Abstract
The aim of this study was to evaluate the effect of enzymolytic soybean meal (ESBM) on antioxidant capacity, intestinal morphology and caecal microbiota in Rex rabbits. One-hundred-twenty 40-day-old healthy Rex rabbits were randomly divided into four groups with thirty replicates in each group and one rabbit per replicate. The control group was fed the basal diet and the ESBM treated groups (T1, T2 and T3) were fed 0.5%, 1% and 1.5% ESBM instead of the equivalent amount of soybean meal (SBM) in the basal diet, respectively. The study lasted 61 days, including a pre-feeding period of 5 days and a formal trial period of 56 days. Compared with the control group, the activity of superoxide dismutase and glutathione peroxidase in the liver, as well as total antioxidant capacity were significantly increased in the T3 group (p < 0.05). The malondialdehyde concentrations in the liver and jejunum were significantly decreased in the T1 group (p < 0.05). There were no significant differences in the antioxidant capacity of jejunum, intestinal morphology index and intestinal pH among all groups (p > 0.05). Compared with the control group, the relative abundance of Ruminococcaceae_UCG-013 was increased significantly in both T2 and T3 groups (p < 0.05), and the relative abundance of Ruminococcaceae_unclassified was decreased significantly in all treatment groups (p < 0.05). In conclusion, the replacement of equivalent amounts of SBM with ESBM in the diet of Rex rabbits can increase the antioxidant capacity of the liver and improve the caecal microbiota, and the optimal replacement level was 1.5%.
Enzymolytic soybean meal (ESBM) increased the liver antioxidant capacity of Rex rabbits.
ESBM improved the caecal microbiota of Rex rabbits.
Effects of ESBM on the intestinal morphology and pH of Rex rabbits were evaluated.
HIGHLIGHTS
Introduction
Soybean meal (SBM) is the most widely used plant-derived protein feedstuff and plays a vital role in rabbit ration (Garcia-Santos et al. Citation2021). However, various anti-nutritional factors (such as glycinin, β-conglycinin and lectins) in SBM that may cause adverse effects after feeding to animals (Nguyen et al. Citation2018). For this reason, it has become a spotlight of research on how to reduce the anti-nutritional factors in SBM and improve the application value of SBM.
In rabbit production, intestinal problems caused by early weaning are one of the main causes of diarrhoea and death of rabbits. During the weaning period, digestive tracts of Rex rabbits were not fully developed and were subjected to multiple stresses of weaning, changes of feed and cage at the same time, which have an impact on the intestinal health and growth of the rabbit and lead to diarrhoea due to poor digestion (Velasco-Galilea et al. Citation2020). By enzymatic hydrolysis, the soybean protein is degraded into small peptides, and its functional properties have been improved, including solubility, emulsifying and foaming capacities as well as its biological properties, such as anti-oxidative activities (Zhang et al. Citation2010). Moreover, in the process of enzymatic hydrolysis, various anti-nutritional factors are able to be degraded or eliminated (Zhou et al. Citation2010). It is important to explore relevant products to benefit the antioxidant capacity and intestinal health of Rex rabbits during their weaning period.
We compared the nutrient composition and biochemical characteristics of SBM and enzymolytic soybean meal (ESBM), and the comparison results are shown in Table . The results showed a substantial increase in small peptides and a noticeable decrease in two anti-nutritional factors (glycinin and β-conglycinin) in ESBM. As a kind of potential functional feedstuff, ESBM can improve the body’s antioxidant capacity and enhance gut health (Ma et al. Citation2019; Uczay et al. Citation2019). ESBM has been used with excellent effect on other animals, however, there is no relevant research report on rabbits. If the ESBM is used to replace the equivalent amount of SBM in Rex rabbit feed, it may be beneficial to the body health of Rex rabbits. Therefore, this study replaced the equivalent amount of SBM in the diet with different proportions of ESBM to determine its effects on the antioxidant capacity of liver and jejunum, intestinal morphology and caecal microbiota of Rex rabbits.
Table 1. Nutrient composition and biochemical characteristics of SBM and ESBM.
Materials and methods
Animal care
The experimental protocols were approved by the Animal Care and Use Committee of Hebei Agriculture University (Baoding, China). The care and use of animals fully complied with local animal welfare laws, guidelines and policies.
Animals and experimental design
In the present study, the ESBM and protease (the activity of alkaline protease was 200,000 u/g and the activity of neutral protease was 50,000 u/g) were provided by Qihao Biotechnology Company Limited (Qinhuangdao, China). ESBM was made by crushing soybean meal, then adding enzyme preparations for enzymatic digestion, after forming, drying, crushing again, screening and packaging into products.
In this study, one hundred and twenty healthy weaned Rex rabbits with suitable weight (771.55 ± 12.30 g) at the age of 40 days were randomly divided into four groups (Each group had fifteen males and fifteen females, thirty replicates per group and one rabbit per replicate, each group was randomly distributed according to the average weight of male and female). The basal diet was free of antibiotics and mildew inhibitors. The control group was fed the basal diet and the ESBM treatment groups (T1, T2 and T3) were fed 0.5%, 1% and 1.5% ESBM substitute the equivalent amount of SBM in the basal diet, respectively. The basal diet was formulated to meet the nutritional requirements of rabbits recommended by National Research Council (Citation2012). The ingredients and chemical composition of the diets are shown in Table .
Table 2. Composition and nutrient levels of the experimental diets (air-dry basis).
The feeding experiment lasted for 61 days, including a pre-feeding period of 5 days and a formal trial period of 56 days. Before the feeding experiment, the experiment pieces of equipment (including cages, water nipples and feeders, etc.) were disinfected. During the feeding experiment, the Rex rabbits were given free access to feed and water, and each rabbit lived in a single cage. The natural light and ventilation were maintained as well as the rabbits were immunised according to rabbit vaccination regulations.
Biochemical analysis
Dry matter, ash, ether extract, calcium, total phosphorus of soybean meal, ESBM and the diets were analysed following the procedures of the Association of Official Analytical Chemists (Citation2000). Gross energy was determined by the YX-ZR automatic calorimeter (Youxin Instrument Manufacturing Company Limited, Changsha, China). The crude fibre was measured by the A2000I automated fibre analyser (ANKOM Technology, New York, USA). Crude protein was determined by the Kjeltec 8400 fully automated Kjeldahl analyser (Foss Company, Hillerød, Denmark). The contents of small peptides were analysed according to the method using electrophoresis in sodium dodecyl sulphate-polyacrylamidegels (Fling and Gregerson Citation1986). The concentrations of glycinin and β-conglycinin were analysed using commercial ELISA kits (Longkexinyu Biotechnology Company Limited, Yantai, China), following the kit instructions.
Intestinal pH
At the end of the feeding experiment, after the Rex rabbits fasted for 12 h, 6 rabbits from each group were randomly selected, euthanized and slaughtered, the pH of the jejunum, ileum and caecum was determined by a Testo 205 pH metre (Testo, Lenzkirch, Germany).
Antioxidant capacity analysis
Liver and jejunum samples were collected from each rabbit, wrapped in tinfoil and placed in 5 mL sterile cryopreservation tubes, temporarily placed in liquid nitrogen and then kept at a temperature of −80 °C for further analysis. The liver and jejunum samples were weighed, then added PBS buffer (Solarbio Science Technology Company Limited, Beijing, China), and centrifuged the upper liquid after working in a cryogenic tissue grinder. The activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), total antioxidant capacity (T-AOC) and the concentration of malondialdehyde (MDA), as important antioxidant indices, were detected in liver and jejunum by using commercial ELISA kits (Boruichangyuan Technology Company Limited, Beijing, China), following the kit instructions.
Intestinal morphology
Intestinal segments of about 3 cm were collected from the middle portion of the jejunum and ileum respectively, rinsed with saline slowly, injected with a small amount of 10% neutral formaldehyde and stored in 10% neutral formaldehyde for histology. The samples were sequentially dehydrated by an automatic tissue dehydrator (Junjie Electronic Company Limited, Wuhan, China), embedded by a BMJ-A embedding machine (Zhongwei Electronic Instrument Company Limited, Changzhou, China), sectioned by an automated rotary microtome (Leica Biosystems, Wetzlar, Germany) to the thickness of 5 µm and dyed by an RS36 dyer (P.S.J Medical Equipment Company Limited, Changzhou, China). The images were captured using a BA210 digital triple vision camera microscope (Motic Digital Pathology, San Francisco, USA), with 40× images of the area to be observed. Images were analysed using Motic Images Advanced (version 3.2) to measure intestinal villus height and crypt depth, as well as to calculate the ratio of villus height to crypt depth. Each slice was measured 10 times to obtain the averaged results.
Caecal microbiota
The contents of the caecum about 5 mL were collected in sterile cryopreservation tubes and stored at −80 °C. The bacterial genomic DNA of caecum chyme was extracted by Stool DNA Kit (Omega Bio-Tek Incorporated, Norcross, USA). Using the isolated genomic DNA as a template, the V3-V4 region of bacterial 16S rRNA gene was amplified by polymerase chain reaction (PCR) using 341 F (5′-CCTACGGGNGGCWGCAG-3′) and 805 R (5′-GACTACHVGGGTATCTAATCC-3′) primers combined with adapter and barcode sequences. High-throughput sequencing analysis of the bacterial rDNA gene was performed using the Illumina NovaSeq PE250 platform (Lianchuan Biotechnology Company Limited, Hangzhou, China).
Statistical analysis
Descriptive statistics were performed to evaluate whether the data were normally distributed using the statistical software SPSS version 20.0 (SPSS Incorporated, Chicago, USA) (for antioxidant capacity analysis, n = 6; for intestinal morphology, n = 6; for intestinal pH, n = 6; for caecal microbiota, n = 4). Duncan’s method was used to conduct multiple comparisons, and a one-way analysis of variance (ANOVA) was used to test the significant difference among the groups. The data of caecal microbiota were analysed by GraphPad Prism (version 8.0.1) as a non-parametric test. Statistical differences were considered significant with p < 0.05.
Results
Effect of enzymolytic soybean meal on the antioxidant capacity of Rex rabbits
The effect of ESBM on the antioxidant capacity of Rex rabbits is shown in Table . Compared with the control group, the activities of SOD and GSH-Px in the liver, as well as T-AOC were significantly increased in the T3 group (p < 0.05), but there were no significant differences in the T1 and T2 groups (p > 0.05). The concentration of MDA in the liver was significantly decreased in the T1 group (p < 0.05) compared to the control group, and there were no significant differences in both the T2 and T3 groups (p > 0.05). There were no significant differences in T-AOC and the activity of T-SOD and GSH-Px in jejunum among the groups (p > 0.05). There was no significant difference in the concentration of MDA in jejunum among all treatment groups compared to the control group (p > 0.05), however, the T1 group was significantly decreased compared to the T3 group (p < 0.05).
Table 3. Effect of enzymolytic soybean meal on the antioxidant capacity of Rex rabbits (n = 6).
Effect of enzymolytic soybean meal on the intestinal morphology of Rex rabbits
Table shows the effect of ESBM on the intestinal morphology of Rex rabbits. There were no significant differences in the villus height, crypt depth and ratio of villus height to crypt depth of jejunum and ileum among all groups (p > 0.05).
Table 4. Effect of enzymolytic soybean meal on the intestinal morphology of Rex rabbits (n = 6).
Effect of enzymolytic soybean meal on the intestinal pH of Rex rabbits
The effect of ESBM on the intestinal pH of Rex rabbits is shown in Table . There were no significant differences in the pH of jejunum, ileum and caecum among all groups (p > 0.05).
Table 5. Effect of enzymolytic soybean meal on the intestinal pH of Rex rabbits (n = 6).
Effect of enzymolytic soybean meal on the caecal microbiota of Rex rabbits
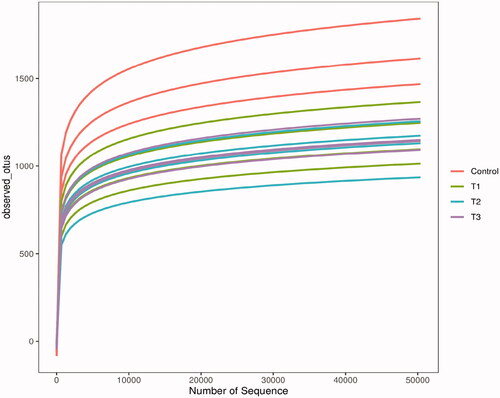
Figure shows the rarefaction curves of samples. As shown in Figure , the sequence dilution curves reached a flat trend, indicating that the amount of sequencing data was reasonable and the diversity of the samples was close to saturation, the tested sequences were sufficient to cover the species composition of the microbial community and were able to reflect the proportional relationship among the species in the community.
Figure 1. Rarefaction curves of samples. The Illumina NovaSeq PE250 sequencing platform was used to detect the V3-V4 region of 16S rDNA in the caecum content samples of Rex rabbits. The sequences were selected randomly, the number of species they represented was counted and the dilution curves were plotted by the number of selected sequences and corresponding species. T1 means 0.5% enzymolytic soybean meal addition group, T2 means 1.0% enzymolytic soybean meal addition group, and T3 means 1.5% enzymolytic soybean meal addition group.
The Venn diagram of the feature is shown in Figure . The number of features that were unique to the control, T1, T2 and T3 groups were 3349, 1396, 1453 and 1305, respectively.
Figure 2. Venn diagram of feature. According to the feature abundance table, the number of features in each group was calculated, and the number of common and unique features in each group was visualised by Venn diagram, if the feature existed in only one group, it was unique to that group. T1 means 0.5% enzymolytic soybean meal addition group, T2 means 1.0% enzymolytic soybean meal addition group, and T3 means 1.5% enzymolytic soybean meal addition group.
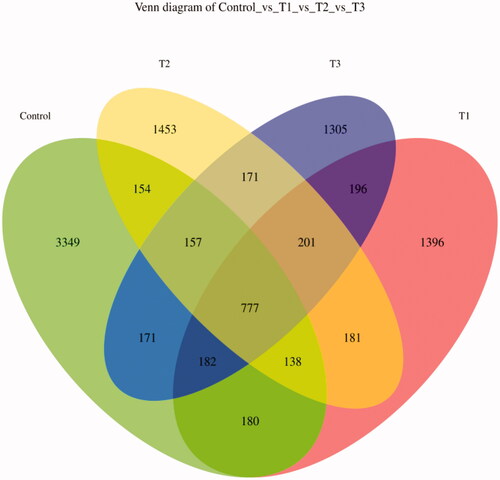
Table shows the alpha diversity indices of caecal microbiota. In terms of species richness, there were no significant differences in the Goods_coverage, Chao1 and Observed_otus indices of Rex rabbits among all groups (p > 0.05), and the Goods_coverage index was more than 0.996 in all groups. In terms of species diversity, the Shannon and Simpson indices were no significant differences among all groups (p > 0.05).
Table 6. Alpha diversity indices of caecal microbiota (n = 4).
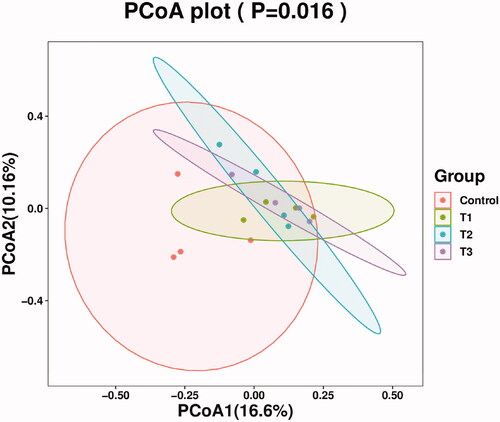
The Principal Coordinates Analysis diagram of caecal microflora composition is shown in Figure . In the present study, the degree of aggregation of samples within the T1, T2 and T3 groups was higher, with less difference and more stability; the degree of aggregation in the control group was lower and the difference was present; the control group was able to be distinguished clearly from the T1, T2 and T3 groups, indicating that the difference was observed in the microbial structure of the caecum between the control group and treatment groups of Rex rabbits.
Figure 3. Principal co-ordinates analysis diagram of caecal microflora composition. Principal Coordinate Analysis (PCoA) is based on a sample distance matrix for ranking. The PCoA analysis allows the difference among the groups to be observed. The different colours in the results represent the different groupings and the closer the distance, the more similar the structure of the microbial composition and the less difference. T1 means 0.5% enzymolytic soybean meal addition group, T2 means 1.0% enzymolytic soybean meal addition group, and T3 means 1.5% enzymolytic soybean meal addition group.
The TOP 10 microbial abundance at the phylum level was used as the predominant bacteria phylum in each group for analysis. As shown in Figure , the caecal microbiota of Rex rabbits consisted mainly of Firmicutes, Bacteroidetes and Verrucomicrobia at the phylum level, and the proportions of the 3 dominant bacterial phyla in control, T1, T2 and T3 groups were 92.08%, 91.55%, 92.66% and 91.78%, respectively. The results of further analysis of the proportion of microbial community composition were shown in Table . The relative abundance of caecal microbiota at the phylum level was no significant difference among all groups (p > 0.05).
Figure 4. Relative abundance of caecal microbiota at phylum level. T1 means 0.5% enzymolytic soybean meal addition group, T2 means 1.0% enzymolytic soybean meal addition group, and T3 means 1.5% enzymolytic soybean meal addition group.
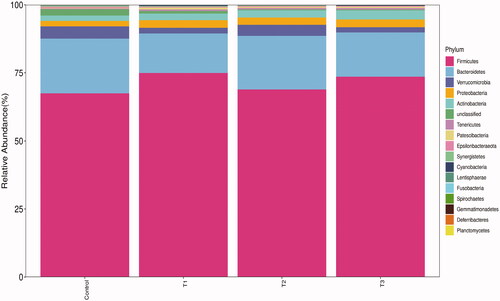
Table 7. Distribution of caecal microbiota at phylum level (%) (n = 4).
The caecal microbiota of Rex rabbits was analysed at the genus level using the microbial abundance TOP 30 as the predominant genus for each group (Figure ) and the genus with more than 1% abundance was selected for significant analysis. As shown in Table , the relative abundance of Ruminococcaceae_UCG-013 was increased significantly (p < 0.05) in both T2 and T3 groups compared to the control group. The relative abundance of Ruminococcaceae_unclassified was decreased significantly in all treatment groups compared to the control group (p < 0.05). There were no significant differences in other genera among all groups (p > 0.05).
Figure 5. Relative abundance of caecal microbiota at genus level. T1 means 0.5% enzymolytic soybean meal addition group, T2 means 1.0% enzymolytic soybean meal addition group, and T3 means 1.5% enzymolytic soybean meal addition group.
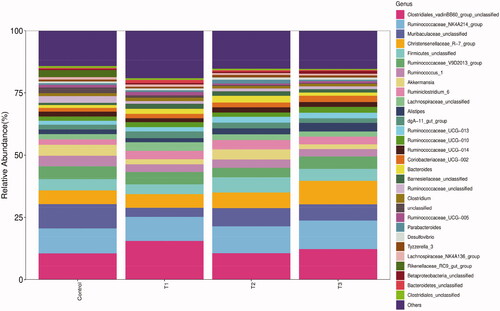
Table 8. Distribution of caecal microbiota at genus level (%) (n = 4).
Discussion
Effect of enzymolytic soybean meal on the antioxidant capacity of Rex rabbits
If the cell is stimulated by intracellular and extracellular environmental factors such as nitric oxide, calcium and pathogenic organisms, then the oxidative balance between the production of reactive oxygen species (ROS) and the antioxidant defence system will be disrupted, which will lead to irreparable oxidative damage and cell death, thus affecting the function and survival of entire body (Ngo et al. Citation2010; Jomova and Valko Citation2011). Oxidative stress is linked closely to numerous diseases, so the body needs to improve its antioxidant capacity (Jomova et al. Citation2012). During the enzymatic hydrolysis of soy protein, the structure of soy protein is altered and the more active amino acid R group will be exposed (Singh et al. Citation2014). The size of soybean peptides could be reduced to 100–1000 Da by microbial fermentation and proteolysis during the production of ESBM, containing abundant bio-active peptides, such as QRPR and lunasin (Pan et al. Citation2019; Fernández-Tomé and Hernández-Ledesma Citation2019). The enhanced antioxidant enzyme system may respond to the elevation of ROS (Song et al. Citation2016), and protect hepatocytes from oxygen-free radicals. When the level of replacement was 1.5% in the present study, ESBM significantly increased the liver SOD and GSH-Px activities and T-AOC in Rex rabbits; when the level of replacement was 0.5%, ESBM decreased significantly the concentration of liver MDA to improve their liver antioxidant capacity; there was no significant difference on the intestinal antioxidant capacity. Uczay et al. (Citation2019) replaced the fish meal with an enzyme-hydrolyzed soybean meal that helped the growth of silver catfish and improved its antioxidant capacity, and this was similar to our study. ESBM is able to produce small peptides with radical scavenging, inhibition of lipid peroxidation and metal ion chelation properties, which enhances the antioxidant capacity of the body (You et al. Citation2010). At the same time, ESBM increased the expression of Gpx4 mRNA in the liver of weaned piglets, suggesting that the improvement of antioxidant capacity may be associated with the upregulation of GPx4 genes caused by ESBM (Ma et al. Citation2019).
Effect of enzymolytic soybean meal on the intestinal morphology of Rex rabbits
Intestinal morphology is a useful marker for assessing the digestive and absorptive capacity of the intestine and the state of intestinal health (Xiong et al. Citation2015; Zhu et al. Citation2017). The changes in the villus height and crypt depth reflect the change in the ability of the intestine to digest and absorb nutrients, and an increment in the ratio of villus height to crypt depth indicates that the intestine wall is in more contact with the chyme, thus making it easier to absorb nutrients (Yang and Liao Citation2019). Some research reports pointed out that, excessive SBM has a negative impact on intestinal structure, which may be linked to the presence of many anti-nutritional factors in SBM (Feng et al. Citation2007). Therefore, before conducting this study, we speculated that after enzymatic hydrolysis of SBM, the content of anti-nutritional factors will be reduced, the oxidative stress on the intestine will also be reduced, meanwhile enzymatic hydrolysis of SBM will produce a large number of small peptides and other active factors, which may play a certain role in promoting intestinal development. However, our research showed that there was no significant improvement in villus height, crypt depth and the ratio of villus height to crypt depth in the jejunum and ileum of Rex rabbits among all groups. A possible reason for this result is that the ESBM was added at a low level in this study, which has not yet reached a dose that could improve intestinal morphology. Ma et al. (Citation2019) reported that ESBM increased significantly the villus height and the ratio of villus height to crypt depth in the duodenum of weaned piglets, but did not improve significantly the intestinal morphology of the jejunum and ileum. Ruckman et al. (Citation2020) found that ESBM had no significant effect on villus height, crypt depth and the ratio of villus height to crypt depth in the ileum of nursery pigs, which was similar to our study. Droy-lefaix et al. (Citation1991) reported that SOD could reduce small intestinal damage and improve intestinal morphology. In the present study, there was no significant difference in the activity of intestinal SOD among all groups, it was also confirmed that the dose of ESBM we used did not improve the intestinal morphology of Rex rabbits.
Effect of enzymolytic soybean meal on the intestinal pH of Rex rabbits
The health of the intestine is not only related to the integrity of the intestinal morphology but also closely related to intestinal pH and caecal microbiota. The level of intestinal pH affects the digestion and absorption of substances in the intestine, and the suitable pH is closely related to intestinal microecology. The pH in the digestive gut depends on the amount of gastric HCl secretion and the action of bile and pancreatic juices. As shown in Table , the intestinal pH of all treatment groups was not different significantly compared to the control group, indicating that ESBM did not induce gastric acid secretion significantly in the body (Ansia et al. Citation2020). Our study showed that all intestinal pH values were within the normal range for healthy Rex rabbits and therefore ESBM does not affect the micro-ecological environment in Rex rabbits.
Effect of enzymolytic soybean meal on the caecal microbiota of Rex rabbits
The majority of studies have reported on the intestinal microbiota of rabbits focussing on the caecum because the caecum is the main fermentation organ in rabbits (Velasco-Galilea et al. Citation2018). The intestinal microbiota is a complex ecosystem that plays an important role in the intestinal health of animals (Cao et al. Citation2019), it also plays an indispensable role in the metabolic, nutritional and physiological processes of mammals (Flint et al. Citation2012). Alpha diversity is the diversity within a particular environment or ecosystem and is primarily used to reflect species richness and evenness (Sun et al. Citation2020). ESBM increased significantly the intestinal microbial Chao1 index of weaned piglets and improved the abundance and diversity of the intestinal microbiota (Li et al. Citation2021a). In our study, ESBM did not influence significantly the correlative index of Alpha diversity in the caecum of Rex rabbits. Fan et al. (Citation2021) reported that there was no significant effect of ESBM on the associated index of Alpha diversity in Pacific white shrimp Litopenaeus vannamei, which was similar to our study. In our study, it was possible to observe by PCoA analysis that the control group could be distinguished significantly from the treatment groups, suggesting that ESBM played a role in regulating the microbial structure of the caecum of Rex rabbits.
The caecal microbiota plays an important role in the growth and survival of Rex rabbits (Liu et al. Citation2019). Our study found that the caecal microbiota of Rex rabbits remained stable at the phylum level, consisting mainly of Firmicutes, Bacteroidetes and Verrucomicrobia, which was consistent with other studies (Yang et al. Citation2020). Firmicutes are predominant in the rabbit caecal bacterial flora and contain a large number of the fibre-degrading genus, which are beneficial to the breakdown of cellulose (Monteils et al. Citation2008). Bacteroidetes are known to promote carbohydrates to be fermented and have the ability to degrade polysaccharides (Thomas et al. Citation2011). The main metabolites of Firmicutes and Bacteroidetes are short-chain volatile fatty acids, and probiotics are able to promote colonisation. Soluble carbohydrates are degraded by Verrucomicrobia which is closely linked to intestinal health (Kylie et al. Citation2018). Our study found that ESBM had no effect on the relative abundance of caecal microbiota at the phylum level in Rex rabbits. The reason for this result could be explained by the fact that ESBM had no significant effect on the intestinal pH of Rex rabbits in our study, as intestinal pH is closely related to the health of the host and the stability of caecal microbiota.
At the genus level, our study found that Clostridiales_vadinBB60_group_unclassified, Ruminococcaceae_NK4A214_group, Muribaculaceae_unclassified and Christensenellaceae_R-7_group were predominant genera in Rex rabbits. Hao et al. (Citation2020) reported that Clostridiales_vadinBB60_group was correlated positively with the production of short-chain fatty acids and bile acids. The majority of members of Ruminococcaceae are essential for the degradation of cellulose as well as Ruminococcaceae_NK4A214_group is correlated positively with the production of butyric acids (Li et al. Citation2021b). Muribaculaceae is linked to the production of propionic acids (Smith et al. Citation2019) and may play a key metabolic role in extending lifespan (Sibai et al. Citation2020). Christensenellaceae is a beneficial bacteria that assist in protecting the health of the body (Zhao et al. Citation2017). Our study found that the relative abundance of Christensenellaceae_R-7_group increased with the dose of ESBM. Ruminococcaceae_UCG-013 plays an important role in the regulation of carbohydrate metabolic pathways (Chen et al. Citation2020). When the level of replacement was 1% and 1.5% in our study, the relative abundance of Ruminococcaceae_UCG-013 was increased significantly by ESBM; the relative abundance of Ruminococcaceae_unclassified was decreased significantly in ESBM treatment groups. Chen and Yu (Citation2021) reported that the relative abundance of Ruminococcaceae_unclassified in the caecum of broilers was correlated negatively with the growth, speculating that the decreased relative abundance of Ruminococcaceae_unclassified in our study might be helpful for the healthy growth of Rex rabbits. Jeong and Kim (Citation2015) reported that ESBM increased significantly the number of beneficial bacteria in the intestine of weaned piglets, thereby protecting intestinal health, which was similar to our study.
To sum up, ESBM could improve the caecal microbiota of Rex rabbits and have a beneficial effect on intestinal health. The reason that ESBM protects the intestinal health of Rex rabbits can be attributed to its ability to produce bioactive small peptide, which is responsible for promoting the growth of beneficial bacteria and improving the micro-ecological environment of the intestine.
Conclusion
The present study showed that the replacement of equivalent amounts of SBM with ESBM in the diet of Rex rabbits can increase the antioxidant capacity of the liver and improve the caecal microbiota. However, there was no significant effect on the intestinal pH and intestinal morphology of Rex rabbits. Under this experimental condition, the optimal replacement level of ESBM in the diet of Rex rabbits was 1.5%.
Ethical approval
The protocol used in this experiment was approved by the Institutional Animal Care and Use Committee of Institute of Animal Science and Veterinary Medicine, Hebei Agricultural University (Baoding, Hebei, China). The authors confirm that the procedures imposed on the animals were carried out to meet the Directive 2010/63/EU of the European Parliament and the Council of 22 September 2010 on the protection of animals used for scientific purposes.
Acknowledgements
The authors thank all those who contributed to this experiment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Ansia I, Stein HH, Vermeire DA, Brøkner C, Drackley JK. 2020. Ileal digestibility and endogenous protein losses of milk replacers based on whey proteins alone or with an enzyme-treated soybean meal in young dairy calves. J Dairy Sci. 103(5):4390–4407.
- Association of Official Analytical Chemists. 2000. Official methods of analysis. 17th ed. Gaithersburg (MD): Association of Official Analytical Chemists.
- Cao GT, Dai B, Wang KL, Yan Y, Xu YL, Wang YX, Yang CM. 2019. Bacillus licheniformis, a potential probiotic, inhibits obesity by modulating colonic microflora in C57BL/6J mice model. J Appl Microbiol. 127(3):880–888.
- Chen HL, Zhao XY, Zhao GX, Huang HB, Li HR, Shi CW, Yang WT, Jiang YL, Wang JZ, Ye LP, et al. 2020. Dissection of the cecal microbial community in chickens after Eimeria tenella infection. Parasit Vectors. 13(1):56.
- Chen JY, Yu YH. 2021. Bacillus subtilis–fermented products ameliorate the growth performance and alter cecal microbiota community in broilers under lipopolysaccharide challenge. Poult Sci. 100(2):875–886.
- Droy-lefaix MT, Drouet Y, Geraud G, Hosford D, Braquet P. 1991. Superoxide dismutase (SOD) and the PAF-antagonist (BN 52021) reduce small intestinal damage induced by ischemia-reperfusion. Free Radic Res Commun. 13(1):725–735.
- Fan Y, Luo K, Guo YL, Gao WH, Xu QQ, Zhang WB, Mai KS. 2021. Replacement of fish meal by enzyme-treated soybean on the growth performance, intestine microflora, immune responses and disease resistance of Pacific white shrimp Litopenaeus vannamei. Aquac Res. 52(10):4619–4628.
- Feng J, Liu X, Xu ZR, Lu YP, Liu YY. 2007. Effect of fermented soybean meal on intestinal morphology and digestive enzyme activities in weaned piglets. Dig Dis Sci. 52(8):1845–1850.
- Fernández-Tomé S, Hernández-Ledesma B. 2019. Current state of art after twenty years of the discovery of bioactive peptide lunasin. Food Res Int. 116:71–78.
- Fling SP, Gregerson DS. 1986. Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris buffer system without urea. Anal Biochem. 155(1):83–88.
- Flint HJ, Scott KP, Louis P, Duncan SH. 2012. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol. 9(10):577–589.
- Garcia-Santos S, Almeida M, Closson M, Guedes CM, Barros A, Ferreira LM, Trindade H, Pinheiro V. 2021. Effect of total replacement of the soya bean meal by lupine seeds (L. albus and L. luteus) on performance and digestion characteristics of growing rabbits. Anim Feed Sci Technol. 278:114996.
- Hao WJ, Zhu HY, Chen JN, Kwek E, He ZY, Liu JH, Ma N, Ma KY, Chen ZY. 2020. Wild melon seed oil reduces plasma cholesterol and modulates gut microbiota in hypercholesterolemic hamsters. J Agric Food Chem. 68(7):2071–2081.
- Jeong JS, Kim IH. 2015. Comparative efficacy of up to 50% partial fish meal replacement with fermented soybean meal or enzymatically prepared soybean meal on growth performance, nutrient digestibility and fecal microflora in weaned pigs. Anim Sci J. 86(6):624–633.
- Jomova K, Baros S, Valko M. 2012. Redox active metal-induced oxidative stress in biological systems. Transition Met Chem. 37(2):127–134.
- Jomova K, Valko M. 2011. Advances in metal-induced oxidative stress and human disease. Toxicology. 283(2–3):65–87.
- Kylie J, Weese JS, Turner PV. 2018. Comparison of the fecal microbiota of domestic commercial meat, laboratory, companion, and shelter rabbits (Oryctolagus cuniculi). BMC Vet Res. 14(1):143.
- Li Y, Lv M, Wang JQ, Tian ZH, Yu B, Wang B, Liu JX, Liu HY. 2021a. Dandelion (Taraxacum mongolicum Hand.-Mazz.) supplementation-enhanced rumen fermentation through the interaction between ruminal microbiome and metabolome. Microorganisms. 9(1):83.
- Li YJ, Liu Y, Wu JN, Chen QH, Zhou Q, Wu FL, Zhang RN, Fang ZF, Lin Y, Xu SY, et al. 2021b. Comparative effects of enzymatic soybean, fish meal and milk powder in diets on growth performance, immunological parameters, SCFAs production and gut microbiome of weaned piglets. J Anim Sci Biotechnol. 12(1):106.
- Liu L, Zeng D, Yang MY, Wen B, Lai J, Zhou Y, Sun H, Xiong LC, Wang J, Lin YC, et al. 2019. Probiotic Clostridium butyricum improves the growth performance, immune function, and gut microbiota of weaning Rex rabbits. Probiotics Antimicrob Proteins. 11(4):1278–1292.
- Ma XK, Shang QH, Hu JX, Liu HS, Brøkner C, Piao XS. 2019. Effects of replacing soybean meal, soy protein concentrate, fermented soybean meal or fish meal with enzyme-treated soybean meal on growth performance, nutrient digestibility, antioxidant capacity, immunity and intestinal morphology in weaned pigs. Livest Sci. 225:39–46.
- Ma XK, Shang QH, Wang QQ, Hu JX, Piao XS. 2019. Comparative effects of enzymolytic soybean meal and antibiotics in diets on growth performance, antioxidant capacity, immunity, and intestinal barrier function in weaned pigs. Anim Feed Sci Technol. 248:47–58.
- Monteils V, Cauquil L, Combes S, Godon JJ, Gidenne T. 2008. Potential core species and satellite species in the bacterial community within the rabbit caecum. FEMS Microbiol Ecol. 66(3):620–629.
- Ngo DH, Qian ZJ, Ryu BM, Park JW, Kim SK. 2010. In vitro antioxidant activity of a peptide isolated from Nile tilapia (Oreochromis niloticus) scale gelatin in freeradical-mediated oxidative systems. J Funct Foods. 2(2):107–117.
- Nguyen NV, Hoang L, Khanh TV, Hai PD, Hung LT. 2018. Utilization of fermented soybean meal for fishmeal substitution in diets of Pacific white shrimp (Litopenaeus vannamei). Aquacult Nutr. 24(3):1092–1100.
- National Research Council. 2012. Nutrient requirements of rabbits. 2nd ed. Washington (DC): The National Academy Press.
- Pan FG, Wang L, Cai ZZ, Wang YN, Wang YF, Guo JX, Xu XY, Zhang XG. 2019. Soybean peptide QRPR activates autophagy and attenuates the inflammatory response in the RAW264.7 cell model. Protein Pept Lett. 26(4):301–312.
- Ruckman LA, Petry AL, Gould SA, Kerr BJ, Patience JF. 2020. The effects of enzymatically-treated soybean meal on growth performance and intestinal structure, barrier integrity, inflammation, oxidative status, and volatile fatty acid production of nursery pigs. Transl Anim Sci. 4(3):txaa170.
- Sibai M, Altuntaş E, Yıldırım B, Öztürk G, Yıldırım S, Demircan T. 2020. Microbiome and longevity: high abundance of longevity-linked Muribaculaceae in the gut of the long-living rodent Spalax leucodon. OMICS. 24(10):592–601.
- Singh BP, Vij S, Hati S. 2014. Functional significance of bioactive peptides derived from soybean. Peptides. 54:171–179.
- Smith BJ, Miller RA, Ericsson AC, Harrison DC, Strong R, Schmidt TM. 2019. Changes in the gut microbiome and fermentation products concurrent with enhanced longevity in acarbose-treated mice. BMC Microbiol. 19(1):130.
- Song ZD, Li PY, Wang JY, Huang BS, Li BS, Wang SX, Zhang Y, Gong XP, Li XL, Tan Q. 2016. Effects of seaweed replacement by hydrolyzed soybean meal on growth, metabolism, oxidation resistance and body composition of sea cucumber Apostichopus japonicus. Aquacult. 463:135–144.
- Sun XM, Shen JL, Liu C, Li S, Peng YX, Chen CZ, Yuan B, Gao Y, Meng XM, Jiang H, et al. 2020. L-arginine and N-carbamoylglutamic acid supplementation enhance young rabbit growth and immunity by regulating intestinal microbial community. Asian-Australas J Anim Sci. 33(1):166–176.
- Thomas F, Hehemann JH, Rebuffet E, Czjzek M, Michel G. 2011. Environmental and gut Bacteroidetes: the food connection. Front Microbiol. 2:93.
- Uczay J, Battisti EK, Lazzari R, Pessatti ML, Schneider TLS, Hermes LB, Peixoto NC, Fabregat TEHP. 2019. Fish meal replaced by hydrolysed soybean meal in diets increases growth and improves the antioxidant defense system of silver catfish (Rhamdia quelen). Aquac Res. 50(5):1438–1447.
- Velasco-Galilea M, Guivernau M, Piles M, Viñas M, Rafel O, Sánchez A, Ramayo-Caldas Y, González-Rodríguez O, Sánchez JP. 2020. Breeding farm, level of feeding and presence of antibiotics in the feed influence rabbit cecal microbiota. Anim Microbiome. 2(1):40.
- Velasco-Galilea M, Piles M, Viñas M, Rafel O, González-Rodríguez O, Guivernau M, Sánchez JP. 2018. Rabbit microbiota changes throughout the intestinal tract. Front Microbiol. 9:2144.
- Xiong X, Yang HS, Wang XC, Hu Q, Liu CX, Wu X, Deng D, Hou YQ, Nyachoti CM, Xiao DF, et al. 2015. Effect of low dosage of chito-oligosaccharide supplementation on intestinal morphology, immune response, antioxidant capacity, and barrier function in weaned piglets. J Anim Sci. 93(3):1089–1097.
- Yang GQ, Zhao F, Tian H, Li JT, Guo DX. 2020. Effects of the dietary digestible fiber-to-starch ratio on pellet quality, growth and cecal microbiota of Angora rabbits. Asian-Australas J Anim Sci. 33(4):623–633.
- Yang Z, Liao SF. 2019. Physiological effects of dietary amino acids on gut health and functions of swine. Front Vet Sci. 6:169.
- You LJ, Zhao MM, Regenstein JM, Ren JY. 2010. Purification and identification of antioxidative peptides from loach (Misgurnus anguillicaudatus) protein hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Res Int. 43(4):1167–1173.
- Zhao L, Zhang Q, Ma WN, Tian F, Shen HY, Zhou MM. 2017. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct. 8(12):4644–4656.
- Zhang L, Li JR, Zhou KQ. 2010. Chelating and radical scavenging activities of soy protein hydrolysates prepared from microbial proteases and their effect on meat lipid peroxidation. Bioresour Technol. 101(7):2084–2089.
- Zhou SF, Sun ZW, Ma LZ, Yu JY, Ma CS, Ru YJ. 2010. Effect of feeding enzymolytic soybean meal on performance, digestion and immunity of weaned pigs. Asian Australas J Anim Sci. 24(1):103–109.
- Zhu JJ, Gao MX, Zhang RL, Sun ZJ, Wang CM, Yang FF, Huang TT, Qu SQ, Zhao L, Li YW, et al. 2017. Effects of soybean meal fermented by L. plantarum, B. subtilis and S. cerevisieae on growth, immune function and intestinal morphology in weaned piglets. Microb Cell Fact. 16(1):191.
