Abstract
In many European countries, the common pheasant is one of the most popular game bird species. Despite its popularity, little information has been published related to its sperm profile, including the common semen parameters used to characterise the quality of this material. Additionally, the spermatozoon’s ultrastructure has never been characterised, and very few data on reproductive performance in the male bird are available. The aim of the present study was to provide these data and fill these gaps in the literature. Semen was collected by the dorso-abdominal massage technique for pheasants and evaluated during the late spring period (May to mid-June). For the ultrastructure and morphometric descriptions, scanning electron and transmission electron images were obtained. The data show that pheasants produce ejaculates of a smaller volume (average volume = 131 µL) but with a higher sperm concentration compared with roosters. Semen pH values tended towards alkaline values (average pH = 8.3). The general shape of the pheasant spermatozoon resembles that of roosters and turkeys, although a bigger sub-acrosomal space was observed compared with these species. As a consequence, its perforatorium occupies two-thirds of the acrosome. A lower number of mitochondria (20–24) was also detected compared with roosters and turkeys. The pheasant spermatozoon’s dimensions are most similar to those observed in turkeys and guineafowl. The present study provides basic information useful for assisted reproductive techniques and conservation programmes.
Basic information on the common pheasant semen is given.
A morphometric description of the common pheasant sperm cell is reported.
The characterisation of the sperm ultrastructure by scanning and transmission electron microscopy is shown.
HIGHLIGHTS
Introduction
Of the various game bird species, the common pheasant is one of the most popular across many European countries, and its presence is highly dependent on croplands and agricultural landscapes (Draycott et al. Citation2008; Holá et al. Citation2015). Despite its popularity, little information is available on its reproductive biology, more precisely on its sperm profile, which includes the common semen parameters used to evaluate the quality of the material (Mantovani et al. Citation1993; Herrera et al. Citation2005; Castillo et al. Citation2009; Citation2021). For example, ejaculate volumes and sperm concentration, viability and mobility are some of the essential parameters required in order to optimise its use for artificial insemination (Faustino et al. Citation2015) or for the cryopreservation of the male gametes as part of a conservation programme (Santiago-Moreno et al. Citation2016). Indeed, the conjunction of qualitative sperm assays may be used to reliably predict the fitness of the evaluated material (Santiago-Moreno et al. Citation2016).
Considering poultry species of particular economic interest, the ultrastructure of the rooster spermatozoon was first described in 1949 (Grlgg and Rodge Citation1949), and several studies were published thereafter (Bakst and Howarth Citation1975; Gunawardana and Scott Citation1977; Bakst and Sexton Citation1979; Thurston and Hess Citation1987; Froman Citation2007; Aire Citation2014; Soley and du Plessis Citation2020). The ultrastructure of the spermatozoon has also been described in turkeys (Marquez and Ogasawara Citation1975; Thurston and Hess Citation1987), quail (Korn et al. Citation2000), guinea fowl (Thurston et al. Citation1982; Thurston and Hess Citation1987), drakes (Simões et al. Citation2012; Soley and du Plessis Citation2020) and ostriches (Soley Citation1993; Soley and Roberts Citation1994). Similarities, but also evident differences, have been observed in the spermatozoon from these species, as well as between the three domestic exponents of the Phasianidae family, i.e. chicken, turkey and quail (Thurston and Hess Citation1987; Jamieson Citation2011). The pheasant (Phasianus colchicus mongolicus) is representative of another important genus of this taxonomic family, though little information is available regarding the morphometry of its spermatozoon (Ducci et al. Citation1998; Immler et al. Citation2007). Moreover, to the best of our knowledge, no study has reported its three-dimensional and ultrastructural features.
Information about spermatozoon morphology and morphometry is valuable for a number of reasons. For instance, spermatozoon morphology may be useful for predicting the “freezabilty” of the cell (i.e. its resilience to withstand cryopreservation) This capacity can be affected by spermatozoon head size, which may influence its water content and the plasma membrane permeability to the cryoprotectant, which may, in turn, affect cell viability following cryopreservation (Esteso et al. Citation2006; Santiago-Moreno et al. Citation2016). Knowledge of normal sperm morphology is also useful as a biological marker of an animal’s exposure to environmental pollutants, which have the potential to affect the different spermatogenesis stages; for example, by causing changes to the spermatozoon total length (Riana Bornman and Bouwman Citation2012; Santiago-Moreno et al. Citation2016). Spermatozoon ultrastructure can also be useful as a tool in taxonomic and phylogenetic relationship studies (Faustino et al. Citation2015; Soley and du Plessis Citation2020).
The aim of this study was to characterise spermatozoon morphology in the common pheasant by means of scanning (SEM) and transmission (TEM) electron microscopy and to evaluate some basic qualitative semen parameters attesting the validity of the collected material and the presence of performing cells.
Materials and methods
Reagents
All chemicals were purchased from Sigma Aldrich (Milano, Italy), with the exception of Accudenz, a cell separation media, that was purchased from Accurate Chemical and Scientific Corp. (Westbury, NY, USA).
Birds
Eighteen male pheasants (Phasianus colchicus mongolicus) were housed for their first reproductive season in a peaceful location, far from public roads, in open-air aviaries containing perches; each male was housed separately in a 6 m2 allocated space (Castillo et al. Citation2009). Birds were fed a basal commercial feed ad libitum which provided 11.51 MJ/kg of M.E. and 19% of C.P. Semen was collected eleven times (every 3–4 days) over a 35-day period. This trial was carried out at the University of Pisa Avian Research Station “Podere le Querciole,” situated in San Piero a Grado, Pisa, Italy; 43°39'54.5"N 10°20'40.0"E.
Semen collection
Semen collection was performed eleven times over a 35-day period in late spring, from May to June, by means of the dorso-abdominal massage technique as described for pheasants (Castillo et al. Citation2021). Semen from each male was collected directly into the collection tube containing 50 µL of Lake’s diluent (Lake Citation1968). Once all donations had been collected, they were subjected to macroscopic selection, such that only dense, milky white ejaculates were used for further analysis.
Evaluation of semen characteristics
Ejaculate volume was assessed by weighing the tubes before and after collection (Sartorius BL 150S precision balance, readability ± 0.001 g). The pH was measured for three random samples in the absence of diluent (Hamilton pH Electrode 238140, Hanna Instruments, Italy). Sperm concentration was assessed in duplicate using a Bürker-Türk counting chamber (in 5% formalin/0.9% NaCl solution). The sperm viability percentage and sperm morphology were evaluated in triplicate in samples of approximately 500 cells using the eosin-nigrosin staining technique (Bakst Murray and Cecil Citation1997). Viable cells did not stain at all, whereas those which stained totally or partially pink were considered dead. The percentage of normal sperm was calculated relative to total live sperm. Spermatozoa mobility was assessed in triplicate using the Accudenz methodology (Froman and McLean Citation1996).
Ultrastructure of the spermatozoon
Samples were subjected to both scanning electron (SEM) and transmission electron microscopy (TEM) to obtain a detailed description of normal sperm morphology from fresh semen. Samples were fixed for 2 h in a mixture of 4% paraformaldehyde and 5% glutaraldehyde in sodium cacodylate, 0.1 M buffer solution, pH 7.2 (Karnovsky Citation1965). Then, after washing in the same buffer overnight at 4 °C, samples were post-fixed in 1% osmium tetroxide and 0.075% ruthenium red in sodium cacodylate, 0.1 M buffer solution, pH 7.2, for 1 h at room temperature. After three washes in distilled water (15 min each at 4 °C), samples were block-stained for 1 h at 4 °C with 1% uranyl acetate in distilled water. They have washed again in distilled water and dehydrated using a graded series of acetone solutions (from 30 to 100%). For SEM, spermatozoa were let to adhere onto poly-l-lysine coated coverslips and critical-point dried in a Balzer Union CPD 020 apparatus. They were mounted on aluminium stubs, coated with gold using the Balzer Union MD 010 sputtering device, and observed under a JEOL JSPM-5200 scanning electron microscope. For TEM, after the dehydration step, samples were infiltrated using a series of EPON-based resin and acetone mixtures, with increasing concentrations of resin, before being immersed in pure resin overnight. Infiltration was performed in a rotator at room temperature. Embedding was performed by placing the samples into new pure resin in moulds and leaving them to polymerise in an oven at 60 °C for two days. Ultra-thin sections (60–80 nm) were cut using a Reichert Yung ultramicrotome and collected on copper grids, then stained with 2% uranyl acetate and lead citrate and examined in a JEOL 1200 EX II electron microscope. Micrographs were acquired using an Olympus SIS VELETA CCD camera equipped with iTEM software.
Spermatozoon morphometry
Spermatozoon electron micrographs analysed by ImageJ software (http://rsb.info.nih.gov/ij/) permitted measurement of the following cell parts: total length, maximum cell width, and head, acrosome, nucleus, midpiece and flagellum length. A total of 150 spermatozoa were evaluated.
Statistical analysis
The results are presented as means ± standard deviation (SD). All analyses were performed at a significance level of p < 0.05 using JMP Statistical Discovery (SAS Institute Inc., Cary, NC, USA, v. 5.0.1.). The parameters of the fresh semen obtained on the eleven collection days were analysed by one-way ANOVA, followed by Tukey’s test for the comparison of means. The day of collection was considered the main factor. The correlation between the semen qualitative parameters was evaluated using the CORR procedure (SAS Studio, v. 3.8; https://www.sas.com/it_it/software/studio.html).
Results
Semen qualitative parameters
Table reports pheasant semen qualitative parameter mean data for the 18 birds in their first reproductive season over the 35-day collection period in late spring (May to mid-June). To facilitate their comparison with other species, published data for roosters and turkeys are also shown. The mean ejaculate volume obtained in pheasants was closer to the minimum value reported for both roosters (Santiago-Moreno et al. Citation2018) and turkeys (Iaffaldano et al. Citation2021). The pheasant's mean pH (8.3) tended toward alkalinity, whereas it is closer to neutral in the other two species, especially in the rooster (6.8–7.8) (Castillo et al. Citation2010; Hu et al. Citation2013; Attia et al. Citation2019). Mean sperm concentration in pheasant semen (8.6 × 109 sperm/mL) was almost double the maximum value reported for rooster semen (4.7 × 109 sperm/mL (Castillo et al. Citation2010). The difference between the minimum and the maximum sperm concentration values in the pheasant was 3.7 × 109 sperm/mL, whereas in the turkey this range is much wider at 5.9 × 109 sperm/mL.
Table 1. Pheasant semen qualitative parameters (mean data; n = 182 ejaculates from 18 birds in their first reproductive season) and analogous data for roosters and turkeys were taken from the literature.
Table reports the semen qualitative parameter mean data for each of the 11 sampling days over the course of the 35-day collection period. No, statistically significant differences were observed across the sampling period in ejaculate volume, pH, sperm concentration, or sperm viability. Sperm mobility, assessed as absorbance units at 550 nm, did show significantly different values (p < 0.01), with a higher number of mobile sperm observed on days 10, 17 and 31. A statistical difference was also observed in the percentage of normal sperm cells (p < 0.01), with a higher mean number of normal cells found from day 17 onward. A significant correlation was observed between the pH and the percentage of normal cells (p < 0.01). For all the other parameters, no correlation was detected.
Table 2. Pheasant semen qualitative parameters over the course of a 35-day period in the birds’ first reproductive season (data mean ± SD).
Morphological and ultrastructural characteristics of the common pheasant spermatozoon
Scanning electron micrographs revealed the distinctive filiform shape of the sperm cell. The mean sperm cell length was 79.2 ± 1.6 µm (Figures and ). The sperm head is slender, with a mean length of 11.8 ± 0.7 µm, and its anterior end consists of a conical acrosome. The maximum sperm cell width, at the junction of the nucleus to the midpiece, was 0.63 ± 0.1 µm. The mean flagellum length was 63.7 ± 1.9 µm.
Figure 1. The common pheasant spermatozoon ultrastructure: SEM images (A–C) and TEM images of longitudinal sections (A1, D–F, M) and transversal sections (G–L). (A) general view of the spermatozoon (scale bar = 10 µm); (A1) TEM micrograph of the endpiece. (B) acrosome and sperm head (scale bar = 1.5 µm). (C) part of the flagellum with the midpiece (scale bar = 1 µm). (D) acrosomal region and anterior part of the nucleus (scale bar = 0.5 µm). (E) nucleus base and centriolar region (scale bar = 500 nm). (F) the midpiece with the mitochondria (scale bar = 1 µm). (G) acrosomal region (scale bar = 200 nm). (H) acrosome and perforatorium section (scale bar = 200 nm). (I) perforatorium and nucleus section (scale bar = 200 nm). (J) nucleus section (scale bar = 500 nm). (K) midpiece section with mitochondria and axoneme (scale bar = 200 nm). (L) principal piece section (scale bar = 200 nm). (M) centriolar region. f = flagellum; ep = endpiece; a = acrosome; mp = midpiece; p = perforatorium; n = nucleus; pc = proximal centriole; dc = distal centriole; m = mitochondria; axe = axoneme.
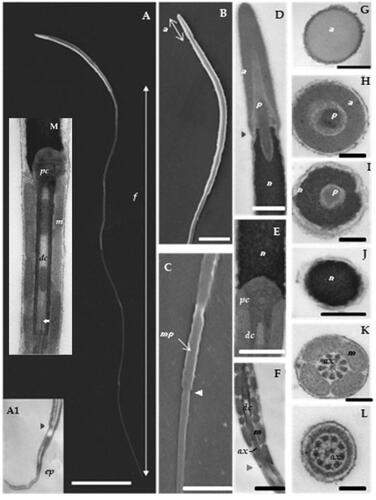
As shown by TEM (Figure ), the acrosome consists of an elongated cone structure with a mean length of 2.2 ± 0.1 µm. Its content appears homogeneous and moderately electron dense, and the transverse section reveals its circular profile (Figure ). The posterior end of the acrosome is attached to the nucleus by a small overlapping joint (Figure ). More than two-thirds of the subacrosomal space is occupied by the perforatorium, which is also held by the nucleus in a conical depression of approximately one-third its length, (Figure ). As in other Galliformes (Jamieson Citation2011), the perforatorium broadens at the nuclear rostrum level (Figure ).
The nucleus is a cylinder-like structure, measuring 9.5 ± 0.9 µm in length, with an anterior rostrum (Figure ), a base formed by a shallow implantation fossa (Figure ), and condensed, electron-dense chromatin (Figure and ).
TEM revealed a typical centriolar complex structure (Figure and ). The proximal centriole is linked to the nucleus by the non-striated connecting piece (Bakst and Howarth Citation1975), and it is orientated perpendicular to the distal centriole. The distal centriole, anteriorly adjacent to the proximal centriole, is on the long axis (Figure and ). Posteriorly, it seems that the central singlets penetrate the distal end of the distal centriole, as shown in micrographs 1-F and 1-M.
The midpiece measures 4.2 ± 0.3 µm in length (Figure ), and its transverse section shows four mitochondria surrounding the axoneme (Figure ). Five or six mitochondria can be counted along its length, resulting in a total of approximately 20–24 (Figure ). The posterior end of the midpiece is characterised by a small annulus (arrow Figure ).
The principal piece has the characteristic structure of the axoneme surrounded by a fibrous sheath (Figure ). The endpiece is demarcated by the loss of the fibrous sheath and, posteriorly, by the progressively disruption of the doublets (Figure ).
Discussion
The present study evaluated the qualitative parameters of pheasant semen collected from 18 captive birds bred in open-air conditions and exposed to a natural photoperiod. The birds were fully sexually mature, and semen samples were collected in late spring (May to early June), which is the height of the breeding season for the geographic location in consideration (Pisa, Italy), which begins in early spring (mid-March) and ends in mid- June. The daily mean ejaculate volumes ranged from a minimum of 116 µL to a maximum of 163 µL, and the 35-day period means the value was 131 µL. The largest individual ejaculate volume was 245 µL of dense semen. This value is high compared with other reports in the literature for pheasants: 17 µL (Herrera et al. Citation2005) and 210 µL (Mashaly et al. Citation1983). Mean ejaculate volumes of 90, 110 and 210 µL were reported for caged birds in the previously cited study carried out on pheasants (Mashaly et al. Citation1983). However, their use of cages makes us suppose that those pheasants would have presented more docile temperaments making them more cooperative than the “wild-temperament birds” our research group is used to working with. Indeed, our experience has evidenced the fundamental importance of the handler’s competence to obtain good quality ejaculates from the latter (Castillo et al. Citation2009), and the housing of pheasants in large over-air aviaries is greatly favourable from the welfare perspective as it reduces the stress and eliminates the high risk of injury birds housed in cages (Castillo et al. Citation2009). Moreover, ejaculate volume is only one indicator of the quality of the collected material. A more important measure is sperm concentration. Unfortunately, Mashaly et al. (Citation1983) do not report this information, and the value reported by Herrera et al. (Citation2005) is four times less concentrated. Mantovani et al. (Citation1993) also reported a mean sperm concentration that was approximately 33% lower than that of the present study. Therefore, it seems that the only analogous values in the literature are those published by our own group using similar rearing conditions, such as Castillo et al. (Citation2021) – a paper in which we discuss the importance of the semen collection method for ensuring a high-quality ejaculate.
Compared with sperm concentrations obtained from roosters (Castillo et al. Citation2010; Saemi et al. Citation2012; Hu et al. Citation2013; Santiago-Moreno et al. Citation2018; Attia et al. Citation2019), pheasant semen is more concentrated, whereas that collected from toms varies according to their genetic origin (Kotłowska et al. Citation2005; Iaffaldano et al. Citation2021), age and diet (Słowińska et al. Citation2011). Thus, the sperm concentration of ejaculates from the pheasants in the present study can be considered as both higher than that reported for turkeys (Bakst and Cecil Citation1981; Kotłowska et al. Citation2005; Słowińska et al. Citation2011; Iaffaldano et al. Citation2021) and lower (Noirault and Brillard Citation1999). However, according to our previously reported data for pheasants (Castillo et al. Citation2009), mean sperm concentration values may reach values of 12.5 × 109 sperm/mL, which is higher than the maximum value reported in toms of 11.4 × 109 sperm/mL (Noirault and Brillard Citation1999). Furthermore, sperm concentration may vary between breeding seasons. In a previous study, we reported a mean increase of 3.5 × 109 sperm/mL between two successive breeding seasons (Castillo et al. Citation2009).
The positive correlation between semen pH and the percentage of normal cells observed in this study lies in accordance with previous data reported in pheasants (Castillo et al. Citation2021). The higher percentage of normal cells was particularly evident in the second half of the evaluated period, which coincided with higher and more constant pH values. The increased number of normal cells could also be related to the breeding season stage. For example, in cranes, the stage of the breeding season was observed to affect the proportion of normal sperm cells, with fewer observed mid-season, followed by a tendency for a higher proportion as the season progressed (Brown et al. Citation2011).
The ultrastructural data show that the general shape of the common pheasant spermatozoon is similar to that of both the turkey and the rooster, with the usual non-passerine components (Jamieson Citation2011). The length of the acrosome (2.2 µm) is comparable to values reported for roosters (2–2.5 µm) (Marquez and Ogasawara Citation1975; Thurston et al. Citation1982; Jamieson Citation2011). A larger range (1–2.6 µm) has been reported for turkeys (Marquez and Ogasawara Citation1975). The base of the vesicle in the pheasant spermatozoon (Figure ), which overlaps the anterior region of the nucleus, seems to be shorter than that reported for roosters and turkeys (Jamieson Citation2011). The pheasant sperm cell has a large sub-acrosomal space, occupying two-thirds of the acrosome; whereas in the rooster it occupies just one-third (Jamieson Citation2011). The distal end of the pheasant perforatorium, which occupies the anterior depression of the nucleus, is similar to that of both the turkey and the rooster sperm cell (Jamieson Citation2011), although that of the rooster is probably slightly wider.
The pheasant spermatozoon nucleus length (9.5 µm) is not dissimilar to that reported for turkeys (7–9 µm) (Thurston and Hess Citation1987), although other authors have reported a larger range in the latter species (7.2–11 µm) (Marquez and Ogasawara Citation1975). By contrast, a longer nucleus has been reported for both roosters and guineafowl (10–14 µm) (Thurston and Hess Citation1987). The length of the common pheasant sperm head (11.8 µm) is comparable to that seen in other pheasant species (Immler et al. Citation2007) with comparable total sperm lengths (79 µm).
As in rooster and turkey sperm (Jamieson Citation2011), the proximal centriole of the pheasant sperm is orientated perpendicular to the distal centriole. However, unlike rooster sperm, in which there is considerable penetration of the central singlets into the distal end of the centriole (Jamieson Citation2011), little penetration was observed in the pheasant sperm, thus making it more similar to turkey sperm (Jamieson Citation2011). According to various authors, the central singlets commence at the posterior end of the distal centriole (Bakst and Howarth Citation1975; Gunawardana and Scott Citation1977).
The length of the midpiece of the pheasant spermatozoon (around 4.2 µm) is similar to the values reported by Immler et al. (Citation2007) for various pheasant species and roosters, whereas the data reported by Jamieson (Citation2011) for roosters was greater at 3.7 µm. Four mitochondria surround the pheasant sperm cell axoneme, the same as in roosters, but the total number of mitochondria per sperm cell is smaller at 20–24 compared with 28–32 in roosters (Bakst and Howarth Citation1975; Jamieson Citation2011) and 25–30 in turkeys (Thurston and Hess Citation1987).
The length of the pheasant spermatozoon flagellum (63.7 µm) is similar to that reported for turkeys, guineafowl (60–65 µm) (Marquez and Ogasawara Citation1975; Thurston and Hess Citation1987) and other pheasant species (62–65 µm) (Immler et al. Citation2007). In roosters, however, longer flagellums measuring 70 µm (Jamieson Citation2011) and 77 µm (Immler et al. Citation2007) have been reported. Similarly, the pheasant spermatozoon's total length (79.2 µm) is more similar to that of turkeys and guineafowl (75–80 µm) (Marquez and Ogasawara Citation1975; Thurston and Hess Citation1987) and some pheasant species such as Phasianus versicolour (Immler et al. Citation2007), whereas the rooster spermatozoon measures 90–110 µm (Lake et al. Citation1968; Thurston and Hess Citation1987; Immler et al. Citation2007).
In this study, semen was evaluated through basic laboratory analyses being reliable in ascertaining the presence of performing male cells and the overall semen quality. Obviously, other biochemical parameters and qualitative aspects of the sperm cell and the seminal plasma are certainly to be studied, particularly due to the increasing availability of advanced technologies. In this sense, future studies could be addressed to identify the seminal components as well as cell metabolism and function and the relationships between sperm cells and the milieu.
Conclusions
Pheasant semen is mainly characterised by its smaller ejaculate volume and higher sperm concentration with respect to that of roosters. The semen pH values tended to be alkaline. The general shape of the pheasant spermatozoon resembles that of rooster and turkey spermatozoa, although it differs in having a bigger sub-acrosomal space, which results in the perforatorium occupying around two-thirds of the acrosome. A smaller total number of mitochondria were observed compared with rooster and turkey sperm cells. In relation to cell dimensions, the pheasant spermatozoon most closely resembles spermatozoa from turkeys and guineafowl. The present study increases our knowledge of reproductive biology in the common pheasant and provides basic information useful for assisted reproductive techniques and conservation programmes.
Institutional review board statement
This study was approved by the Ethics Committee of Pisa University (OPBA; resolution n. 26/2021), under article 2, paragraph 1, point b, of the Italian legislative decree n. 26/2014.
Disclosure statement
The authors declare no conflict of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author, A.S., upon reasonable request.
Additional information
Funding
References
- Aire TA. 2014. Spermiogenesis in birds. Spermatogenesis. 4(3):e959392.
- Attia YA, El-Naggar AS, Abou-Shehema BM, Abdella AA. 2019. Effect of supplementation with trimethylglycine (Betaine) and/or vitamins on semen quality, fertility, antioxidant status, dna repair and welfare of roosters exposed to chronic heat stress. Animals. 9(8):547.
- Bakst MR, Cecil HC. 1981. Changes in the characteristics of turkey ejaculated semen and ductus deferens semen with repeated ejaculations. Reprod Nutr Dev. 21(6B):1095–1103.
- Bakst MR, Howarth B. 1975. The head, neck and midpiece of cock spermatozoa examined with the transmission electron microscope. Biol Reprod. 12(5):632–640.
- Bakst Murray R, Cecil HC. 1997. Sperm viability 1. Nigrosin/Eosin stain for determining liveydead and abnormal sperm counts. In: Techniques for semen evaluation, semen storage, and fertility determination. 2nd ed. Champaign, IL, USA: The Poultry Science Association. p. 29–34.
- Bakst MR, Sexton TJ. 1979. Fertilizing capacity and ultrastructure of fowl and turkey spermatozoa before and after freezing. J Reprod Fertil. 55(1):1–7.
- Brown ME, Crosier A, Lynch W, Converse SJ, Chandler J, Olsen G, French J, Wildt DE, Songsasen N. 2011. 231 Seminal quality in Whooping Crane (Grus Americana) is affected by stage of breeding season but not by age of individual. Reprod Fertil Dev. 23(1):214.
- Castillo A, Lenzi C, Pirone A, Baglini A, Russo C, Soglia D, Schiavone A, di Cossato MMF. 2021. From the semen collection method to the hatchlings: the use of cryopreserved sperm from pheasants fed an antioxidant-enriched diet. Animals. 11(9):2624.
- Castillo A, Marzoni M, Romboli I. 2009. Some advices to breed common pheasants used as donors of good quality semen. In: Avian biology research. Vol. 2. SAGE Publications Ltd; p. 243–243.
- Castillo A, Romboli I, Marzoni M. 2010. Preliminary investigation on fertility and hatchability by the use of cryopreserved cock semen. In: Incubation and fertility research group. Tours: Avian Biology Research.
- Donoghue AM, Kirby JD, Froman DP, Lerner SP, Crouch AN, King LM, Donoghue DJ, Sonstegard TS. 2003. Field testing the influence of sperm competition based on sperm mobility in breeder turkey toms. Br Poult Sci. 44(3):498–504.
- Draycott RAH, Hoodless AN, Woodburn MIA, Sage RB. 2008. Nest predation of common pheasants Phasianus colchicus. IBIS. 150(SUPPL.1):37–44.
- Ducci M, Villani C, Gazzano A, Coli A, Tedeschi D, Marzoni M, Romboli I, Sighieri C, Frateschi TL, Martelli F. 1998. Valutazione morfologica e morfometrica degli spermatozoi di fagiano. Annali della Facoltà di Medicina Veterinaria di Pisa, vol. 50, pp 229, tot. pag 8, 1998. Annali della Facoltà di Medicina Veterinaria di Pisa [Internet]. 50:229–237. http://hdl.handle.net/11568/176670.
- Esteso MC, Fernández-Santos MR, Soler AJ, Montoro V, Quintero-Moreno A, Garde JJ. 2006. The effects of cryopreservation on the morphometric dimensions of Iberian red deer (Cervus elaphus hispanicus) epididymal sperm heads. Reprod Domest Anim. 41(3):241–246.
- Faustino F, Silva RC, Hilbig CC, Makino LC, Senhorini JA, Ninhaus-Silveira A, Nakaghi LSO. 2015. Spermatozoon ultrastructure and semen parameters of brycon vermelha (Characiformes, Characidae). Anim Reprod Sci. 157:17–23.
- Froman DP. 2007. Sperm motility in birds: insights from fowl sperm. Soc Reprod Fertil Suppl. 65:293–308.
- Froman DP, Feltmann AJ. 1998. Sperm mobility: a quantitative trait of the domestic fowl (Gallus domesticus). Biol Reprod. 58(2):379–384.
- Froman DP, McLean DJ. 1996. Objective measurement of sperm motility based upon sperm penetration of accudenz®. Poult Sci. 75(6):776–784.
- Grlgg GW, Hodge AJ. 1949. Electron microscopic studies of spermatozoa: I. The morphology of the spermatozoon of the common domestic fowl (Gallus domesticus). Aust J Biol Sci. 2(3): 271–286.
- Gunawardana VK, Scott MGAD. 1977. Ultrastructural studies on the differentiation of spermatids in the domestic fowl. J Anat. 124(Pt 3):741–755.
- Herrera JA, Quintana JA, López MA, Betancourt M, Fierro R. 2005. Individual cryopreservation with dimethyl sulfoxide and polyvinylpyrrolidone of ejaculates and pooled semen of three avian species. Arch Androl. 51(5):353–360.
- Holá M, Zíka T, Šálek M, Hanzal V, Kušta T, Ježek M, Hart V. 2015. Effect of habitat and game management practices on ring-necked pheasant harvest in the Czech Republic. Eur J Wildl Res. 61(1):73–80.
- Hu J, Chen JL, Wen J, Zhao GP, Zheng MQ, Liu RR, Liu WP, Zhao LH, Liu GF, Wang ZW. 2013. Estimation of the genetic parameters of semen quality in Beijing-You chickens. Poult Sci. 92(10):2606–2612.
- Iaffaldano N, di Iorio M, Rusco G, Antenucci E, Zaniboni L, Madeddu M, Marelli S, Schiavone A, Soglia D, Buccioni A, et al. 2021. Italian semen cryobank of autochthonous chicken and turkey breeds: a tool for preserving genetic biodiversity. Ital J Anim Sci. 20(1):2022–2033.
- Iaffaldano N, Manchisi A, Gambacorta M, di Iorio M, Rosato MP. 2009. Effect of different sperm concentrations on the post-thaw viability and motility of turkey spermatozoa cryopreserved by the pellet method. Ital J Anim Sci. 8(sup2):760–762.
- Immler S, Saint-Jalme M, Lesobre L, Sorci G, Roman Y, Birkhead TR. 2007. The evolution of sperm morphometry in pheasants. J Evolution Biol. 20(3):1008–1014.
- Jamieson BGM. 2011. Reproductive biology and phylogeny of birds: part A: phylogeny, morphology, hormones and fertilization. Boca Raton, USA: CRC Press.
- Karnovsky MJ. 1965. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J Cell Biol. 27:1A–149A.
- Korn N, Thurston RJ, Pooser BP, Scott TR. 2000. Ultrastructure of spermatozoa from Japanese quail. Poult Sci. 79(1):86–93.
- Kotłowska M, Glogowski J, Dietrich GJ, Kozłowski K, Faruga A, Jankowski J, Ciereszko A. 2005. Biochemical characteristics and sperm production of turkey semen in relation to strain and age of the males. Poult Sci. 84(11):1763–1768.
- Lake PE, Smith W, Young D. 1968. The ultrastructure of the ejaculated fowl spermatozoon. Exp Physiol. 53(4):356–366.
- Lake PE. 1968. Sixth International Congress on Animal Reproduction and Artificial Insemination. In: International Congress on Animal Reproduction and Artificial Insemination. Paris; p. 1633–1635.
- Mantovani C, Cerolini S, Bellagamba F, Mangiagalli MG, Cavalchini LG. 1993. Evaluation of pheasant semen production during the reproductive season. Reprod Nutr Dev. 33(6):503–509.
- Marquez BJ, Ogasawara FX. 1975. Scanning electron microscope studies of turkey semen. Poult Sci. 54(4):1139–1142.
- Mashaly MM, Kratzer KR, Keene OD. 1983. Effect of photoperiod on body weight and reproductive performance of ringneck pheasants. Poult Sci. 62(10):2109–2113.
- Ngu GT, Etchu KA, Butswat ISR, Woogeng IN. 2014. Semen and microbial characteristics of two breeds of Turkeys in an arid tropical environment of Bauchi State, Nigeria. Afr J Microbiol Res. 8(21):2174–2182.
- Noirault J, Brillard JP. 1999. Effects of frequency of semen collection on quantitative and qualitative characteristics of semen in turkey breeder males. Poultr Sci. 78(7):1034–1039.
- Riana Bornman MS, Bouwman H. 2012. Environmental pollutants and diseases of sexual development in humans and wildlife in South Africa: harbingers of impact on overall health? Reprod Domest Anim. 47(SUPPL.4):327–332.
- Saemi F, Zamiri MJ, Akhlaghi A, Niakousari M, Dadpasand M, Ommati MM. 2012. Dietary inclusion of dried tomato pomace improves the seminal characteristics in Iranian native roosters. Poultr Sci. 91(9):2310–2315.
- Santiago-Moreno J, Esteso M, Villaverde-Morcillo S, Toledano-Díaz A, Castaño C, Velázquez R, López-Sebastián A, Goya A, Martínez J. 2016. Recent advances in bird sperm morphometric analysis and its role in male gamete characterization and reproduction technologies. Asian J Androl. 18(6):882–888.
- Santiago-Moreno J, Gil MG, Dávila SG, Campo JL, Castãno C, Toledano-Díaz A, Prieto MT, Blesbois E. 2018. Access to pasture in an outdoor housing system affects welfare indicators and improves rooster sperm quality in two native Mediterranean breeds. Poultr Sci. 97(12):4433–4441.
- Simões K, Orsi AM, Artoni SMB. 2012. Ultrastructure of the spermatozoa of the domestic duck (Anas platyrhynchos sp). J Vet Med C: Anat Histol Embryol. 41(3):202–208.
- Słowińska M, Jankowski J, Dietrich GJ, Karol H, Liszewska E, Glogowski J, Kozłowski K, Sartowska K, Ciereszko A. 2011. Effect of organic and inorganic forms of selenium in diets on turkey semen quality. Poultr Sci. 90(1):181–190.
- Soley JT. 1993. Ultrastructure of ostrich (Struthio camelus) spermatozoa: I. Transmission electron microscopy. Onderstepoort J Vet Res. 60(2):119–130.
- Soley JT, du Plessis L. 2020. Ultra-imaging in applied animal andrology: the power and the beauty. Anim Reprod Sci. 220:106306.
- Soley JT, Roberts DC. 1994. Ultrastructure of ostrich (Struthio camelus) spermatozoa. II. Scanning electron microscopy. Onderstepoort J Vet Res. 61(3):239–246.
- Thurston RJ, Hess RA. 1987. Ultrastructure of spermatozoa from domesticated birds: comparative study of turkey, chicken and guinea fowl. Scanning Microsc. 1(4):1829–1838.
- Thurston RJ, Hess RA, Hughes BL, Froman DP. 1982. Ultrastructure of the guinea fowl (Numidia meleagris) spermatozoon. Poult Sci. 61(8):1738–1743.
