Abstract
We evaluated the fects of different levels of dietary silver nanoparticle (AgNP) powder on performance, intestinal microflora, carcass traits and blood parameters of broiler chickens. Three hundred seven-day-old Ross broiler chicks were randomly divided into five groups, each group replicated three times with 20 birds per replication. Chickens were fed the basal diet with 2.5, 5, 10 and 20 mg AgNPs per kg feed. Dietary inclusion of AgNPs improved the final body weight, cumulative weight gain and feed conversion ratio. The best broiler performance, carcass traits, and relative organ weights were observed in the group supplemented with 2.5 ppm AgNPs. Increasing the AgNP dose resulted in a significant decrease in the caecal lactose positive and enterococci bacteria populations, while lactobacilli counts were numerically increased. The silver residues in the breast and thigh muscle significantly increased (p < .05) in a dose-dependent manner. Dietary inclusion of AgNPs induced dose-dependent lesions in liver, kidney, spleen and duodenum tissues involving degeneration, necrosis, mononuclear infiltration and focal aggregation of inflammatory cells. In conclusion, despite its potential positive impacts on growth performance, carcass traits and caecal microbial population diversity at a dose of 2.5 ppm, dietary inclusion of AgNPs had the following negative effects on broilers: 1) silver residues in breast and thigh muscle, which may result in AgNPs transmission to consumers, and 2) cytotoxicity in intestinal, liver, spleen and kidney cells in a dose-dependent manner. Therefore, we suggest the use of lower doses of AgNPs (< 2.5 ppm diet) in poultry production in the future studies.
Dietary inclusion of silver nanoparticles (AgNPs) in broiler diets more than 2.5 mg/kg diets had many negative effects represented by accumulation of silver residue in broiler meat and the possibility of transmission of nanosilver to consumers.
AgNPs had a cytotoxic effect on intestine, liver, spleen and kidney cells in a dose-dependent manner in broilers and might be harmful to chicken and human health.
Therefore, we do not recommend using AgNPs as a dietary growth promotor or antibacterial agent in broiler diets and their use and marketing should be controlled and restricted.
HIGHLIGHTS
Introduction
Antibiotic growth promoters have long been used in chicken feed to enhance feed efficiency and minimise the occurrence of certain diseases (Chattopadhyay Citation2014). However, to avoid the development of microbial resistance, their usage in animal production has been restricted to varying degrees. As a result, alternatives to antibiotics are urgently needed. Numerous nutritional supplements are currently available in the market, including organic acids, probiotics, prebiotics, essential oils (Awad et al. Citation2009; Al-Sultan et al. Citation2016) and nanoparticles.
Nanoparticles are considered promising in livestock and poultry production due to their chemical and physical properties. They are used in various applications in nutrition, therapy, and medicine. Although it has not yet been made mandatory to use AgNPs and neither it has been recommended by NRC. It has been suggested by researchers to be used in animal production and chicken feed additives for improving various aspects as a result of their antibacterial, antifungal and immune stimulatory capabilities (Małaczewska Citation2014; Hassanpour et al. Citation2015; Patra and Lalhriatpuii Citation2020). Silver nanoparticles (AgNPs) have a diameter of less than 100 nanometres, making them ideal for penetration and accumulation in bacteria (improving antibacterial action) and enhancing absorption into the intestinal lining epithelia (Atiyeh et al. Citation2007). Due to their toxicity to microorganisms, AgNPs are used as growth promoters in animal nutrition and to modify the gut flora (Percival et al. Citation2005; Bolandi et al. Citation2021). According to previous studies, the use of nanoparticles improved the growth performance, immune response, digestive efficiency and reduced the mortality rate of the poultry (Kumar and Bhattacharya Citation2019, Kumar et al. Citation2020, Dosoky et al. Citation2021).
On the other hand, supplementation of the larger dose of AgNPs (8 mg/kg feed) resulted in deterioration in growth performance and significant negative impacts on all the measured parameters in broilers (Awaad et al. Citation2021). Similarly, the use of AgNPs in drinking water at a dosage of 50 ppm decreased broiler growth, diminished immune function, and had no antibacterial effect on various intestinal bacterial groups (Vadalasetty et al. Citation2018).
Many researchers used varied concentrations of AgNPs in the drinking water of broiler chickens and detected residues in the edible parts of broiler muscle at all concentrations (Ahmadi and Rahimi Citation2011, Kulak et al. Citation2018, Salem et al. Citation2021). There was a linear increase in AgNPs retention in meat with increasing dose in growing rabbits (Abdelsalam et al. Citation2019) and in poultry (Ahmadi and Rahimi Citation2011, Saleh and El-Magd Citation2018). Ag retention was higher in liver followed by spleen than that in muscular tissue in broiler chickens (Fondevila Citation2010, Wang et al. Citation2013). In addition, Nabinejad et al. (Citation2016) stated that the muscles and organs of the poultry may transfer AgNPs to consumers which may cause negative effects.
Several studies on the effects of silver nanoparticles on chicken growth performance, intestinal histomorphology and microflora, tissue residue, and carcass characteristics have recently been published, in spite of that there is insufficient data as the results are inconsistent. Therefore, our primary goal for this study was to elucidate the effect of dietary AgNP powder on growth performance, carcass traits, intestinal histopathology and microflora, silver residues in meat and blood metabolites.
Materials and methods
Statement of animal rights
The ethics committee for Laboratory Animal Research at King Faisal University approved all study procedures (KFU-REC-2022-JAN-EA000337). The institutional and national guidelines that ensure animal rights were strictly followed during the study.
Nanoparticles
Chemical reduction was used to synthesise AgNPs, as described by Pal et al. (Citation2009). AgNPs were created by exposing a silver nitrate solution to microwave radiation in an ethanolic medium, including polyvinylpyrrolidone as a stabilising agent. In the presence of microwave, ethanol was applied as a reducing agent. To obtain AgNPs in powdered form, the solvent was evaporated at a mild temperature (mild degree of microwave oven at 2450 MHz for 5 seconds). Particle sizes were measured using a JEOL JEM-2100 high-resolution transmission electron microscope with an accelerating voltage of 80 kV (). The AgNPs were spherical, with particle size ranging from 11.9 to 45 nm, according to morphological examination.
Animal and dietary management
Three hundred (50% male) seven-day-old Ross 308 broiler chickens were raised until the age of 42 days. Broiler chicks were randomly allocated into five treatment groups, each replicated three times with 20 birds per replication. Five AgNP concentrations (0, 2.5, 5, 10, and 20 ppm, respectively) were added to the basal diet from day 7 to day 42 post-hatching. Five broiler starter (8–28 d) and finisher (28–42 d) diets were formulated incorporating five concentrations of AgNPs (0, 2.5, 5, 10 and 20 ppm diet). The following were the five dietary treatments: 1) basal diet without any addition (C), 2) basal diet containing 2.5 ppm AgNPs (NS 2.5), 3) basal diet containing 5 ppm AgNPs (NS5), 4) basal diet containing 10 ppm AgNPs (NS10), and 5) basal diet containing 20 ppm AgNPs (NS20). The birds were housed in standard conditions in a building with controlled temperature and humidity in pens with sawdust litter. Water and experimental diets were offered ad libitum. The experimental diet formulations satisfied the nutritional requirements for broiler chickens according to the NRC (Citation1994) guidelines (Table ). During the first week, the temperature was maintained at 32 ± 1 °C, then reduced to 27 °C in the second week and to 24 °C in the third week of age. From the start of the 4th week the temperature was set to 23 ± 1 °C until the end of the experiment. From 7 to 42 days of age, the broilers’ body weight (BW) and feed intake were measured and recorded weekly.
Table 1. Composition and chemical analysis of basal diets (% as fed basis).
Growth performance
To calculate feed consumption each week, we measured daily feed intake per replicate. A digital balance was used to record chick body weight at the time of delivery and every week thereafter. FCR was calculated using the formula FCR = g feed/g weight gain.
Carcass yield
At the end of the experiment, broiler carcass characteristics and organ weights were assessed by randomly selecting and euthanizing two chickens from each replicate. A digital balance was used to weigh the warm carcass, heart, liver, breast, thigh, and abdominal fat to the nearest 0.01 g. Dressing percentage is the ratio of dressed carcass weight to the weight of the live bird, expressed as a percentage.
Water-holding capacity
To estimate the water-holding capacities (WHCs) of post-rigor chilled chicken leg and breast muscle the method Honikel and Hamm (Citation1994) was applied. By squeezing the sample (≈300 mg) between 2 filter papers (70 mmφ) and ceramic plates for 4 min. The compressed sample was carefully separated from the moist filter paper after pressing and weighed immediately. RW % was estimated by the formula: RW%= (W1-W2)/W1X 100; where W1= Weight of meat sample, W2= Weight of meat sample after pressing. WHC was expressed as percent water retained by the meat sample; calculated as following: WHC (% water retained) = 100 – RW%.
Evaluation of silver residue from nanoparticles
Meat sample from breast and thigh muscle (1 gram) was placed in a 30-ml galzzed porcelain crucible then the crucible placed in muffle furnace at 500 °C for 2 hours. After cooling the crucible was removed from the muffle furnace and 3 mL of HNO3 (1 + 1 (deionised water (DW)) was added and heating on a hot plate at 100–120 °C until dryness. Then the crucible was set back into the muffle furnace at 500 °C for additional 1 hour. After cooling crucible was removed and 10 mL Hcl (1 + 1 DW) was added. Then the sample transferred into a 50 ml volumetric flask and dilute to the volume with deionised water and well mixed. Then the sample was placed in the inductively coupled plasma optical emission spectrometry to evaluate total silver concentration in breast and thigh muscle samples, as described in a previous study (Isaac and Johnson Citation2019).
Histopathological studies
Duodenum, liver, kidney, and spleen specimens were fixed in a 10% neutral buffered formalin solution. Increasing concentrations of ethyl alcohol were used to dehydrate the specimens. Samples were then clarified with xylene before being embedded in paraffin, sectioned 4–5 µm thick and stained with haematoxylin and eosin, then Masson trichrome for fibrosis detection (Banchroft et al. Citation1996).
Transmission electron microscopes (TEM)
Tissue samples (1 mm) were obtained from the duodenum. They were fixed in 3% buffered glutaraldehyde for 2 hours, then washed three times (10 minutes each) with cacodylate buffer. The post was then fixed for 1.5 hours in 1% buffered osmic acid, then washed three times with distilled water (10 minutes each time). The samples were dehydrated using increasing alcohol concentrations (30–50–70–90–100%), changed every 15 minutes. The samples were embedded in a mixture of epon and araldite. From the embedded blocks, ultrathin (70 nm) sections were cut and stained with uranyl acetate and lead citrate. Finally, the TEM 100 eXII electron microscope in the Electron Microscope Unit, Assiut University, Assiut, Egypt, was used to examine the samples at 80 kV according to the protocol of Bozzola and Russell (Citation1999).
Enumeration of bacteria
Intestinal content from the caecum was collected shortly after slaughter in weighted screw-capped sterile Falcon tubes (50 ml capacity). The collected digesta were stored on ice until they were brought to the lab for enumeration. microbial population enumeration. The sealed containers were kept at 4 °C in the laboratory, and the fresh mass was diluted 1:10 with a sterile 0.1% peptone solution. ten-fold serial dilutions up to 107 of each sample were made. The spread-plate technique was used to count coliform bacteria, lactose-negative enterobacteria, enterococci, and lactobacilli. Coliform and lactose-negative enterobacteria were enumerated as red and colourless colonies on MacConkey agar (BBL), respectively. Enterococci were enumerated as red colonies mostly surrounded by a yellow zone on KF Streptococcus agar basis (Merck, 110707). By the use of DeMan, Rogosa, and Sharpe (MRS) agar (Biolife) lactobacilli was enumerated and the plates were incubated with 5% CO2 for 48 hours. Lactobacilli appear as little opaque and white colonies that are compact or feathery. Colonies were counted after incubation based on colony morphology. The average of the counts from two plates was calculated. The number of colony-forming units per gram of digesta was given as log colony-forming units.
Blood biochemical parameters
At day 42, 2 birds from each replicate were randomly chosen for collection of blood samples from wing vien. The serum was obtained by centrifugation of the collected blood samples at 4000 x g for 15 min and saved at −20 °C until further analysis. Blood metabolites (total Protein, albumin, cholesterol, triglycerides, uric acid (Spectrum Egypt), urea, creatine (Diamond, Egypt), calcium, phosphorus (Spinreact, Spain), Igg, IgA (QCA, Spain) were investigated by spectrophotometer (Unico, USA) using commercial test kits in accordance with the manufacturer company procedures.
Statistical analysis
Kolmogorov–Smirnov tests were used to examine all data for normality and homogeneity of variance. All bacterial enumeration information was converted to log10 CFU/mL. Data were analysed by ANOVA using SPSS software (version 19) (SPSS Citation2010). We used the Duncan multiple range test post-hoc when significant differences (p < .05) were found (Steel and Torrie, Citation1980). The data are presented for each variable as means with pooled standard errors.
Results
Effect of AgNPs on the growth performance parameters
The effects of AgNP concentration on the growth performance of broiler chickens are shown in Table . No significant differences were observed between AgNP concentration groups in live body weight (g) in the starter phase (initially and at day 28) and body weight gain (g) for the periods of 8–28 days and the finisher phase of 28–42 days. However, there were significant increases in the final body weight and weight gain with increasing AgNP dose from 2.5 to 10 mg/kg, followed by a decrease at 20 mg/kg. The highest body weight (2600 g) and weight gain (2480 g) were observed after 42 days in the group consuming 2.5 mg/kg AgNPs. Feed consumption (g/bird) was not affected (p > .05) by AgNP concentration during all phases of the experiment. However, the FCR was significantly (p < .05) improved by dietary treatments compared to the control group from 8–42 days. Moreover, birds receiving 2.5 mg AgNPs/kg diet demonstrated the lowest (p < .05) FCR value (1.89) from 8–42 days (Table ). In addition, the FCR was not significantly (p > .05) affected by the dietary treatments during the periods from 8–28 and 28–42 days.
Table 2. Effect of different dietary treatments on body weight, feed intake and feed conversion ratio in broiler chickens.
Effect of AgNPs on carcass traits and water holding capacity
The data in Table indicated that the AgNP concentration significantly (p < .05) affected worm carcass weight, dressing percentage and relative organ weights (Table ). The dressing percentages were significantly (p < .05) increased by the addition of 2.5 mg AgNPs/kg diet compared to the control and other treatment groups. The relative weights of the liver, gizzard, kidney, spleen and heart were significantly (p < .05) decreased by the dietary inclusion of 20 mg AgNPs/kg diet. The relative weight for the proventriculus and bursa were not significantly (p > .05) affected by the concentration of AgNPs. The percentages of WHC of both thigh and breast muscle were significantly (p < .05) decreased at 10 and 20 mg AgNPs/kg diet.
Table 3. Effect of different dietary treatments on carcass traits and relative organ weight and WHC in broiler chickens.
Effect of AgNPs on silver residues in broiler meat
Silver residues were detectable (in ppm) in all samples, even the control group. The average silver residue in the breast and thigh muscle significantly increased (p < .05) with increasing AgNP dosage (Table ).
Table 4. The effect of dietary AgNPs level on residues of silver (mg/kg) in meat of broilers.
Effect of AgNPs on caecal microbial population
The populations of caecal lactose-negative, lactose-positive, and enterococcal bacteria on day 28 significantly decreased with dietary inclusion of AgNPs, but not (p > .05) on day 42 (Table ). A numerical increase in the lactobacilli population was observed in groups fed 2.5 to 10 mg/kg dietary AgNPs; however, this increase was not statically significant (p > .05).
Table 5. Numbers of dominant bacterial groups in the contents of caeca [log cfu/g] of broilers after treatment with increasing concentration of AgNano.
Effect of AgNPs on blood serum constituents
The results of blood serum analyses of 6-week-old broiler chickens are shown in Table . Total protein (mg/dl), total cholesterol, urea, creatinine, and phosphorus exhibited significant (p < .05) differences between treated and control broiler groups. However, differences in other blood serum constituents were insignificant. Groups supplemented with 2.5 and 20 mg/kg dietary AgNPs had the highest total serum protein (4 mg/dl) compared to the control group (2.73 g/dl). Serum total cholesterol significantly decreased between the groups fed 2.5 to 20 mg/kg dietary AgNPs. Groups supplemented with AgNPs had lower (p < .05) serum phosphorus than the control group.
Table 6. Effect of different dietary treatments on blood metabolites (mg/dL) in broiler chickens.
Histopathological evaluation
Duodenum
Figure shows that the duodenum from the control group had normal epithelium with no lesions (A). The duodenum from birds supplemented with a dose of 2.5 mg/kg AgNPs were observed with normal intestinal villi structure (B). The duodenum from the 5 mg/kg supplemented group showed necrosis and sloughing of the epithelium (arrow) (C). The duodenum from the 10 mg/kg AgNPs group exhibited epithelial metaplasia (arrow) (D). The duodenum from 20 mg/kg AgNPs also showed epithelial metaplasia (arrow) (E).
Figure 2. Representative micrographs for duodenum (A–E) and spleen (F-G) of broiler chickens treated with different levels AgNPs for 42 days, H&E stain. A) Duodenum from control group showing normal epithelium with no lesions. (B) Duodenum from 2.5 mg supplemented group showing normal structure of intestinal villi. (C) Duodenum from 5 mg group showing necrosis and sloughing of epithelium (arrow). (D) Duodenum from 10 mg showing epithelial metaplasia (arrow). (E) Duodenum from 20 mg group showing epithelial metaplasia (arrow). F) Spleen from control group showing normal red and white pulp with normal lymphoid follicle. (G) Spleen from 2.5 mg group showing normal red and white pulp with normal lymphoid follicle. (H) Spleen from 5 mg group showing congestion of the blood vessels, desquamation of endothelium (arrow) and perivascular infiltration. (I) Spleen from 10 mg group showing necrosis of the lymphoid follicle (arrow). (G) Spleen from 20 mg group showing necrosis of the lymphoid follicle (arrow).
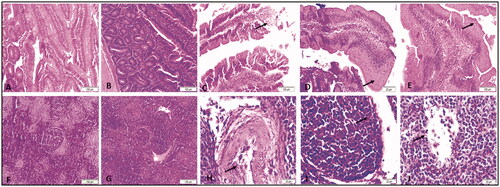
Spleen
As seen in Figure , the spleen from the control group demonstrated normal red and white pulp with normal lymphoid follicles (F). The spleen from the 2.5 mg/kg supplemented group had normal red and white pulp with normal lymphoid follicles (G). The spleen from the 5 mg supplemented group showed congestion of the blood vessels, desquamation of endothelium (arrow) and perivascular infiltration (H). The spleen from the 10 mg/kg supplemented group demonstrated necrosis of the lymphoid follicle (arrow) (I), as did the spleen from the 20 mg/kg supplemented group (arrow) (G).
Representative micrographs for liver (A–E) and kidney (F–G) of broiler chickens treated with different dietary AgNP doses for 42 days, H&E stain ().
Figure 3. Representative micrographs for liver (A–E) and kidney (F-J) of broiler chickens treated with different levels AgNPs for 42 days, H&E stain. A) Liver from control group showing normal hepatic architecture. (B) Liver from 2.5 mg group showing focal mononuclear infiltration (arrow). (C) Liver from 5 mg group showing congestion and vacuolar degeneration. (D) Liver from 10 mg group showing hepatocellular vacuolation (arrow). (E) Liver from 20 mg group showing hepatocellular vacuolation (arrow). F) Kidney from control group showing normal glomerulus and renal tubules. (G) Kidney from 2.5 mg group showing normal glomerulus and renal tubules. (H) Kidney from 5 mg group showing congestion of the glomerulus (arrow). (I) Kidney from 10 mg group showing severe congestion of the blood vessels (arrow). (J) Kidney from 20 mg group showing congestion of the glomerulus (arrow).
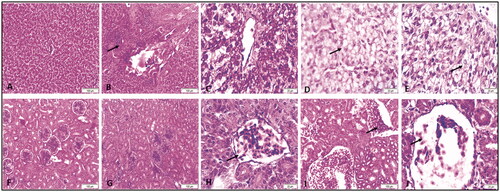
Liver: The liver from the control group showed normal hepatic architecture (A). The liver from the 2.5 mg/kg group demonstrated focal mononuclear infiltration (arrow) (B). The liver from the 5 mg/kg supplemented group exhibited congestion and vacuolar degeneration (C). The liver from the 10 mg/kg supplemented group showed hepatocellular vacuolation (arrow) (D), as did the liver from the 20 mg/kg group (arrow) (E).
Kidney: In the control group, the kidney exhibited normal glomerulus and renal tubules (F). Kidneys from the 2.5 mg/kg group showed normal glomerulus and renal tubules (G). Kidneys from the 5 mg/kg group presented congestion of the glomerulus (arrow) (H). Kidneys from the 10 mg/kg group showed severe congestion of the blood vessels (arrow) (I). Kidneys from the 20 mg/kg group showed congestion of the glomerulus (arrow) (G).
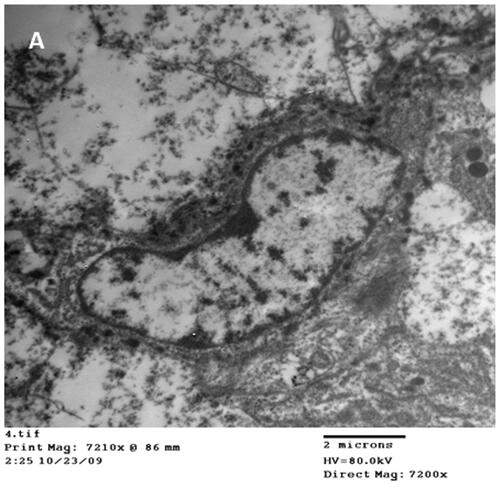
Representative transmission electron microscope micrographs of duodenums from broiler chickens treated with 20 mg/kg AgNPs for 42 days showing AgNPs of different sizes inside the nucleus (N) of the enterocyte (E) ().
Figure 4. Representative micrographs of Transmission electron microscope of duodenum from broiler chickens treated with 20 mg AgNPs for 42 days showing nano particles of different size inside the nucleus (A) of the enterocyte.
A summary of the lesions at 42 days in all examined organs is presented in Table . The severity of lesions increased in all examined tissues and organs (duodenum, liver and kidney) in a dose-dependent manner. The smallest lesions were observed at 2.5 mg/kg dietary AgNPs.
Table 7. Summary of lesion score at 42 days in all studied groups.
Discussion
Several authors have suggested that the addition of a solution of AgNPs to broilers under ideal conditions either inhibits or stimulates growth performance (Saleh and El-Magd Citation2018; Kumar and Bhattacharya Citation2019; Kumar et al. Citation2020; Bolandi et al. Citation2021; Dosoky et al. Citation2021). In the current study, growth performance improvement observed after incorporating different levels of AgNPs ( Saleh and El-Magd Citation2018 [50 ppm]; Kumar and Bhattacharya Citation2019 [50 ppm], Kumar et al. Citation2020 [50 ppm], Bolandi et al. Citation2021 [25, 50, 75 ppm]; Dosoky et al. Citation2021 [2, 4, 8 ppm] ) was consistent with the results of these previous studies. The improvement could be attributed to the antimicrobial effect of AgNPs on harmful intestinal bacteria, improving gut health and the absorption of nutrients at low doses 2.5 ppm because the higher dose > 2.5 ppm induce had a negative impact. The improvement in gut health leads to enhanced nutrient absorption, as seen by increased weight gain, feed conversion ratio and feed intake in broilers fed AgNP diets (Andi et al. Citation2011).
AgNPs also have anti-inflammatory properties because they regulate the expression of matrix metalloproteinases, proteolytic enzymes involved in various inflammatory and healing processes (Nadworny et al. Citation2010). Stimulating digestive enzyme activity is another proposed reason for the AgNPs growth stimulatory effect. AgNPs improve the animal’s health and immunological state by allowing them to expend fewer nutrients for the metabolic effort required for immunological control, allowing them to utilise those extra nutrients for other physiological and productive functions (Fondevila Citation2010). In contrast, other studies found that a diet supplemented with AgNPs negatively impacted chicken performance (Ahmadi and Rahimi Citation2011 [0, 4, 8, 12 ppm]; Pineda et al. Citation2012 [0, 10, 20 ppm]; Vadalasetty et al. Citation2018 [50 ppm]). These inconsistent results are possibly attributable to differences in nanoparticle size, the method of synthesis, the dosage, or the means of administration.
The decrease in cumulative body weight and relative organ weights of organs in broilers supplemented with a high dose (20 mg/kg dietary AgNP) might be due to AgNPs reducing protein enzymatic digestibility and blocking the absorption of sugars and amino acids. Another possible reason might be that the birds undergo more cellular stress and excessive cellular interactions when consuming AgNPs (Vadalasetty et al. Citation2018).
The improvement in dressing percentages and relative organ weight in chickens administered low AgNP doses is partially consistent with Elkloub et al. (Citation2015). In contrast, Ahmadi and Rahimi (Citation2011) reported that the levels of AgNPs 4, 8 and 12 ppm silver nanoparticles increased the weight of small intestine and abdominal fat and had non-significant effects on liver and gizzard % weight. Moreover, Felehgari et al. (Citation2013) found that the relative weight of the small intestine and liver increased, but the gizzard, proventriculus and pancreas were not affected by the addition of different levels of AgNPs and inorganic selenium.
The improvement in the water holding capacity of breast and thigh muscle from chickens administered the lowest dose of AgNPs (2.5 mg/kg) is consistent with results obtained previously (Hashemi Citation2014; Hashemi et al. Citation2017). These results indicate that the lower dose induces low protein oxidation levels (Hashemi Citation2014).
The histopathology of the duodenum, liver, kidneys, and spleen tissues from control and NS2.5 chickens were within normal histologic limits without particular abnormalities, indicating that AgNPs have no deleterious influence on the histologic structure at such dose levels. However, birds given higher doses of AgNPs (5, 10 and 20 mg/kg) had mild to moderate pathologic lesions ranging from the congestion of blood vessels, necrosis and sloughing of epithelium, necrosis in the lymphoid tissue, and focal accumulation of mononuclear cells. Similar findings were reported previously (Loghman et al. Citation2012; Samani et al. Citation2018; Dosoky et al. Citation2021). Those researchers found that the administration of high doses (Loghman et al. Citation2012 [8 and 12 ppm]; Samani et al. Citation2018 [10 and 100 µg/ml]; Dosoky et al. Citation2021 [8 and 12 ppm]) of AgNPs induced mild necrotic changes and inflammatory cell infiltration in the liver, spleen and kidney tissues of broiler chickens. Several authors proposed that the toxic action of AgNPs was due to its ability to generate reactive oxygen species and consequently cause DNA damage by oxidative stress in mammalian cells (Hussain et al. Citation2005; Choi et al. Citation2010; Chen et al. Citation2014; El Mahdy et al. Citation2015). These findings could also be explained by AgNPs reaching the cells of numerous organs, such as the kidneys, liver and lymphoid organs, by binding to plasma proteins (Wijnhoven et al. Citation2009). In contrast, other authors found that a variable dose of oral AgNPs (2.87–63.74 mg/bird) or 50 mg/kg in drinking water did not result in silver accumulation in broiler breast muscles and did not affect tissue histology or morphology (Kulak et al. Citation2018; Kumar et al. Citation2020).
Previous research has shown that adding dietary AgNP powder reduces the population of lactose-positive and enterococcal bacteria and increases the Lactobacillus population after three weeks from treatments (day 28) (Ahmadi and Kurdestany Citation2010; Elkloub et al. Citation2015; Bolandi et al. Citation2021). Results of several studies (Fondevila Citation2010, Vadalasetty et al. Citation2018) indicated that the application of AgNPs in drinking water at 5, 15 and 25 mg/L had no impact on growth or intestinal microbial count, but increased the number of lactobacilli. Differences in AgNP concentrations, species, dietary ingredients, and even bacterial counting methods could explain some discrepancies in the results in different studies.
The addition of AgNPs significantly impacted the mean values of various blood parameters (Table ). This finding is consistent with Ahmadi (Citation2012), who fed broiler chicks with feed supplemented with 20, 40, 60 ppm AgNPs and observed significant changes in total protein, albumin, and globulin. Serum total cholesterol significantly (p < .05) and triglycerides non-significantly (p > .05) decreased in all treatments relative to the control. Those results indicate that AgNPs had no negative effect on lipid profile or blood indices as reported previously (Sawosz et al. Citation2009; Andi et al. Citation2011; Ognik et al. Citation2016; Kumar et al. Citation2020). Ognik et al. (Citation2016) reported that the decrese in the total cholesterol could be due to degradation of polyunsaturated fatty acid and as a result of lipid peroxidation induced by AgNPs.
Previous research demonstrated that AgNP dietary supplementation resulted in silver residue accumulation in broiler tissues and organs in a dose-dependent manner and that this accumulation is beneficial, which supports the current findings (Kulak et al. Citation2018; Kumar et al. Citation2020). Our data support evidence from previous studies that excess silver is not efficiently removed (Hadrup and Lam Citation2014). Furthermore, because silver has a long elimination half-life, it accumulates in the human body. The European Food Safety Authority established a maximum silver concentration of 0.05 mg/L in water and 0.05 mg/kg in food (EFSA. Citation2016). For chronic oral silver exposure, the United States Environmental Protection Agency established a reference dose of 5 g/kg body weight/d (SCENIHR Citation2014). Although the level of silver in broiler breast and thigh muscle may be below the safety limits set for silver in food, it may still be toxic because of its small size and Ag + release capabilities. Incorporating AgNPs into a poultry diet can induce a wide range of adverse effects and can be harmful to both birds and humans (Leino et al. Citation2021). The results of the present study indicate that the accumulation of AgNPs increases in a dose dependent manner in different broiler meat parts and the hazards from the transmission of nanosilver to humans requires that their use and marketing as feed additives or growth promotors be controlled and restricted.
Conclusions
We found that despite its potential effects on growth performance, carcass traits and caecal microbial population diversity at a dose of 2.5 ppm diet, dietary inclusion of AgNPs in broiler diets had many negative effects in terms of 1) silver residue in breast and thigh muscle in a dose dependent manner and the possibility of transmission of nanosilver to consumers and 2) the cytotoxic effects of AgNPs on intestinal, liver, spleen and kidney cells. Thus, the use of dietary AgNP powder might be harmful to chicken and human health. Therefore, we suggest the use of lower dose of AgNPs (< 2.5 ppm) to be tried in further studies.
Ethical approval
All experimental protocols were approved by the ethics committee for Laboratory Animal use and care at King Faisal University in accordance with the institutional and national guidelines of King Faisal University for laboratory animal research.
Author contributions
All of the authors contributed equally to the experimental design, sample collection and analysis, and manuscript writing and revision. All authors approved the final draft of the work.
Acknowledgments
The authors acknowledge the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia for the financial support of this research through the Annual Funding track (GRANT306).
Disclosure statement
The authors report no potential conflicts of interest regarding the publication of this paper.
Data availability statement
The corresponding author is committed to providing data that support the findings of the study upon request.
Additional information
Funding
References
- Abdelsalam M, Al-Homidan I, Ebeid T, Abou-Emera O, Mostafa M, El-Razik A, Shehab-El-Deen M, Abdel Ghani S, Fathi M. 2019. Effect of silver nanoparticle administration on productive performance, blood parameters, antioxidative status, and silver residues in growing rabbits under hot climate. Animals. 9(10):967.
- Ahmadi F. 2012. Impact of different levels of silver nanoparticles (Ag-NPs) on performance, oxidative enzymes and blood parameters in broiler chicks. Pak Vet J. 32:325–328.
- Ahmadi F, Kurdestany AH. 2010. The impact of silver nano particles on growth performance, lymphoid organs and oxidative stress indicators in broiler chicks. Glob Vet. 5(6):366–370.
- Ahmadi F, Rahimi F. 2011. The effect of different levels of Nano Silver on performance and retention of silver in edible tissues of broilers. World Appl. Sci J. 12(1):1–4.
- Al-Sultan SI, Abdel-Raheem SM, El-Ghareeb WR, Mohamed MH. 2016. Comparative effects of using prebiotic, probiotic, synbiotic and acidifier on growth performance, intestinal microbiology and histomorphology of broiler chicks. JPN J Vet Res. 64(Supplement 2):S187–S95.
- Andi MA, Hashemi M, Ahmadi F. 2011. Effects of feed type with/without nanosil on cumulative performance, relative organ weight and some blood parameters of broilers. Glob Vet. 7(6):605–609.
- Atiyeh BS, Costagliola M, Hayek SN, Dibo SA. 2007. Effect of silver on burn wound infection control and healing: review of the literature. Burns. 33(2):139–148.
- Awaad MH, Moustafa KE, Zoulfakar SA, Elhalawany MS, Mohammed FF, El-Refay RM, Morsy EA. 2021. The role of silver nanoparticles in the reluctance of colisepticemia in broiler chickens. J Appl Poult Res. 30(2):100155.
- Awad WA, Ghareeb K, Abdel-Raheem S, Böhm J. 2009. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult Sci. 88(1):49–56.
- Banchroft JD, Stevens A, Turner DR. 1996. Theory and practice of histological techniques 4th ed. New York, London, San Francisco, Tokyo: Churchil Livingstone.
- Bolandi N, Hashemi SR, Davoodi D, Dastar B, Hassani S, Ashayerizadeh A. 2021. Performance, intestinal microbial population, immune and physiological responses of broiler chickens to diet with different levels of silver nanoparticles coated on zeolite. Ital J Anim Sci. 20(1):497–504.
- Bozzola JJ, Russell LD. 1999. Electron microscopy: principles and techniques for biologists. Boston: Jones and Bartlett. p. 670.
- Chattopadhyay MK. 2014. Use of antibiotics as feed additives: a burning question. Front Microbiol. 5:334.
- Chen Q, Xue Y, Sun J. 2014. Hepatotoxicity and liver injury induced by hydroxyapatite nanoparticles. J Appl Toxicol. 34(11):1256–1264.
- Choi JE, Kim S, Ahn JH, Youn P, Kang JS, Park K, Yi J, Ryu DY. 2010. Induction of oxidative stress and apoptosis by silver nanoparticles in the liver of adult zebrafish. Aquat Toxicol. 100(2):151–159.
- Dosoky WM, Fouda MM, Alwan AB, Abdelsalam NR, Taha AE, Ghareeb RY, El-Aassar MR, Khafaga AF. 2021. Dietary supplementation of silver-silica nanoparticles promotes histological, immunological, ultrastructural, and performance parameters of broiler chickens. Sci Rep. 11(1):1–15.
- EFSA. 2016. Panel on Food Additives and Nutrient Sources added to Food (ANS), 2016. Scientific opinion on the re‐evaluation of silver (E 174) as food additive. EFSA J. 14(1):4364.
- El Mahdy MM, Eldin TA, Aly HS, Mohammed FF, Shaalan MI. 2015. Evaluation of hepatotoxic and genotoxic potential of silver nanoparticles in albino rats. Exp Toxicol Pathol. 67(1):21–29.
- Elkloub K, El Moustafa M, Ghazalah AA, Rehan AA. 2015. Effect of dietary nanosilver on broiler performance. Inter J Poultry Sci. 14(3):177–182.
- Felehgari K, Ahmadi F, Rokhzadi A, Kurdestany AH, Khah MM. 2013. The effect of dietary silver nanoparticles and inorganic selenium supplementation on performance and digestive organs of broilers during starter period. Bull Env Pharmacol Life Sci. 2(8):104–108.
- Fondevila M. 2010. Potential use of silver nanoparticles as an additive in animal feeding. In: David Pozo (Eds.), Silver Nanoparticles. Vol 1. Rijeka, Croatia: InTech. p. 325–334.
- Hadrup N, Lam HR. 2014. Oral toxicity of silver ions, silver nanoparticles and colloidal silver-a review. Regul Toxicol Pharmacol. 68(1):1–7.
- Hashemi SR. 2014. Meat quality attributes of broiler chickens fed diets supplemented with silver nanoparticles coated on zeolite. Poult Sci J. 2(2):183–193.
- Hashemi SR, Davoodi D, Dastar B. 2017. Effect of clinoptilolite coated with silver nanoparticles on meat quality attributes of broiler chickens during frozen storage. Iran J Appl Anim Sci. 7(2):321–328.
- Hassanpour H, Mirshokraei P, Sadrabad EK, Dehkordi AE, Layeghi S, Afzali A, Mohebbi A. 2015. In vitro effect of nanosilver on gene expression of superoxide dismutases and nitric oxide synthases in chicken Sertoli cells. Animal. 9(2):295–300.
- Honikel, K.O. and R. Hamm. 1994. Measurement of water-holding capacity, and juiciness. In A.M. Pearson, and T.R. Dutson (Eds.), Quality attributes and their measurement in meat, poultry and fish products, Advances in Meat Research (Vol. 9, pp. 125-161). Blackie Academic, and Professional.
- Hussain SM, Hess KL, Gearhart JM, Geiss KT, Schlager JJ. 2005. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol Vitro. 19(7):975–983.
- Isaac RA, Johnson WC. J 1998. Elemental determination by inductively coupled plasma atomic emission spectrometry. I. In:Handbook of reference methods for Plant Analysis; Kalra, Y.P., (Eds.).; CRC Press: Boca Raton, FL, USA, pp. 165–170.
- Kulak E, Ognik K, Stępniowska A, Sembratowicz I. 2018. The effect of administration of silver nanoparticles on silver accumulation in tissues and the immune and antioxidant status of chickens. J Anim Feed Sci. 27(1):44–54.
- Kumar I, Bhattacharya J. 2019. Assessment of the role of silver nanoparticles in reducing poultry mortality, risk and economic benefits. Appl Nanosci. 9(6):1293–1307.
- Kumar I, Bhattacharya J, Das BK, Lahiri P. 2020. Growth, serum biochemical, and histopathological responses of broilers administered with silver nanoparticles as a drinking water disinfectant. 3 Biotech. 10(3):1–12.
- Leino V, Airaksinen R, Viluksela M, Vähäkangas K. 2021. Toxicity of colloidal silver products and their marketing claims in Finland. Toxicol Rep. 8:106–113.
- Loghman A, Iraj SH, Naghi DA, Pejman M. 2012. Histopathologic and apoptotic effect of nanosilver in liver of broiler chickens. Afr. J. Biotechnol. 11(22):6207–6211.
- Małaczewska J. 2014. Impact of noble metal nanoparticles on the immune system of animals. Medycyna Weterynaryjna. 70(4):204–208.
- Nabinejad AR, Noaman V, Khayyam Nekouiee M. 2016. Evaluation of silver residues accumulation in tissues of Broilers treated with nanosilver using MNSR (A Clinical Trial). Arch. Razi Inst. 71(1):51–55.
- Nadworny PL, Wang J, Tredget EE, Burrell RE. 2010. Anti-inflammatory activity of nanocrystalline silver-derived solutions in porcine contact dermatitis. J Inflamm. 7(1):1–20.
- NRC 1994. Nutrient requirements of poultry. 9th Rev ed. Washington, DC: National Academic Press.
- Ognik K, Sembratowicz I, Cholewińska E, Wlazło Ł, Nowakowicz-Dębek B, Szlązak R, Tutaj K. 2016. The effect of chemically-synthesized silver nanoparticles on performance and the histology and microbiological profile of the jejunum in chickens. Ann Anim Sci. 16(2):439–450.
- Pal A, Shah S, Devi S. 2009. Microwave-assisted synthesis of silver nanoparticles using ethanol as a reducing agent. Mater Chem Phys. 114(2-3):530–532.
- Patra A, Lalhriatpuii M. 2020. Progress and prospect of essential mineral nanoparticles in poultry nutrition and feeding—A review. Biol Trace Elem Res. 197(1):233–253.
- Percival SL, Bowler PG, Russell D. 2005. Bacterial resistance to silver in wound care. J Hosp Infect. 60(1):1–7.
- Pineda L, Chwalibog A, Sawosz E, Lauridsen C, Engberg R, Elnif J, Hotowy A, Sawosz F, Gao Y, Ali A, et al. 2012. Effect of silver nanoparticles on growth performance, metabolism and microbial profile of broiler chickens. Arch Anim Nutr. 66(5):416–429.
- Saleh AA, El-Magd MA. 2018. Beneficial effects of dietary silver nanoparticles and silver nitrate on broiler nutrition. Environ Sci Pollut Res Int. 25(27):27031–27038.
- Salem HM, Ismael E, Shaalan M. 2021. Evaluation of the effects of silver nanoparticles against experimentally induced necrotic enteritis in broiler chickens. Int J Nanomed. 16:6783–6796.
- Samani PY, Samani PY, Arabi M, Shadkhast M, Samani PY, Piraei E. 2018. Repeated-dose toxicity in mouse liver and kidney after skin exposure to silver nanoparticles. J Clin Diagnostic Res. 12(1):1–4.
- Sawosz E, Grodzik M, Zielińska M, Niemiec T, Olszańska B, Chwalibog A. 2009. Nanoparticles of silver do not affect growth, development and DNA oxidative damage in chicken embryos. Arch. für Geflugelkunde. 73(3):208–213.
- SCENIHR 2014. Opinion on Nanosilver: Safety, Health and Environmental Effects and Role in Antimicrobial Resistance, European commission.
- SPSS 2010. IBM Corp. Released 2010. IBM SPSS statistics for windows, version 19.0. Armonk, NY: IBM Corp.
- Steel RG, Torrie JH. 1980. Principles and procedures of statistics a biometrical approach. 2nd ed. New York, NY: Mc Grow- Hill Book Co.
- Vadalasetty KP, Lauridsen C, Engberg RM, Vadalasetty R, Kutwin M, Chwalibog A, Sawosz E. 2018. Influence of silver nanoparticles on growth and health of broiler chickens after infection with Campylobacter jejuni. BMC Vet Res. 4(1):1–11.
- Wang B, He X, Zhang Z, Zhao Y, Feng W. 2013. Metabolism of nanomaterials in vivo: blood circulation and organ clearance. Acc Chem Res. 46(3):761–769.
- Wijnhoven SW, Peijnenburg WJ, Herberts CA, Hagens WI, Oomen AG, Heugens EH, Roszek B, Bisschops J, Gosens I, Van De Meent D, et al. 2009. Nano-silver–a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology. 3(2):109–138.


