Abstract
This study aims to investigate the effects of in-feed additive synergistic blend of short and medium-chain organic acids (SGG) in sows during late gestation and lactation as well as litter performance. On day 107 of gestation, a total of 150 multiparous (Landrace × Yorkshire) sows were blocked according to parity (2.6) and allocated to one of three dietary treatments: CON – basal diet, SGG-Low – CON + 0.1% SGG and SGG-High – CON + 0.3% SGG. Sows supplemented with SGG-High consumed more lactation feed (p = .04) than sows fed the CON diet. The body weight (BW) and back fat (BF) loss during the lactation period were lower (p ≤ .05) in sows fed SGG supplements. Additionally, sows fed with different levels of SGG-supplement reduced (p = .04) the number of mummified and birth coefficient of variation (p = .03) and improved (p ≤ .05) the survivability of piglets. Also, piglets born to SGG group sows and fed a creep diet from days 5 to 21 of age, showed a tendency to increase (p = .07) BW on day 7, and significant improvements on days 14 (p = .02) and 21 (p < .001), and average daily gain (p < .001) during the overall experimental period. Furthermore, the SGG supplement significantly reduced (p ≤ .05) the number of Clostridium perfringens in faeces of sows on day 7 of lactation. Thus, we infer that the application of 0.1–0.3% of SGG supplement in sow diet and subsequently feeding their offsprings with creep diet would serve as the best option for optimum sow productivity and to enhance pre-weaning growth rate.
The breeding efficiency of sow and the growth rate of piglets are very important for successful pig production.
Sows fed with a synergistic blend of short and medium-chain organic acids (SGG) during late gestation and lactation improved the survivability of piglets.
Piglets born to sows supplemented with different levels of SSG gained more weight and had a higher litter weight during weaning.
HIGHLIGHTS
Introduction
The reproductive performance of sows and the growth rate of piglets are considered to be the major factors for successful pig production. However, sows undergo dramatic changes in foetal and mammary gland growth during the gestation period (Kim et al. Citation2016). In fact, changes in a sow’s diet during pregnancy may affect its fertility (Lawlor and Lynch Citation2007) and the growth rate of piglets (McNamara et al. Citation2011). Therefore, high nutrient feed intake at gestation is very essential to meet the nutrient requirement of foetal development and for storing body fat that can be used during negative energy balance caused by lactation (Young et al. Citation2004). Some studies have shown that modern sows are extremely productive during the lactation period (Kim et al. Citation2016). Yet, the lactation diet plays a major role in breeding performance, especially after weaning. Over the past decades, livestock producers have used antibiotics as growth promoters (AGPs) in animals’ feedstuff to increase productivity and prevent disease (Dibner and Richards Citation2005). However, excessive use of certain AGPs generate bacterial resistance in animals and cause serious health issue to consumers through food chain. Accordingly, many countries including European Union (EU) and South Korea have banned the use of certain AGPs in animal feeds since 2011 (Sampath et al. Citation2021). The prohibition on AGP and the mounting anxieties about the safety and quality of livestock products have prompted many researchers and feed manufacturers to look for alternative additives that can improve production performance. Numerous feed additives like pre-and probiotics, medium chain fatty acids, yeast, enzymes, essential oils and plant extracts (Mroz Citation2005; Devi et al. Citation2016; Upadhaya et al. Citation2016) are in vogue for achieving this target, but organic acids and their salts have been accepted globally as one of the best alternatives
Organic acids (OAs) are weak acids with one carboxylic group (–COOH) and a carbon chain having one to seven carbon atoms (Upadhaya et al. Citation2016). Notably, OAs have a similar antimicrobial activity to antibiotics like penetrating the bacterial cell wall and interrupting the normal actions of Salmonella spp., Escherichia coli, Clostridia spp. and some coliforms bacteria (Suryanarayana and Ramana Citation2015). Besides, the main action of OAs in swine nutrition is to reduce the gastro-intestinal pH (Desai et al. Citation2007). Apart from this, short-chain fatty acids like formic acid, acetic acid, lactic acid, propionic acid, citric acid and sorbic acid have been extensively used in pig diet to improve their production performance (Nguyen et al. Citation2020). For instance, Suryanarayana and Ramana (Citation2015) reported that pigs fed a diet supplemented with 0.1% of Luprosil-NC (a product containing 53.5% propionic acid) had significantly reduced the E. coli counts. Similarly, Maribo et al. (Citation2000) stated that the inclusion of 0.7%, 1.4% or 2.8% lactic acid in pigs’ diet showed significant changes in their gastrointestinal characteristics. Additionally, our previous study demonstrates that protected OA and its blends have the potential to improve the performance of sows and their litters (Devi et al. Citation2016). However, Mroz (Citation2005) reported that the inclusion of 1% formic acid has no effect on the body mass of sows from pregnancy to lactation, whereas it significantly increased the feed intake during lactation, and slightly improved litter size and piglet birth weight. Recently, medium-chain fatty acids (MCFAs) particularly saturated with 6–12 carbon-long chains have been demonstrated as the best feed additives to improve animal health, production and feed digestibility (Baltić et al. Citation2017). From the above-mentioned literature, we hypothesised that adding in-feed additives based on a synergistic blend of short- and medium-chain organic acids (SGG) in the sows’ diet during the late gestation and lactation phases may enhance their performance. However, there were no studies existing on analysing the effect of the combination of short and medium chain OAs, particularly on sows, therefore, we aimed to examine the effect of SSG supplementation on sow reproduction and litter performance.
Materials and methods
Ethical authorisation
Animal handling procedures and protocol of this study were revised and approved (No: DK-2-2005) by Animal Ethics and the Welfare Committee of Dankook University – Republic of Korea.
Source of the main additive
SGG is a free-flowing powder based on a synergistic blend of short-chain organic acids (formic acid, acetic acid, lactic acid, propionic acid, citric acid and sorbic acid) combined with medium-chain fatty acids (C8 – caprylic acid, C10 – caproic acid and C12 – lauric acid). The SSG additive used in this study was obtained from Trouw Nutrition, Co., Ltd. (Amsterdam, The Netherland).
Trial design, diets and dietary regimens
On day 107 of gestation, a total of 150 multiparous sows (Landrace × Yorkshire, with the average parity of 2.6; 34 sows in first pregnancy, 31 sows in second pregnancy, 43 sows in third pregnancy and 42 sows in fourth pregnancy) were assigned to one of three dietary treatments with 50 replicates of 1 sow and their litter. The dietary treatments included: (1) CON – basal diet, (2) SGG-Low – CON + 0.1% SGG and (3) SGG-High – CON + 0.3% SGG. The basal and creep diet for sows and their offspring, respectively, were formulated to be isocaloric and isonitrogenous according to the NRC (Citation2012) recommendations (Tables and ). A master batch of the basal diet was prepared and SGG supplement was added, mixed well using DDK-801 series feed mixer (Daedong Tech, Gyeonggi-do, South Korea), and put into pre-marked feed bags.
Table 1. Composition of the basal diet (as fed basis).
Table 2. Composition of creep feed (as fed basis).
Sows and litter management
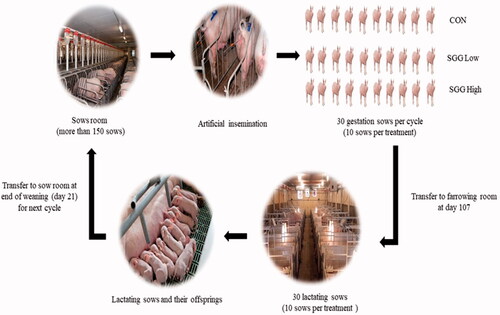
One hundred and fifty sows were artificially inseminated (AI) twice (12–24 h) with the semen of Duroc boars. After AI (21 days later), sows were diagnosed for pregnancy and their health status was closely monitored throughout the 15 weeks of pregnancy. A schematic view of the experiment was shown in Figure . On day 105 of pregnancy, all sows were weighed individually, moved to farrowing crates, and stayed until the end of lactation period. The farrowing crates had 2.21 × 1.60 m2 space on each side for newborn piglets. Before farrowing, rubber mats were placed down as surface for piglets to lie and the farrowing room temperature was maintained at 20 °C. Sows were offered a common commercial gestation diet at 2.5 kg daily in two equal meals up to day 106, from day 107 dietary supplements were included in the sow diet and continued until the end of lactation day 21.
After parturition, all piglets were taken away from their dam and wiped with a clean surgical towel, check for mummification, splayed leg and ear-notched for identity. Then piglets’ individual birth weights and total birth weight were measured. The number of alive, dead and mummified piglets in each pen was calculated to determine the survival rate during the farrowing period. All litters (∼12 piglets) were cross-fostered within the same treatment from 24 to 48 h of parturition. Additional heat was given to newborn piglets for 72 h using heat lamps and they were treated with regular management practices such as teeth cutting and tail docking. 1 mL (per pig) intravenous iron dextran injection was also given within 24 h of birth. The male piglets were castrated on day 5 of postpartum. Piglets were provided with a common creep diet from days 5 to 21 at 9.00am and 6.00pm. After day 1 of parturition, sows were fed with a lactation diet which was gradually increased from 2.5 kg/day to ad libitum feed access by day 5 of lactation. Each crate had a sole feeder and nipple drinker for sows and piglets.
Reproductive analysis
The body weight (BW), body weight change (BW loss), and body condition score (BCS) were measured at pre- and post-farrowing and weaning (day 21). The back fat thickness (BFT) (6–8 cm from the midline of the 10th rib) of each sow was measured using piglet 105, SFK Tech real-time ultrasonic instrument (Herlec, Denmark) on the 107th day of gestation, pre- and post-farrowing and weaning to determine the back fat thickness loss (BFTL). During the gestation and lactation period, the feed intake and the leftovers were calculated to determine the average daily feed intake (ADFI). Birth weight, total birth, alive, stillbirth and mummified piglets were recorded to calculate the litter size. Also, the starter and fostered piglets until weaning were recorded to calculate the survival rate (SUR%). Individual piglet’s BW, average daily gain (ADG), ADFI and gain to feed ratio (G:F) were measured on days 1, 7, 14 and 21. The ADGs of the piglets were calculated as the difference between birth weight (kg) and weaning weight (kg)/length of the lactation period. While ADFI was calculated from the feed offering and remaining feed in the feeder. 21 days after weaning, sows were taken to the breeding room (day 22) and rested for about 2 weeks. Later they were examined for standing response caused by a back-pressure test in presence of mature Duroc boars (twice a day) for oestrus detection.
Faecal microbial analysis
Fresh and clean faecal samples (200 g) were randomly collected from 20 sows per treatment pre- and post-farrowing, and on day 7 of lactation, and in litters on days 7 and 21 to determine the presence of Lactobacillus, E. coli and Clostridium perfringens. The collected sample was taken to the laboratory within 20 min. 1 g of composite faecal samples were diluted with 9 mL of 1% peptone broth and mixed with a vortex mixer. Viable bacterial plating was conducted (101 to 109) onto Lactobacilli spp. medium III agar plates (Medium 638, DSMZ, Braunschweig, Germany), MacConkey agar plates (Difco, Laboratories, Detroit, MI, USA), and C. perfringens agar plates (MB cell, Korea) to isolate Lactobacillus, E. coli and C. perfringens. respectively. The Lactobacillus agar plates were incubated anaerobically at 37–38 °C for 24 h, whereas MacConkey and C. perfringens agar plates were incubated anaerobically at 37 °C for 24–48 h. Finally, colonies were enumerated and log transferred for statistical analysis.
Statistical analysis
The collected data were analysed by the mixed GLM procedure of SAS version 9.2 (SAS Institute Inc., Cary, NC, USA) using individual sows and their offsprings as an experimental unit. Creep diet was considered as a fixed effect while, parity and grouping were considered as a random effect. The normality of data was verified using the UNIVARIATE procedure of SAS. Differences among the treatments were separated by Tukey’s range test. Variability in the data is expressed as the standard error means. A p-value of ≤0.05 was considered significant and ≤0.10 as a trend.
Results
Sow performance
The effect of the SGG supplement on a sow’s reproductive performance is shown in Table . The daily feed intake of sows during the gestation period remains (p > .05) similar in all treatment groups whereas during the lactation period SGG groups sows had greater (p = .04) daily feed intake compared to the CON group. The inclusion of SGG supplementation in the sow diet did not exhibit a significant difference in BW during pre-and post-farrowing, and at weaning but it has highly reduced (p ≤ .0003) the BW changes during the lactation period compared to those fed the CON diet. Also, the addition of SGG-Low and SGG-High supplementation in the sow diet tended to increase (p = .08) the back fat thickness at weaning and showed less (p ≤ .001) BFT loss at the end of the lactation period. However, the sow body condition score (BCS) and the days to oestrus interval of all sows remain similar (p > .05) until the end of the experiment. Furthermore, sows fed diet containing SGG-Low and SGG-High supplementation did not affect (p > .05) the birth weight of pigs, the total number of pigs born, the number of born alive, and stillbirth but, it has highly reduced (p = .04) the number of mummified and improved (p ≤ .05) the survivability of piglets during the first 24 h after birth.
Table 3. Effect of SGG supplement on sow reproductive performance.
Litter performance
The effect of the SGG supplement on litter performance is shown in Table . Piglets born to SGG group sows and fed a creep diet from days 5 to 21 of age, showed a tendency to increase the BW on day 7 (p = .07), and significantly higher BW on day 14 (p = .02), and 21 (p < .001). In addition, piglets born to SGG group sows and fed a creep diet showed significantly (p < .001) increased daily gain during the overall experimental period. Though, SGG group piglets showed a tendency to increase (p = .07) G:F from days 14 to 21 compared to the control group the daily feed intake of piglets remains unaffected (p > .05) until the end of the trial. The coefficient of variation (CV) of birth weight was lower (p = .03) in litters born to sows supplemented with SGG, but similar among the treatments at weaning (p > .05).
Table 4. Effect of SGG supplementation on litter performance.
Faecal bacterial count in sow
The dietary treatments did not (p > .05) influence the Lactobacillus and E. coli counts at pre- and post-farrowing and on day 7 of lactation (Table ). Also, the number of C. perfringens was similar (p > .05) during the pre- and post-farrowing. However, on day 7 of lactation, the C. perfringens counts were significantly reduced (p ≤ .05) in sows fed an SGG-High supplemental diet.
Table 5. Effect of SGG supplementation on bacteria counts in the faeces of sows.
Faecal bacterial count in litters
There were no differences (p > 0.05) in the number of faecal Lactobacillus, E. coli and C. perfringens bacterial counts in litters throughout the experiment (Table ).
Table 6. Effect of SGG supplementation on bacteria counts in the faeces of piglets.
Discussion
In this study, sows were selected as an experimental model to evaluate the efficacy of in-feed additive SGG on reproductive performance. Gestation sows in all treatment groups consumed a moderate level of feed compared to the lactation period suggesting that a moderate level of feed intake during gestation has highly helped the sows to avoid more weight gain and to overcome the farrowing complications while increased feed intake during lactation period helped the sows to maximise the productivity was reliable with the reports of Maes et al. (Citation2004).
Generally, acidifiers or products based on organic acids (OAs) serve to be an alternative choice to replace AGP in pigs’ diets (Li et al. Citation2018). In particular, OAs are established to improve the overall performance and immune status of pigs by reducing the inhabitants of harmful bacterial compounds and subclinical infections. Even earlier studies proved that OA supplementation had boosted the growth performance of pigs (Ngoc et al. Citation2020). For instance, Devi et al. (Citation2016) reported that 0.2% of OA supplementation increased the white blood cell (WBC) and immunoglobulin (IgG) counts in suckling piglets. On the other hand, Walsh et al. (Citation2007) demonstrate that weaning pigs fed 0.4% of blended OAs (citric, fumaric, lactic and benzoic acid) showed a better growth rate, feed intake and feed efficiency. In contrast with the current finding, Devi et al. (Citation2016) reported that sows fed diet supplements with OA blends had no impact on their feed intake during pre- and post-farrowing, and at weaning time and the proposed reason for this discrepancy might be due to the environmental factors, feeding patterns, or due to the neural and hormonal status of individual animals.
Body weight of sows in the present study remains similar and no adverse effects were found until the end of the trial but sows that were fed a diet supplemented with SGG-Low and SGG-High reduce BF loss at the end of the lactation period and tend to have higher body condition score (BCS) and back fat thickness at post-farrowing and at weaning, respectively. The current finding was not relatable with Devi et al. (Citation2016) who found no difference in the BW and BFT loss in sows fed 0.2% protected organic acid. The optimum BW and BCS of sows during gestation and lactation is very important to increase productivity and ensure the efficient utilisation of feed (Kim et al. Citation2016). Recently, modern swine industries focus to evaluate BW and BFT of sows at various production phases in order to adjust feeding levels that could maintain the body condition to achieve adequate reproductive efficiency, litter performance and sow longevity (Theil et al. Citation2014). Previously, Zaleski and Hacker (Citation1993) reported that excess body weight of sows during the last phase of gestation led to reproductive disorders such as difficulty in farrowing and stillborn piglets. While, Kim et al. (Citation2016) reported that the excess BWL of sows during lactation had extended the weaning to oestrous intervals, however in this study weaning to oestrus interval remains similar among all treatment groups suggesting that no improvements in weaning to oestrous interval might be due to the less BFT loss at the end of lactation period or due to the effect of creep feeding to piglets that may influence the behaviour and reproductive performance of sow (Yan et al. Citation2011).
Body weight and birth weight uniformity become the most important factors for piglet survival (Quiniou et al. Citation2002). However, the birth weight uniformity of piglets is mainly associated with the size of litter and parity (Damgaard et al. Citation2003). In this study, sows fed SGG-Low and -High supplementation improved the survivability of piglets during the first 24 h after birth and also enhance the litter weaning weight at day 21. Also, piglets born to SGG-high and low group sows and fed a creep diet from days 5–21 showed increased BW and ADG which was as not agreed with Devi et al. (Citation2016) who found no improvements in BW and ADG in piglets that were born to sows fed 0.1% and 0.2% of protected OA supplementation diet. Previously, Klindt (Citation2003) reported that feeding the creep diet from day 5 of age had a higher increase in ADG in pre-weaning piglets compared to those fed the creep diet 2 days prior to weaning. Likewise, Wolter et al. (Citation2002) found increased ADG in piglets fed a creep diet during lactation. The increased BW and ADG in this study were primarily due to the inclusion of highly digestible and palatable creep diets during lactation, which could stimulate acid production, and accelerate the induction of amylase and protease enzymes in their intestine (De Passille et al. Citation1989). Unlike, significant improvements in the BW and ADG, piglets born to SGG-Low and -High group sows showed a tendency to increase the G:F ratio and this finding was correlated with Canibe et al. (Citation2005) who found a similar result in growing pigs fed 1.8% formic acid supplemented diet. Correspondingly, Metzler and Mosenthin (Citation2007) and Suryanarayana et al. (Citation2012) reported that the addition of OA to the basal diet increased the G: F in growing pigs. While piglets born to sows fed different levels of SGG and had a creep diet showed no improvements in daily feed intake agreed with Balamuralikrishnan et al. (Citation2017) who observed no improvements in nursery pigs fed a creep diet. In the earlier study, Wattanakul et al. (Citation2005) demonstrate that feeder design including conventional creep hopper and shallow tray had influenced the piglets to consume more creep diet, from this we speculate that lack of feed intake in this present study might be due to the feeder type or feeding method that fails to influence the piglets to consume more feed and the exact reason for lack of ADFI is unknown currently, thus it needs further investigation.
Gut health plays a vital role in regulating the pig’s performance (Nguyen et al. Citation2020). Recently, several feed additives have been proposed to enhance the growth performance of animals by modulating the gut microbiota. For example, Devi et al. (Citation2016) reported that the addition of protected organic acid supplements in sows’ diet increased Lactobacillus and reduced the E. coli counts during the farrowing and weaning period. Thus, we hypothesised that the synergistic blend of OA would exert a beneficial effect on the gut microbiota of sows. As anticipated, dietary SGG supplementation has significantly reduced C. perfringens residents in sows during day 7 of lactation. Clostridium perfringens is a gram-positive, anaerobic and spore-forming bacillus that may produce major toxin microbiota in animals and humans (Silva et al. Citation2015). In 2020, Nguyen et al. (Citation2020) demonstrated that OA could penetrate through the cell wall of bacteria and prevent bacterial growth in the intestine. From this, we assume that the reduced C. perfringens count in sows at day 7 of lactation might be due to the effect of medium chain fatty acid in SGG that helps to prevent the entering of gram-positive C. perfringens bacteria into the gut. Though SGG supplemental diet reduces the gram-positive bacteria, there was no significant difference observed in the Lactobacillus and E. coli counts at pre-and post-farrowing, and on day 7 of lactation. The present outcome was not correlated with Dibner and Buttin (Citation2002) who observed increased Lactobacillus and reduced E. coli counts in pigs fed OAs supplement. Similarly, Upadhaya et al. (Citation2014) also reported that the inclusion of a 0.1% organic acid mixture (10% malic, 13% citric and 17% fumaric acids) had decreased E. coli and increased Lactobacillus counts in growing and finishing pigs. The proposed reason for this inconsistent result might be due to the anti-microbial activities of OA which differ from acid to acid depending upon concentration and pH of the gut or due to the difference in animals age. Moreover, our research team have planned to do further research to find out the exact cause for this inconsistent result.
Conclusion
Our study demonstrates the inclusion of a 0.3% synergistic blend of short and medium-chain organic acids (SGG) in the sow diet from late gestation to post-partum day 21, and subsequently feeding their off-springs with a creep diet would be beneficial for both sow productivity and to enhance pre-weaning growth rate.
Ethical approval
Animal handling and the experimental procedures of this study were revised and approved (No: DK-2-2005) by Animal Ethics and the Welfare Committee of Dankook University – Republic of Korea.
Author contributions
Vetriselvi Sampath, Jae Hong Park and Lane Pineda: investigation, data curation, formal analysis, writing – original draft preparation. Yanming Han: conceptualisation, methodology, software, writing – review & editing. In Ho Kim: conceptualisation, methodology, writing – review & editing, supervision.
Disclosure statement
Lane Pineda and Yanming Han were employed at Trouw Nutrition, R&D, Co., Ltd.
All authors have read and approved the final manuscript and affirmed that there are no potential conflicts of interest towards publication of the same.
Data availability statement
The data presented in this study are available on request from the corresponding author.
References
- Balamuralikrishnan B, Park JH, Kim IH. 2017. Interactive effects of weaning age and creep feed on the performance of sows and their piglets. Indian J Anim Sci. 87(10):1259–1263.
- Baltić B, Starčević M, Đorđević J, Mrdović B, Marković R. 2017. Importance of medium chain fatty acids in animal nutrition. IOP Conf Ser: Earth Environ Sci. 85:012048.
- Canibe N, Højberg O, Højsgaard S, Jensen BB. 2005. Feed physical form and formic acid addition to the feed affect the gastrointestinal ecology and growth performance of growing pigs. J Anim Sci. 83(6):1287–1302.
- Damgaard LH, Rydhmer L, Lovendahl P, Grandinson K. 2003. Genetic parameters for within-litter variation in piglet birth weight and change in within-litter variation during suckling. J Anim Sci. 81(3):604–610.
- De Passille AMB, Pelletier G, Menard J, Morisset J. 1989. Relationships of weight gain and behaviour to digestive organ weight and enzyme activities in piglets. J Anim Sci. 67(11):2921–2929.
- Desai DN, Patwardhan DS, Ranade AS. 2007. Acidifiers in poultry diets and poultry production. In: Luckstadt C, editor. Acidifiers in animal nutrition: a guide for feed preservation and acidification to promote animal performance. Nottingham (UK): Nottingham University Press; p. 63–69.
- Devi SM, Lee KY, Kim IH. 2016. Analysis of the effect of dietary protected organic acid blend on lactating sows and their piglets. R Bras Zootec. 45(2):39–47.
- Dibner JJ, Buttin P. 2002. Use of organic acids as a model to study the impact of gut microflora on nutrition and metabolism. J Appl Poult Res. 11(4):453–463.
- Dibner JJ, Richards JD. 2005. Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci. 84(4):634–643.
- Kim JS, Yang X, Baidoo SK. 2016. Relationship between body weight of primiparous sows during late gestation and subsequent reproductive efficiency over six parities. Asian Australas J Anim Sci. 29(6):768–774.
- Klindt J. 2003. Influence of litter size and creep feeding on preweaning gain and influence of preweaning growth on growth to slaughter in barrows. J Anim Sci. 81(10):2434–2439.
- Lawlor PG, Lynch PB. 2007. A review of factors influencing litter size in Irish sows. Ir Vet J. 60(6):359–366.
- Li S, Zheng J, Deng K, Chen L, Zhao XL, Jiang X, Fang Z, Che L, Xu S, Feng B, et al. 2018. Supplementation with organic acids showing different effects on growth performance, gut morphology, and microbiota of weaned pigs fed with highly or less digestible diets. J Anim Sci. 96(8):3302–3318.
- Maes DGD, Janssens GPJ, Delputte P, Lammertyn A, de Kruif A. 2004. Backfat measurements in sows from three commercial pig herds: relationship with reproductive efficiency and correlation with visual body condition scores. Livest Prod Sci. 91(1–2):57–67.
- Maribo H, Jensen BB, Hedemann MS. 2000. Different doses of organic acids to piglets. Danish Bacon and Meat Council no. Report no. 469, Copenhagen, Denmark.
- McNamara LB, Giblin L, Markham T, Stickland NC, Berry DP, O’Reilly JJ, Lynch PB, Kerry JP, Lawlor PG. 2011. Nutritional intervention during gestation alters growth, body composition and gene expression patterns in skeletal muscle of pig offspring. Animal. 5(8):1195–1206.
- Metzler B, Mosenthin R. 2007. Effects of organic acids on growth performance and nutrient digestibilities in pigs. In: Luckstadt C, editor. Acidifiers in animal nutrition: a guide for feed preservation and acidification to promote animal performance. Nottingham (UK): Nottingham University Press; p. 39–54.
- Mroz Z. 2005. Organic acids as potential alternatives to antibiotic growth promoters for pigs. Advances in pork production. 16(1), 169–182.
- Ngoc TTB, Oanh DT, Pineda L, Suparlark A, de Nienke G, Yanming H. 2020. The effects of synergistic blend of organic acid or antibiotic growth promoter on performance and antimicrobial resistance of bacteria in grow–finish pigs. Transl Anim Sci. 4(4):211.
- Nguyen DH, Seok WJ, Kim IH. 2020. Organic acids mixture as a dietary additive for pigs—a review. Animals. 10(6):952.
- NRC. 2012. Nutrient requirements of swine. 11th ed. Washington (DC): National Academy Press.
- Quiniou N, Dagorn J, Gaudré D. 2002. Variation of piglet’s birth weight and consequences on subsequent performance. Livest Prod Sci. 78(1):63–70.
- Sampath V, Han K, Kim IH. 2021. Influence of yeast hydrolysate supplement on growth performance, nutrient digestibility, microflora, gas emission, blood profile, and meat quality in broilers. J Anim Sci Technol. 63(3):563–574.
- Silva ROS, Junior CAO, Guedes RMC, Lobato FCF. 2015. Clostridium perfringens: a review of the disease in pigs, horses and broiler chickens. Microbiology. 45(06):927.
- Suryanarayana MVAN, Ramana JV. 2015. A review of the effects of dietary organic acids fed to swine. J Anim Sci Biotechnol. 6:45.
- Suryanarayana MVAN, Suresh J, Rajasekhar MV. 2012. Organic acids in swine feeding – a review. Agric Sci Res J. 2:523–533.
- Theil PK, Lauridsen C, Quesnel H. 2014. Neonatal piglet survival: impact of sow nutrition around parturition on fetal glycogen deposition and production and composition of colostrum and transient milk. Animal. 8(7):1021–1030.
- Upadhaya SD, Lee KY, Kim IH. 2014. Protected organic acid blends as an alternative to antibiotics in finishing pigs. Asian Australas J Anim Sci. 27(11):1600–1607.
- Upadhaya SD, Lee KY, Kim IH. 2016. Effect of protected organic acid blends on growth performance, nutrient digestibility and faecal micro flora in growing pigs. J Appl Anim Res. 44(1):238–242.
- Walsh MC, Sholly DM, Hinson RB, Saddoris KL, Sutton AL, Radcliffe JS, Odgaard R, Murphy J, Richert BT. 2007. Effects of water and diet acidification with and without antibiotics on weanling pig growth and microbial shedding. J Anim Sci. 85(7):1799–1808.
- Wattanakul W, Bulman CA, Edge HL, Edwards SA. 2005. The effect of creep feed presentation method on feeding behaviour, intake and performance of suckling piglets. Appl Anim Behav Sci. 92(1–2):27–36.
- Wolter BF, Ellis M, Corrigan BP, Decker JM. 2002. The effect of birth weight and feeding supplemental milk replace to piglets during lactation on pre-weaning and post-weaning growth performance and carcass characteristics. J Anim Sci. 80(2):301–308.
- Yan L, Jang HD, Kim IH. 2011. Effects of varying creep feed duration on pre-weaning and post-weaning performance and behaviour of piglet and sow. Asian Australas J Anim Sci. 24(11):1601–1606.
- Young MG, Tokach MD, Aherne FX, Main RG, Dritz SS, Goodband RD, Nelssen JL. 2004. Comparison of three methods of feeding sows in gestation and the subsequent effects on lactation performance. J Anim Sci. 82(10):3058–3070.
- Zaleski HM, Hacker RR. 1993. Variables related to the progress of parturition and probability of stillbirth in swine. Can Vet J. 34(2):109–113.