in the chicken/animal diet: from the past to the future. J Anim Physiol Anim Nutr (Berl)). 98(1):19–31.
- Tangney CC, Rasmussen HE. 2013. Polyphenols, inflammation, and cardiovascular disease. Curr Atheroscler Rep. 15(5):324.
- Tateo A, Maggiolino A, Domínguez R, Lorenzo JM, Dinardo FR, Ceci E, Marino R, Malva A. d, Bragaglio A, Palo PD. 2020. Volatile organic compounds, oxidative and sensory patterns of vacuum aged foal meat. Animals. 10(9):1495.
- Varatharajan R, Sattar MZA, Chung I, Abdulla MA, Kassim NM, Abdullah NA. 2013. Antioxidant and pro-oxidant effects of oil palm (Elaeis guineensis) leaves extract in experimental diabetic nephropathy: a duration-dependent outcome. BMC Complement Altern Med. 13:242.
- Wojcikowski K, Gobe G. 2014. Animal studies on medicinal herbs: predictability, dose conversion and potential value. Phytother Res. 28(1):22–27.
- Zhang J-M, An J. 2007. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 45(2):27–37.
- Zhao T, Luo H, Zhang Y, Liu K, Jia H, Chang Y, Jiao L, Gao W. 2013. Effect of vitamin E supplementation on growth performance, carcass characteristics and intramuscular fatty acid composition of Longissimus dorsi muscle in Tan sheep. Chilean J Agric Res. 73(4):358–365.
