Abstract
This aim of the present study was to evaluate the effects of dietary xylooligosaccharides (XOS) on the intestinal morphology, nitrogen metabolism, faecal ammonia release, antioxidant capacity, and immune organ indices of broilers fed corn-soybean meal diet. A total of 240 healthy 1-day-old Arbour Acres (AA) broilers were randomly divided into four treatment groups receiving XOS supplementation of 0 (Control), 150, 300, and 450 mg/kg to basal diet for 6 weeks. Six replicates with 10 birds each were prepared for each treatment. Results showed that the villus height of duodenum, jejunum, and ileum and the villus height to crypt depth ratio of jejunum were increased significantly in the broilers receiving 150 mg/kg XOS compared with those in the Control (p < 0.05). Nitrogen utilisation from day 19 to 21 was higher in the 150 mg/kg XOS group than in the Control (p < 0.05). Moreover, the addition of 150 mg/kg XOS reduced the nitrogen excretion from day 40 to 42 (p < 0.05), and all XOS treatments increased the nitrogen utilisation from day 40 to 42 (p < 0.05). All dietary XOS supplementations decreased the faecal ammonia concentration on day 21 (p < 0.05), and the addition of 150 and 450 mg/kg XOS induced a lower faecal ammonia concentration compared with that of the Control on day 42 (p < 0.05). Dietary supplementation of 300 and 450 mg/kg XOS enhanced the activity of serum total antioxidant capacity compared with that in the Control and 150 mg/kg XOS groups at the age of 42 days (p < 0.05). Supplementation with 450 mg/kg XOS significantly increased the index of thymus (p < 0.05) and the content of total protein compared with those of the Control at the age of 21 days (p < 0.05). Therefore, dietary supplementation with 150 mg/kg XOS have a beneficial effect on broilers.
Dietary supplementation with XOS increased the villus height of duodenum, jejunum and ileum, and the VH/CD ratio of jejunum.
Dietary supplementation with XOS enhanced nitrogen metabolism and reduced faecal ammonia release.
Highlights
Introduction
Intestinal disease, oxidant damage, and poor immune defense systems have become major problems in poultry production (Borsoi et al. Citation2015; Zhang et al. Citation2018; Tarradas et al. Citation2020). Once the animals suffer from diseases, they will be harmed to varying degrees whether acute or chronic and infectious or non-infectious and bring certain losses to the farmers and the breeding industry. Antibiotics are widely used as animal feed additives because of their role in promoting growth and resisting diseases (Dibner and Richards Citation2005; Afsharmanesh et al. Citation2013) for the rapid development of animal husbandry. Unfortunately, the exposure of livestock and poultry to antibiotics poses risks to human health due to the emergency of antibiotic-resistant bacteria and the passage of antibiotics through faeces and residues in food of animal origin from organisms to humans (Hu and Cowling Citation2020). The long-term use of antibiotics has caused a series of negative problems, such as intestinal flora imbalance, decreased immunity, drug residues, drug-resistant strains, and environmental pollution. Therefore, exploring and promoting non-polluting and non-residue antibiotic alternatives are necessary for the further development of green ecological animal husbandry.
Gibson and Roberfroid (Citation1995) defined prebiotics as a non-digestible food component that produce beneficial effects on the health of the host by selectively stimulating the growth and/or activity of one or a limited number of gastrointestinal microflora already existing in the colon. In general, non-digestible oligosaccharides are prebiotics. At present, some functional oligosaccharides, such as mannooligosaccharides (MOS), fructooligosaccharides (FOS), chitooligosaccharides (COS), pectic oligosaccharides (POS), and soybean oligosaccharides (SBO), have been widely used in poultry diets as growth promoters instead of antibiotics (Corrigan et al. Citation2015; Shang et al. Citation2018; Xu et al. Citation2020; Liu et al. Citation2021; Wang et al. Citation2021). Xylooligosaccharides (XOS) are composed of 2–8 xylose molecules linked by β-1,4 glycosidic bonds, and its main components include xylobiose, xylotriose and xylotetraose (Pu et al. Citation2016; Zidan et al. Citation2021). XOS are mainly produced by enzymatic hydrolysis and thermal cracking from lignin-rich fibre raw materials, such as corn cob, peach palm waste, cauliflower stalk, wheat bran, and sugarcane bagasse (Seesuriyachan et al. Citation2017; Majumdar et al. Citation2021; Sonkar et al. Citation2021; Vieira et al. Citation2021; Nascimento et al. Citation2022). They have good stability and heat resistance under acidic conditions, a low calorie content, non-toxicity, and biological effects even at low daily doses (Carvalho et al. Citation2013). Given that XOS cannot be digested and absorbed via the gastrointestinal tract of animals, the short-chain fatty acids (SCFAs) produced through XOS fermentation by beneficial bacteria regulate lipid metabolism, antioxidant defense systems, and immune factors and relieve intestinal inflammation (Wang et al. Citation2011; Hansen et al. Citation2013; De Maesschalck et al. Citation2015; Fei et al. Citation2019). Using a human colon simulator, Christophersen et al. (Citation2013) added XOS to ferment soybean protein by mixing the faecal microbiota and it was found that XOS could modulate the protein-induced genotoxicity of the colonic environment through specific microbiota and SCFAs. XOS administration could beneficially improve the production performance, increase the content of SCFAs in the hindgut, enhance the egg quality, and modulate the nutrient digestibility and ileum morphology of laying hens (Xiao et al. Citation2020; Zhou et al. Citation2021). However, previous studies obtained inconsistent results regarding the effectiveness of XOS as prebiotics in broilers. Suo et al. (Citation2015) reported that XOS supplemented at 75 mg/kg decreases duodenal crypt depth. Yuan et al. (Citation2018) found that the dietary addition of 2 mg/kg XOS affects immune function by stimulating SCFAs to reduce jejunal cytokine gene expression. Sun et al. (Citation2013) observed that supplementation with 10 g/kg XOS could increase growth performance and endocrine metabolism and upregulate the titre of avian influenza H5N1 antibody to enhance humoral immunity. Yang et al. (Citation2022) demonstrated that supplementation with 200 mg/kg XOS could strengthen the activity of total superoxide dismutase (T-SOD) in serum and glutathione peroxidase (GSH-Px) in breast muscle. The above disparities might be related to the supplementation dose and source of XOS and diet type and breed of broilers.
The objective of the current study was to investigate the effects of dietary XOS supplementation of 0, 150, 300 and 450 mg/kg, on the intestinal morphology, nitrogen metabolism, faecal ammonia release, antioxidant capacity, and immune organ indices of broilers.
Materials and methods
Experiment design and dietary treatments
All the animal experiments were approved by the Institutional Animal Care and Use Committee of the Henan University of Science and Technology (HAUST-EAW-2021-C00227). A total of 240 healthy 1-day-old Arbour Acres (AA) broilers purchased from a local breeding farm (Luoyang, China) were randomly divided into four treatment groups with six replicates per treatment and 10 broilers per replicate. The birds were fed a basal diet supplementation with 0 (Control), 150, 300, and 450 mg/kg XOS. The basal diet (Table ) was formulated to meet the nutrient requirement of broilers in accordance with the Management Guide of National Research Council (National Research Council (NRC) Citation1994). XOS was purchased from Zhengzhou Yicong Biotechnology Co., Ltd (Zhengzhou, China). The main components of XOS are xylobiose, xylotriose and xylotetraose, and the content of XOS is 20%. The trial lasted for 42 days and divided into two stages: early (day 1–21) and later (day 22–42). Over the entire period (day 1–42) mortality rate was evaluated.
Table 1. Composition and nutrient levels of the basal diets (air-dry basis).
Experimental conditions
The birds in each replicate were housed in a wire cage (95 cm length × 90 cm width × 40 cm height) with ad libitum access to food and water. Natural light and artificial supplementary light (16 L: 8 D) were adopted. The room temperature was set at 33 °C–35 °C for the 1st week and gradually decreased by 2 °C–3 °C every week until the room temperature reached 25 °C. Good ventilation was kept throughout the trail period.
Sample collection
Blood was collected from the wing vein of broilers on 21 and 42 days of age. Two chickens were selected from each replicate, and 5 mL of blood was collected to a vacuum blood collection tube and centrifuged at 3000 x g/min for 15 min. The serum was stored in a − 20 °C refrigerator. On day 42, two broilers from each replicate were randomly selected and stabbed in the jugular vein to bleed to death. Approximately 2 cm portions in the middle part of duodenum, jejunum, and ileum were excised, and their contents were gently flushed with physiological saline. The intestinal segments were preserved in 4% paraformaldehyde tissue fixing solution. The thymus, spleen, liver, and bursa of Fabricius were weighed and recorded separately.
Intestinal morphology
The fixed intestinal segments were dehydrated, transparent, immersed in wax, embedded, sliced, stained with hematoxylin-eosin, then mounted and observed under a microscope (Olympus, Olympus, Inc., Tokyo, Japan). The villus height (VH), crypt depth (CD) and the VH/CD ratio of duodenum, jejunum and ileum were measured (Motic image plus 2.0, Motic, Inc., Wetzlar, Germany).
Nitrogen metabolism and faecal ammonia release
Total protein (TP), uric acid (UA), and urea nitrogen (UN) levels in serum were determined with an automatic biochemical analyser (Toshiba TBA-2000FR, Japan).
The total faecal method with manure trays set up for each replicate was performed on day 19–21 and 40–42. The daily feed intake was recorded, and the faeces were collected in repeat units. Hairy debris was removed from the collected fresh faecal samples. The samples were fix in nitrogen with 10% HCl (20 mL HCl per 100 g of faecal sample) and mixed thoroughly. Afterward, the faecal sample were taken out, dried in an oven at 65 °C for 72 h to a constant weight, crushed and passed through a 40 mesh sieve, and stored for measurement. The nitrogen content of the rations and manure was determined using a semi-automatic Kjeldahl nitrogen tester (Shengsheng K1301, Shanghai, China). In brief, 100 g of fresh chicken faeces were collected per replicate on day 21 and 42, placed in a 500 ml conical flask, and allowed to stand for 24 h at 25 °C. A portable ammonia gas detector (Xima AR8500, Shenzhen, China) was used to measure ammonia release from the samples.
Nitrogen intake (g/d) = daily feed intake × nitrogen content in the diet
Nitrogen excretion (g/d) = daily excretion × nitrogen content in the fecal
Nitrogen utilization % = (nitrogen intake – nitrogen excretion) / nitrogen intake
Antioxidant capacity
T-AOC, SOD and GSH-Px activities and malondialdehyde (MDA) content in serum were assayed following the instructions of the commercial kits from Nanjing Jiancheng Bioengineering Research Institute (Nanjing, China).
Immune organ indices
Immune organ index = immune organ weight (g)/live body weight (kg)
Statistical analysis
Data were analysed with SPSS 20.0 software (IBM Inc., NY) using one-way ANOVA and Duncan’s multiple range test to identify the differences among treatments. Significant difference was determined as p < 0.05.
Results
Intestinal morphology
The effects of dietary XOS on the morphology of small intestinal are summarised in Table . The dietary supplementation of 150 mg/kg XOS significantly increased the VH of duodenum, jejunum and ileum compared with that of the Control (p < 0.05). All XOS treatments did not have a significant effect on CD regardless of intestine part (p > 0.05). Nonetheless, the VH/CD ratio of the jejunum in the 150 mg/kg XOS treatment group was significantly improved compared with that in the Control (p < 0.05).
Table 2. Effects of dietary supplementation Xylooligosaccharides (XOS) on the intestinal morphology of broilers at 42 days of age.
Nitrogen metabolism and faecal ammonia release
Table shows the serum biochemical parameters on day 21 and 42. The concentration serum TP in 450 mg/kg XOS treatment group was the highest (p < 0.05) at the age of 21 days. XOS had no effect on the concentration of serum UA and UN (p > 0.05). The nitrogen utilisation in day 19–21 was higher (p < 0.05) in the 150 mg/kg treatment group than that in the Control (Table ). The 150 mg/kg XOS supplementation significantly reduced (p < 0.05) the nitrogen excretion from day 40 to 42 and notably increased the nitrogen utilisation from day 40 to 42 (p < 0.05) compared with those of the Control. XOS supplementation decreased the faecal ammonia release at day 21; in particular, the faecal ammonia release in the 150 and 450 mg/kg XOS treatment groups were still lower (p < 0.05) than that in the Control at day 42 (Figure ).
Figure 1. Effects of dietary Xylooligosaccharides (XOS) supplementation on the faecal ammonia release of broilers at 21 and 42 days of age. Basal diet supplementation with 0, 150, 300, and 450 mg/kg XOS. Values are mean ± standard error of mean. Values with different letters superscript have significantly difference.
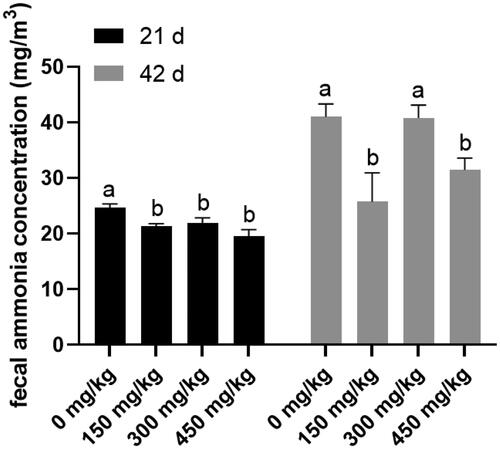
Table 3. Effects of dietary Xylooligosaccharides (XOS) supplementation on the serum biochemical parameters of broilers at 21 and 42 days of age.
Table 4. Effects of dietary Xylooligosaccharides (XOS) supplementation on the nitrogen metabolism of broilers from day 19 to 21 and from day 40 to 42.
Antioxidant capacity
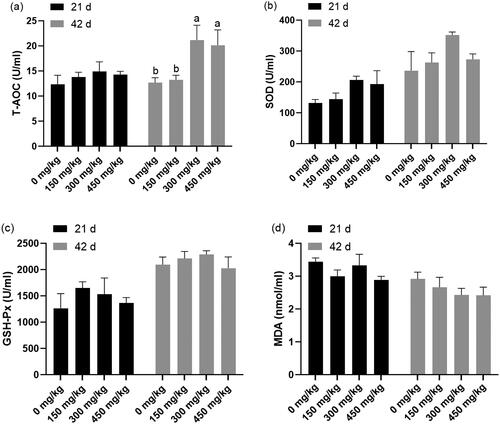
The effects of dietary XOS supplementation on T-AOC, SOD and GSH-Px activities and MDA content in the serum at the age of 21 and 42 days are presented in Figure . No significant difference (p > 0.05) was observed on serum T-AOC, SOD, and GSH-Px activities and MDA concentration among the treatments on day 21. At the age of 42 days, serum T-AOC activity was significantly enhanced (p < 0.05) in the 300 and 450 mg/kg XOS treatment groups compared with that in the Control and 150 mg/kg XOS treatment group.
Figure 2. Effects of dietary Xylooligosaccharides (XOS) supplementation on the antioxidant parameters in serum of broilers at 21 and 42 days of age. Basal diet supplementation with 0, 150, 300, and 450 mg/kg XOS. Values are mean ± standard error of mean. Values with different letters superscript have significantly difference.
Immune organ indices
Table shows that the effects of dietary XOS on the thymus, spleen, liver and bursa of Fabricius of broilers. Compared with that in the Control, the thymus index was significantly improved in the broilers receiving 450 mg/kg XOS (p < 0.05). No significant difference in spleen, liver, and bursa of Fabricius was observed among the treatment groups (p > 0.05).
Table 5. Effects of dietary Xylooligosaccharides (XOS) supplementation on the immune organ indices of broilers at 42 days of age (g/kg).
Discussion
The intestinal is an important place for the digestion and absorption of animal nutrients. Nutrients enter the animal body and are finally absorbed by small intestinal epithelial cells through physical reaction. Intestinal villus height, crypt depth, the villus height to crypt depth ratio are important indicators reflecting the integrity of intestinal structure (Paiva et al. Citation2014). Yang et al. (Citation2022) reported that 200 mg/kg XOS supplementation in broiler diet significantly increased the jejunal and ileal villus height. Other studies also found that the addition of 150 mg/kg XOS in broiler diets increased the ileum villus height (Luo et al. Citation2021). However, Wu et al. (Citation2006) indicated that XOS supplementation had no effect on jejunum morphology of broilers. Long intestinal villus and shallow crypt depth suggest that cells have a high ability to proliferate and absorb nutrients (Ghalwash et al. Citation2022). Current results showed that the villus height of duodenum, jejunum, and ileum and the VH/CD ratio of jejunum were significantly increased in the broilers fed with 150 mg/kg XOS. Similar to our findings, Wang et al. (Citation2021) found that corn-soybean meal diet supplemented with 100 mg/kg XOS has beneficial effects on the intestine of broilers; the villus height and the VH/CD ratio in all intestinal segments displayed notable increases. The improvement in intestinal morphology was due to XOS stimulating the populations of Bifidobacterium and Lactobacilli and the production of acetate and butyrate (Li et al. Citation2015; Ribeiro et al. Citation2018; Yin et al. Citation2019). In vitro experiment showed that probiotics can utilise XOS for growth and reproduction by competing with pathogenic bacteria for substrates and attachment sites, thus leading to the large accumulation of SCFAs and other metabolites of probiotics (Citationde Figueiredo et al. 2020). SCFAs provide energy for the proliferation of intestinal epithelial cells (Venegas et al. Citation2019), the VH/CD ratio is generally considered to be directly connected with epithelial cell turnover (Fan et al. Citation1997). Cell proliferation occurs primarily in the lower half of the crypt, in which the mitotic pressure forces cells to ascend along the crypt axis (Inan et al. Citation2000); the cells that promote crypt division move towards the exfoliated intestinal villus epithelium, thus increasing villus height. Given that all intestinal segment villus height were stimulated, we believed that XOS may accelerate the renewal rate of intestinal epithelial cells. The large absorption area leads to the enhancement of intestinal nutrient absorption ability, thus keeping the broiler intestine in a healthy state.
The digestibility of plant-based protein feeds is lower than that of animal-based protein feeds mainly due to their fibre levels and anti-nutritional factors. During active carbohydrate breakdown, amino acid fermentation end products, such as ammonia, are used by bacteria for protein synthesis during microbial growth; however, amines, ammonia, phenols, and indoles accumulate during carbon-limited fermentation (Cummings and Bingham Citation1987). The combination of xylanase and XOS stimulates the microbial community in the gut to efficiently degrade fibre, thereby increasing the intake of digestible nutrients and energy (González-Ortiz et al. Citation2021).
Prebiotic oligosaccharides may have anti-adhesive activity that allow them to compete with enteric pathogens for binding sites, coat the intestinal epithelial surface, and inhibit pathogen infection (Shoaf et al. Citation2006). XOS are selectively utilised by beneficial gut flora without being fermented by potential pathogens, such as toxigenic Escherichia coli, proteolytic Bacteroides, and toxigenic Clostridium (Manning and Gibson Citation2004). In this study, 150 mg/kg XOS supplementation significantly improved the nitrogen utilisation and reduced the faecal ammonia release of broilers. XOS can exert its effect by inhibiting intestinal ammonia-producing anaerobic bacteria, such as Bacteroides, and improve nutrient utilisation by increasing the activity of intestinal digestive enzymes (Kajihara et al. Citation2000; Li et al. Citation2021). The improved nitrogen utilisation and reduced faecal ammonia could be attributed in part to the increased height of the intestinal villi that widens the absorption area of nutrients, thereby improving protein utilisation. The supplementation of 150 mg/kg XOS can increase the height of small intestinal villi and improve nitrogen utilisation, and a further increase in XOS had no significant difference compared with that in the Control, which may be related to the fact that XOS promotes the proliferation of beneficial bacteria (Bifidobacterium and Lactobacillus). The increase in the number of beneficial bacteria and the secretion of SCFAs reduce the intestinal pH, provide a suitable working environment for digestive enzymes, and promote the absorption and utilisation of nutrients. However, an over acid gut environment can affect digestive enzyme activity and inhibit nutrient absorption.
The oxidation of polyunsaturated phospholipids present in cell membranes causes lipid peroxidation which increases the permeability of cell membranes; protein oxidation changes the structure and impairs the functional properties of the cell membrane, and DNA oxidation produces mutations through aberrations and damage repair mechanisms (Shrivastava et al. Citation2021). T-AOC is a comprehensive index used to measure the antioxidant function and reflects the compensatory ability of the antioxidant enzyme system and non-enzymatic system to external stimuli and the body’s free radical metabolism. SOD and GSH-Px constitute the enzymatic antioxidant defense system that prevents cells and tissues from being damaged by oxidative stress, resists the destruction of peroxides, and balances oxidative and antioxidant status (Xu et al. Citation2021; Wu et al. Citation2022). XOS derived from corn cob has good 2,2′-diphenyl-1-pyridinium hydrazine (DPPH) and 2,2′-azido-bis (3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging activity and iron-reducing antioxidant capacity (FRAP) (Boonchuay et al. Citation2021). The phenolic substituents of XOS show antioxidant activity (Bhatia et al. Citation2019) as confirmed by in vitro experiment (Lasrado and Gudipati Citation2015). Min et al. (Citation2016) found that the dietary supplementation of XOS synbiotics (1 g of probiotics B. subtilis product/kg of diets, 150 mg/kg XOS and 1 g/kg MOS) also enhanced the activities of T-SOD and lysozyme (LZM) in serum, thereby improving the antioxidant capacity of broilers. In the present study, 300 and 450 mg/kg XOS treatment significantly increased the serum T-AOC activity of broilers on day 42. The findings on this topic are inconsistent. Recent studies indicated that broilers fed with 200 mg/kg XOS improved the serum total superoxide dismutase (T-SOD) activity (Yang et al. Citation2022). Broilers receiving 100 mg/kg XOS had increased serum SOD and CAT activities and decreased MDA content (Yang Citation2012). These results indicated that XOS could alleviate the damage caused by oxidative stress. However, only a few reports are available on the antioxidant mechanism of oligosaccharides in animals. SCFAs may be one of the key factors regulating antioxidant capacity. Wang et al. (Citation2019) confirmed that the chelate of pectin oligosaccharides and zinc significantly up-regulates the expression of nuclear factor E2-related factor 2 (Nrf2) gene in the liver, which helps reduce mitochondrial dysfunction and oxidative damage. The improved antioxidant capacity is primarily due to the antioxidant properties of XOS. With the increase in the amount of XOS added, the active ingredients exhibiting antioxidant activity also increase. Our results indicated that supplementation of 300 and 450 mg/kg XOS can be effective to attenuate the oxidative stresses. These inconsistent results may be associated with the type and amount of XOS supplementation and the breed of broilers. Hence, the antioxidant mechanism of XOS needs further study.
The immune organ index is an important indicator of the immune status of poultry, the great absolute weight and relative weight of immune organs indicates the strength of the body’s cellular and humoral immune function. The liver contains the largest collection of phagocytic cells in the body and can generate a rapid and powerful immune response under the right conditions. Spleen is important in regulating the immune system and maintaining peripheral tolerance by eliminating circulating apoptotic cells, differentiating and activating T and B cells, and producing antibodies in the white pulp (Tarantino et al. Citation2013). The bursa of Fabricius is the major lymphoid organ in birds and is responsible for the expansion and differentiation of B lymphoid progenitors in its follicular microenvironment (Fellah et al. Citation2014). Broilers infected with Salmonella typhimurium and fed XOS diets showed a reduced abundance of Salmonella in their immune organs such as liver, spleen, and bursa of Fabricius, indicating their enhanced immune response (Jazi et al. Citation2019). Zhao (Citation2013) indicated that 75 mg/kg superfine XOS had no effect on the thymus and spleen index of broiler chickens at 42 days but significantly increased the bursa of the Fabricius index. Our present results indicated that dietary supplementation with 450 mg/kg XOS could significantly increase the thymus index of broilers. Thymus is closely related to the selection, development, proliferation, and differentiation of T cells (Xue et al. Citation2019). XOS can be used as antigens to effectively and lastingly stimulate the immune system, thus promoting the division and development of immune organ cells (Licht et al. Citation2012). O-acetylated XOS and its deacetylated derivatives formed by almond hulls hydrolysis are linked to (4-O-methyl-D-glucuronide)-D-xylan, exert mitogenic activity, and improve T-mitogen-induced thymocyte proliferation (Bhatia et al. Citation2019). Immune organs play an important role in the defense of poultry against bacterial invasion, so the immune organ index can reflect the immune response ability of birds. In the present work, supplementation of 450 mg/kg XOS significantly increased the thymus index, thereby enhancing the immunity of broilers to a certain extent, and without adversely affecting the development of other immune organs. A possible explanation is that the body obtained the immune ability from thymus and had reached the required level of immunity; therefore, the index of other immune organs can no longer be up-regulated. In summary, XOS might promote the development of immune organs.
Conclusion
This study demonstrated that dietary supplementation with 150 mg/kg XOS in broilers could increase the villus height of duodenum, jejunum, and ileum and the VH/CD ratio of jejunum, enhance the nitrogen utilisation, and reduce the faecal ammonia release. The dietary addition of 450 mg/kg XOS can improve the serum T-AOC activity and thymus index on day 42. Owing to its effects and breeding benefits, the dietary addition level of 150 mg/kg XOS is beneficial to broiler production.
Ethical approval
The experimental protocol in this study was approved by the Animal Care and Use Committee of Henan University of Science and Technology.
Acknowledgments
All authors of this manuscript are grateful to their universities and institutes for their technical assistance and valuable support in completing this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Afsharmanesh M, Sadaghi B, Silversides FG. 2013. Influence of supplementation of prebiotic, probiotic, and antibiotic to wet-fed wheat-based diets on growth, ileal nutrient digestibility, blood parameters, and gastrointestinal characteristics of broiler chickens. Comp Clin Pathol. 22(2):245–251.
- Bhatia L, Sharma A, Bachheti RK, Chandel AK. 2019. Lignocellulose derived functional oligosaccharides: production, properties, and health benefits. Prep Biochem Biotechnol. 49(8):744–758.
- Boonchuay P, Wongpoomchai R, Jaturasitha S, Mahatheeranont S, Watanabe M, Chaiyaso T. 2021. Prebiotic properties,antioxidant activity,and acute oral toxicity of xylooligosaccharides derived enzymatically from corncob. Food Biosci. 40:100895.
- Borsoi A, Quinteiro-Filho WM, Calefi AS, Ferreira AJP, Astolfi-Ferreira CS, Florio JC, Palermo-Neto J. 2015. Effects of cold stress and Salmonella Heidelberg infection on bacterial load and immunity of chickens. Avian Pathol. 44(6):490–497.
- Carvalho AFA, Neto PO, da Silva DF, Pastore GM. 2013. Xylo-oligosaccharides from lignocellulosic materials: chemical structure, health benefits and production by chemical and enzymatic hydrolysis. Food Res Int. 51(1):75–85.
- Christophersen CT, Petersen A, Licht TR, Conlon MA. 2013. Xylo-oligosaccharides and inulin affect genotoxicity and bacterial populations differently in a human colonic simulator challenged with soy protein. Nutrients. 5(9):3740–3756.
- Corrigan A, de Leeuw M, Penaud-Frézet S, Dimova D, Murphy RA. 2015. Phylogenetic and functional alterations in bacterial community compositions in broiler ceca as a result of mannan oligosaccharide supplementation. Appl Environ Microbiol. 81(10):3460–3470.
- Cummings JH, Bingham SA. 1987. Dietary fibre, fermentation and large bowel cancer. Cancer Surv. 6(4):601–621.
- de Figueiredo FC, Ranke FFB, de Oliva-Neto P. 2020. Evaluation of xylooligosaccharides and fructooligosaccharides on digestive enzymes hydrolysis and as a nutrient for different probiotics and Salmonella typhimurium. LWT. 118:108761.
- De Maesschalck C, Eeckhaut V, Maertens L, De Lange L, Marchal L, Nezer C, De Baere S, Croubels S, Daube G, Dewulf J, et al. 2015. Effects of xylo-oligosaccharides on broiler chicken performance and microbiota. Appl Environ Microbiol. 81(17):5880–5888.
- Dibner JJ, Richards JD. 2005. Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci. 84(4):634–643.
- Fan YK, Croom J, Christensen VL, Black BL, Bird AR, Daniel LR, Mcbride BW, Eisen EJ. 1997. Jejunal glucose uptake and oxygen consumption in turkey poults selected for rapid growth. Poult Sci. 76(12):1738–1745.
- Fei Y, Wang Y, Pang Y, Wang W, Zhu D, Xie M, Lan S, Wang Z. 2019. Xylooligosaccharide modulates gut microbiota and alleviates colonic inflammation caused by high fat diet induced obesity. Front Physiol. 10:1601.
- Fellah JS, Jaffredo T, Nagy N, Dunon D. 2014. Chapter 3 - development of the avian immune system. In: Schat KA, Kaspers B, Kaiser P, editors. Avian Immunology. 2nd ed. London: Academic Press; p. 45–63.
- Ghalwash HR, Salah AS, El‐Nokrashy AM, Abozeid AM, Zaki VH, Mohamed RA. 2022. Dietary supplementation with Bacillus species improves growth, intestinal histomorphology, innate immunity, antioxidative status and expression of growth and appetite‐regulating genes of Nile tilapia fingerlings. Aquac Res. 53(4):1378–1394.
- Gibson GR, Roberfroid MB. 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 125(6):1401–1412.
- González-Ortiz G, Santos T, Bedford MR. 2021. Evaluation of xylanase and a fermentable xylo-oligosaccharide on performance and ileal digestibility of broiler chickens fed energy and amino acid deficient diets. Anim Nutr. 7(2):488–495.
- Hansen CHF, Frøkiaer H, Christensen AG, Bergström A, Licht TR, Hansen AK, Metzdorff SB. 2013. Dietary xylooligosaccharide downregulates IFN-γ and the low-grade inflammatory cytokine IL-1β systemically in mice. J Nutr. 143(4):533–540.
- Hu YJ, Cowling BJ. 2020. Reducing antibiotic use in livestock, China. Bull World Health Organ. 98(5):360–361.
- Inan MS, Rasoulpour RJ, Yin L, Hubbard AK, Rosenberg DW, Giardina C. 2000. The luminal short-chain fatty acid butyrate modulates NF-κB activity in a human colonic epithelial cell line. Gastroenterology. 118(4):724–734.
- Jazi V, Mohebodini H, Ashayerizadeh A, Shabani A, Barekatain R. 2019. Fermented soybean meal ameliorates Salmonella Typhimurium infection in young broiler chickens. Poult Sci. 98(11):5648–5660.
- Kajihara M, Kato S, Konishi M, Yamagishi Y, Horie Y, Ishii H. 2000. Xylooligosaccharide decreases blood ammonia levels in patients with liver cirrhosis. Am J Gastroenterology. 95(9):2514–2514.
- Lasrado LD, Gudipati M. 2015. Antioxidant property of synbiotic combination of Lactobacillus sp. and wheat bran xylo-oligosaccharides. J Food Sci Technol. 52(7):4551–4557.
- Li SZ, Liu J, Chen ZM, Zheng AJ, Liu GH, Cai HY, Xu Y, Chang WH. 2021. Effects of xylo-oligosaccharides from poplar on growth performance, intestinal digestive enzyme activity and short-chain fatty acid content and serum hormone levels of broilers. Chin J Anim Nutr. 33(2):832–840.
- Li Z, Summanen PH, Komoriya T, Finegold SM. 2015. In vitro study of the prebiotic xylooligosaccharide (XOS) on the growth of Bifidobacterium spp and Lactobacillus spp. Int J Food Sci Nutr. 66(8):919–922.
- Licht TR, Ebersbach T, Frøkiaer H. 2012. Prebiotics for prevention of gut infections. Trends Food Sci Technol. 23(2):70–82.
- Liu HY, Li X, Zhu X, Dong WG, Yang GQ. 2021. Soybean oligosaccharides attenuate odour compounds in excreta by modulating the caecal microbiota in broilers. Animal. 15(3):100159.
- Luo D, Li J, Xing T, Zhang L, Gao F. 2021. Combined effects of xylo‐oligosaccharides and coated sodium butyrate on growth performance, immune function, and intestinal physical barrier function of broilers. Anim Sci J. 92(1):e13545.
- Majumdar S, Bhattacharyya DK, Bhowal J. 2021. Evaluation of nutraceutical application of xylooligosaccharide enzymatically produced from cauliflower stalk for its value addition through a sustainable approach. Food Funct. 12(12):5501–5523.
- Manning TS, Gibson GR. 2004. Prebiotics. Best Pract Res Clin Gastroenterol. 18(2):287–298.
- Min YN, Yang HL, Xu YX, Gao YP. 2016. Effects of dietary supplementation of synbiotics on growth performance,intestinal morphology, sIgA content and antioxidant capacities of broilers. J Anim Physiol Anim Nutr. 100(6):1073–1080.
- Nascimento CEO, Simões LCO, Pereira JC, da Silva RR, de Lima EA, de Almeida GC, Penna ALB, Boscolo M, Gomes E, da Silva R. 2022. Application of a recombinant GH10 endoxylanase from Thermoascus aurantiacus for xylooligosaccharide production from sugarcane bagasse and probiotic bacterial growth. J Biotechnol. 347:1–8.
- National Research Council (NRC). 1994. Nutrient requirements of poultry. 9th revised ed. Washington (DC): National Academy Press.
- Paiva D, Walk C, McElroy A. 2014. Dietary calcium, phosphorus, and phytase effects on bird performance, intestinal morphology, mineral digestibility, and bone ash during a natural necrotic enteritis episode. Poult Sci. 93(11):2752–2762.
- Pu J, Zhao X, Wang Q, Wang Y, Zhou H. 2016. Development and validation of a HPLC method for determination of degree of polymerization of xylo-oligosaccharides. Food Chem. 213:654–659.
- Ribeiro T, Cardoso V, Ferreira LMA, Lordelo MMS, Coelho E, Moreira ASP, Domingues MRM, Coimbra MA, Bedford MR, Fontes CMGA. 2018. Xylo-oligosaccharides display a prebiotic activity when used to supplement wheat or corn-based diets for broilers. Poult Sci. 97(12):4330–4341.
- Seesuriyachan P, Kawee-ai A, Chaiyaso T. 2017. Green and chemical-free process of enzymatic xylooligosaccharide production from corncob: enhancement of the yields using a strategy of lignocellulosic destructuration by ultra-high pressure pretreatment. Bioresour Technol. 241:537–544.
- Shang Y, Kumar S, Thippareddi H, Kim WK. 2018. Effect of dietary fructooligosaccharide (FOS) supplementation on ileal microbiota in broiler chickens. Poult Sci. 97(10):3622–3634.
- Shoaf K, Mulvey GL, Armstrong GD, Hutkins RW. 2006. Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infect Immun. 74(12):6920–6928.
- Shrivastava A, Mishra SP, Pradhan S, Choudhary S, Singla S, Zahra K, Aggarwal LM. 2021. An assessment of serum oxidative stress and antioxidant parameters in patients undergoing treatment for cervical cancer. Free Radic Biol Med. 167:29–35.
- Sonkar RM, Gade PS, Bokade V, Mudliar SN, Bhatt P. 2021. Ozone assisted autohydrolysis of wheat bran enhances xylooligosaccharide production with low generation of inhibitor compounds: a comparative study. Bioresource Technol. 338(14):125559.
- Sun Z, Lv W, Yu R, Li J, Liu H, Sun W, Wang Z, Li J, Shan Z, Qin Y. 2013. Effect of a straw-derived xylooligosaccharide on broiler growth performance, endocrine metabolism, and immune response. Can J Vet Res. 77:105–109.
- Suo H, Lu L, Xu G, Xiao L, Chen X, Xia R, Zhang L, LUO X. 2015. Effectiveness of dietary xylo-oligosaccharides for broilers fed a conventional corn-soybean meal diet. J Integr Agr. 14(10):2050–2057.
- Tarantino G, Scalera A, Finelli C. 2013. Liver-spleen axis: intersection between immunity, infections and metabolism. World J Gastroenterol. 19(23):3534–3542.
- Tarradas J, Tous N, Esteve-Garcia E, Brufau J. 2020. The control of intestinal inflammation: a major objective in the research of probiotic strains as alternatives to antibiotic growth promoters in poultry. Microorganisms. 8(2):148.
- Venegas DP, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA. 2019. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 10:277.
- Vieira TF, Corrêa RCG, Moreira RFPM, Peralta RA, de Lima EA, Helm CV, Garcia JAA, Bracht A, Peralta RM. 2021. Valorization of peach palm (bactris gasipaes kunth) waste: production of antioxidant xylooligosaccharides. Waste Biomass Valor. 12(12):6727–6740.
- Wang J, Cao Y, Wang C, Sun B. 2011. Wheat bran xylooligosaccharides improve blood lipid metabolism and antioxidant status in rats fed a high-fat diet. Carbohyd Polym. 86(3):1192–1197.
- Wang Q, Wang XF, Xing T, Li JL, Zhu XD, Zhang L, Gao F. 2021. The combined impact of xylo-oligosaccharides and gamma-irradiated Astragalus polysaccharides on growth performance and intestinal mucosal barrier function of broilers. Poult Sci. 100(3):100909.
- Wang Z, Yu H, Xie J, Cui H, Gao X. 2019. Effect of pectin oligosaccharides and zinc chelate on growth performance, zinc status, antioxidant ability, intestinal morphology and short-chain fatty acids in broilers. J Anim Physiol Anim Nutr (Berl). 103(3):935–946.
- Wang J, Zhang C, Zhao S, Ding X, Bai S, Zeng Q, Zhang K, Zhuo Y, Xu S, Mao X, et al. 2021. Dietary apple pectic oligosaccharide improves reproductive performance, antioxidant capacity, and ovary function of broiler breeders. Poult Sci. 100(7):101200.
- Wu Z, Chen J, Pirzado SA, Haile TH, Cai H, Liu G. 2022. The effect of fermented and raw rapeseed meal on the growth performance, immune status and intestinal morphology of broiler chickens. Anim Physiol Nutr. 106(2):296–307.
- Wu YY, Guo YM, Wang Z. 2006. Effect of XOS on growth performance, intestinal physiology and morphology of broilers. J Chin Agric Univ. 11(4):42–46.
- Xiao X, Wang Y, He J, Li Y. 2020. Effects of xylooligosaccharide (XOS) and probiotics (PRO) on production performance, egg quality and intestinal short-chain fatty acids of laying hens in late laying period. Anim Sci. 12(1):21–25.
- Xu Q, Azzam MMM, Zou X, Dong X. 2020. Effects of chitooligosaccharide supplementation on laying performance, egg quality, blood biochemistry, antioxidant capacity and immunity of laying hens during the late laying period. Ital J Anim Sci. 19(1):1181–1188.
- Xu D, Wu J, Sun L, Qin X, Fan X, Zheng X. 2021. Combined stress of acute cold exposure and waterless duration at low temperature induces mortality of shrimp Litopenaeus vannamei through injuring antioxidative and immunological response in hepatopancreas tissue. J Therm Biol. 100:103080.
- Xue Z, Ansari AR, Zhao X, Zang K, Liang Y, Cui L, Hu Y, Cheng R, Zhang X, Zhong J, et al. 2019. RNA-seq-based gene expression pattern and morphological alterations in chick thymus during postnatal development. Int J Genomics. 2019:6905194.
- Yang WB. 2012. Effects of xylooligosaccharides with different particle size on antibacterial function in vitro and applications in broiler diets [MSc]. Nanjing City (China): Nanjing Agricultural University.
- Yang C, Tang XW, Liu X, Yang H, Bin DM, Liu HJ, Tang QH, Tang JY. 2022. Effects of dietary oligosaccharides on serum biochemical index, intestinal morphology, and antioxidant status in broilers. Anim Sci J. 93(1):e13679.
- Yin J, Li F, Kong X, Wen C, Guo Q, Zhang L, Wang W, Duan Y, Li T, Tan Z, et al. 2019. Dietary xylo-oligosaccharide improves intestinal function in weaned piglets. Food Funct. 10(5):2701–2709.
- Yuan L, Li W, Huo Q, Du C, Wang Z, Yi B, Wang M. 2018. Effects of xylo-oligosaccharide and flavomycin on the immune function of broiler chickens. PeerJ. 6(3):e4435.
- Zhang JF, Bai KW, Su WP, Wang AA, Zhang LL, Huang KH, Wang T. 2018. Curcumin attenuates heat-stress-induced oxidant damage by simultaneous activation of GSH-related antioxidant enzymes and Nrf2-mediated phase II detoxifying enzyme systems in broiler chickens. Poult Sci. 97(4):1209–1219.
- Zhao Y. 2013. Effects of superfine xylooligosaccharides on growth performance, antioxidant and immunity function of broiler chickens [MSc]. Nanjing City (China): Nanjing Agricultural University.
- Zhou J, Wu S, Qi G, Fu Y, Wang W, Zhang H, Wang J. 2021. Dietary supplemental xylooligosaccharide modulates nutrient digestibility, intestinal morphology, and gut microbiota in laying hens. Anim Sci. 7(1):152–162.
- Zidan D, Sabran MR, Ramli NS, Shafie SR, Fikry M. 2021. Prebiotic properties of xylooligosaccharide extracted from sugarcane wastes (pith and rind): a comparative study. Int J Food Sci Technol. 56(5):2175–2181.
