Abstract
This study investigated the effects of different doses of rutin on the growth performance, immunity, intestinal barrier, and antioxidant capacity of broilers. A total of two hundred and fifty-six 1-day-old male broilers were divided into 4 groups and fed basal diets supplemented with 0 (control group), 250, 500 and 1,000 mg rutin/kg, respectively, for 42 days. In the starter period (days 1–21) and whole trial period (days 1–42), broilers fed diets containing 500 mg rutin/kg had significantly (p < 0.05) higher average daily feed intake, body weight and average daily gain and better feed conversion ratio. Dietary 500 mg rutin/kg increased the villus height, villus height to crypt depth ratio and villus area, while reducing the B cell lymphoma 2 associated X mRNA expression (p < 0.05). Dietary 500 mg rutin/kg increased the level of IgA in serum and decreased serum tumour necrosis factor-α (TNF-α) content, and the nuclear factor kappa-B, interleukin-2 and TNF-α mRNA expression in jejunal mucosa (p < 0.05). Simultaneously, 500 and 1,000 mg rutin/kg significantly decreased serum diamine oxidase activity, D-lactic acid and lipopolysaccharide concentration, increased activities of total superoxide dismutase and total antioxidant capacity in jejunal mucosa (p < 0.05), and upregulated mRNA expressions of genes related to intestinal barrier and antioxidant capacity in jejunal mucosa (p < 0.05). These findings indicate that dietary rutin, especially at 500 mg/kg, improves broilers’ growth performance, intestinal barrier function, immunity, and antioxidant capability, which may be related to the nuclear factor erythroid-2-related factor 2 (Nrf2) pathway.
Dietary rutin improved growth performance, jejunal morphology, and intestinal barrier function.
Dietary rutin enhanced the immunity via inhibiting NF-κB, and improved antioxidant capacity via activating Nrf2/HO-1 pathway in broilers.
The optimal dose of rutin was 500 mg/kg in broiler diet.
Highlights
Introduction
In the modern poultry industry, many factors such as disease infection, high stocking density and heat stress can all have harmful effects on broilers, leading to poor growth performance, reduced immunity and meat quality, and increased mortality and huge economic losses (Mishra and Jha Citation2019; Rajput et al. Citation2020; Abo-Al-Ela et al. Citation2021). In addition to nutrient digestion and absorption, the intestine also plays a critical role in immunity and defense against the potential stresses for farm animals–especially for broilers and piglets (Gessner et al. Citation2013; Yang, Liu et al. Citation2019; Wang et al. Citation2021). Adding additives into diets to improve intestinal functions, such as the intestinal morphology, barrier, immunity, and antioxidant capacity, is an important and easy way to maintain healthy and promote growth in broilers ( Lykkesfeldt and Svendsen Citation2007; Lin et al. Citation2013; Yang, Liu et al. Citation2019; Wang et al. Citation2021). Due to the increasing demands for food safety, plant extracts and their phytochemicals (such as flavonoids) have attracted extensive attention and are becoming the new trends to be used as growth promoters and meat quality improvers because they are widely available and leave no toxic residue in animal products (Landy et al. Citation2011; Kumar et al. Citation2013; Surai Citation2014; Qi et al. Citation2017; Long et al. Citation2020; Yang et al. Citation2022).
Rutin is widely present in various foods and medicinal plants, as light yellow or light green needle or crystalline powder (Yang et al. Citation2008). It has a number of pharmacological functions, such as anti-oxidation (Dobson et al. Citation2000), anti-inflammation (Yoo et al. Citation2014), anticarcinogenic activities (Alonso-Castro et al. Citation2013) and enhancing immunity (Awad et al. Citation2019; Casa et al. Citation2000). In addition, rutin has the potential for mucosal protection and antiulcer functions (Olaleye and Akinmoladun Citation2013). Yang et al. (Citation2008) and Gautam et al. (Citation2016) reported that rutin has a high degree of reactive oxygen species scavenging activity. Studies have reported that rutin could protect pancreatic islets, liver, kidney, testis, brain, and other tissues and organs from free radicals (Hosseinzadeh and Nassiri-Asl Citation2014), and alleviate oxidative stress, thereby increasing the body’s antioxidant function and strengthening the immune system (Russo et al. Citation2000; Caglayan et al. Citation2019).
For multiple biological functions, rutin, as an inexpensive natural plant extract, has the potential to be used as an efficient and green feed additive in broilers. However, the mechanism of rutin in improving growth performance and its effect on the intestinal function of broilers remain largely unclear. Therefore, this study was carried out to determine the possible effects, and optimal dosage of dietary rutin on boilers and, further, to explore the corresponding mechanism.
Materials and methods
Animal care, diets and experimental design
The Institutional Animal Care and Use Committee of Nanjing Agricultural University (Jiangsu, China) approved all of the procedures of this study (Permit number: SYXK-2020-00176).
Rutin (95% purity) was purchased from Shanghai Aladdin Biotechnology Co., Ltd. (Shanghai, China). A total of two hundred and fifty-six 1-day-old male broilers (Arbor Acres Plus) purchased from a local hatchery were randomly assigned to 4 groups (each group 8 replicates and 8 birds per replicate), which were fed basal diets supplemented with 0 (control group), 250, 500 and 1,000 mg rutin/kg, respectively, for 42 days. All of the broilers were kept in wire cages (8 broilers per cage). Birds were given a starter diet from day 1 to day 21 and a grower diet from day 22 to day 42. The basal diets were formulated to meet or exceed the National Research Council nutrient requirements (NRC, Citation1994). The corresponding ingredients and nutritional levels are listed in . Throughout the experiment, the broilers had unlimited access to feed in a mash form and clean water with a light program (24 h L: 0 h D, 1–3 days; 21 h L: 3 h D, 4–21 days; and 18 h L: 6 h D, 22–42 days). For the first 3 days, the temperature of the room was maintained at 34 ± 1 °C, then steadily decreased by 2 °C to 3 °C every week to the final temperature of 22 °C. The relative humidity of the room was kept at 45–55%.
Table 1. Ingredient and nutrient levels of the basal diets (fed basis).
Growth performance evaluation
The body weight (BW) of broilers was measured on days 1 (after hatching), 21 (fasted for 12 h) and 42 (fasted for 12 h), respectively, and the feed consumption was recorded. Then, the average feed intake (ADFI), average daily gain (ADG) and feed conversion ration (FCR) were calculated and adjusted by mortality during the starter (days 1–21), grower (days 22–42) and whole trial periods (days 1–42).
Sample collection
On day 42, one broiler (having fasted for 12 hours) was randomly chosen from each replication and sacrificed. The serum was collected by centrifuging the blood at 3,000 g for 15 minutes at 4 °C and stored at −20 °C until analysis. A jejunal segment from the middle of the small intestine was removed, and fixed in a 4% paraformaldehyde and 2.5% glutaraldehyde solution to analyse the jejunal morphology and mucosal tight junctions (TJs), respectively. The jejunal mucosa samples were collected and mixed with ice-cold normal saline solution before being homogenised (1:9, wt/vol). After that, the homogenate was centrifuged at 4,000 g for 15 minutes at 4 °C. Then, the supernatant was collected promptly and stored at −80 °C for further investigation. The remaining jejunal mucosa was scratched, quickly frozen in liquid nitrogen and stored at −80 °C. The bursa, thymus and spleen were removed and weighed to calculate the immune organ indexes using the following formula: immune organ index (g/kg) = immune organ weight (g)/body weight (kg).
Jejunal morphology analysis
A previous method was used to analyse the morphology of the intestines (Dong et al. Citation2016). Briefly, the paraformaldehyde-fixed jejunum segment was embedded in paraffin, cut into 5-μm-thick slices, mounted on glass slides, and stained with haematoxylin and eosin. In each section, villus height (VH, the distance from the villus tip to the crypt mouth), villus width (VW) and crypt depth (CD, the distance from the crypt mouth to the base) were measured using a computer-aided light microscope (Nikon, Japan) and Image-Pro Plus 6.0 software (Media Cybernetics, USA). For statistical analysis, the means of each cross section were employed. The villus height to crypt depth ratio (VH:CD) was calculated.
Transmission electron microscopy (TEM) analysis
The TJs between intestinal epithelial cells were characterised by TEM analysis according to Min et al. (Citation2014). Briefly, the fixed jejunum sample in 2.5% glutaraldehyde solution (24 h, 4 °C) was further fixed in 1% osmium for 1 h, dehydrated with a series of hierarchical ethanol solutions, and then fixed in epoxy resin. The sections were cut with a microtome (Boeckeler, Tucson, AZ, USA) and photographed under a TEM (Hitachi H-7650, Tokyo, Japan).
Indices measured by enzyme-linked immunoassay (ELISA)
The serum immunoglobulin A (IgA, Catalogue no. ANG-E32004C), immunoglobulin G (IgG, Catalogue no. ANG-E32009C), immunoglobulin M (IgM, Catalogue no. ANG-E32005C), growth hormone (GH, Catalogue no. ANG-E32049C), insulin-like growth factor-1 (IGF-1, Catalogue no. ANG-E32048C), D-lactic acid (D-LA, Catalogue no. ANG-E32105C), lipopolysaccharide (LPS), tumour necrosis factor-α (TNF-α, Catalogue no. ANG-E32030C) concentration and diamine oxidase (DAO, Catalogue no. AA88-2) activity in serum were measured by ELISA with the corresponding kits provided by Nanjing Qiangke Biotechnology Co., Ltd. (Nanjing, China).
Antioxidative indices in jejunum
The antioxidant parameters of the jejunal mucosa, including malondialdehyde (MDA, Catalogue no. A003-1-2) concentration, and total superoxide dismutase (T-SOD, Catalogue no. A015-2-1), glutathione peroxidase (GSH-Px, Catalogue no. A005-1-2), catalase (CAT, Catalogue no. A007-2-1) and total antioxidant capacity (T-AOC, Catalogue no. A015-2-1) activities were measured by the corresponding kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). To allow for inter-sample comparison, the acquired data were normalised against the total protein content in each sample.
RNA extraction and quantitative real-time PCR analysis
The total RNA was extracted to analyse the relative mRNA expression in jejunal mucosa according to the previous methods (Wang et al. Citation2018; Livak and Schmittgen Citation2001). The primer sequences for the target genes related to the antioxidant (SOD, GSH-Px, Haem oxygenase-1 (HO-1), nuclear factor erythroid-2-related factor 2 (Nrf2) and NAD(P)H dehydrogenase, quinone 1 (NQO1)), immunity (the nuclear factor kappa-B (NF-κB), myeloid differentiation primary response 88 (MyD88), interleukin-2 (IL-2), tumour necrosis factor-α (TNF-α) and interferon-γ (INF-γ)), intestinal barrier (Mucin2 (MUC2), occludin (OCLN), claudin2 (CLDN2), zonula occludens-1 (ZO-1)), and cell proliferation and apoptosis (B-cell lymphoma 2 (Bcl-2), Bcl-2 associated X (BAX), Ki67, and Caspase3) were synthesised by Sangon Biotech Co., Ltd. (Shanghai, China) and are listed in .
Table 2. Sequences used for real-time PCR primers.
Statistical analysis
All the data were firstly processed by Excel 2010 and, further, analysed using one-way ANOVA by SPSS 22.0. The values are presented as means with pooled standard error of means (SEM). Duncan’s multiple comparisons were used to compare differences across groups, and orthogonal polynomials were used to test linear, and quadratic effects, which were considered significant at p < 0.05.
Results
As indicated in , compared to the control group, dietary 250, 500 and 1,000 mg rutin/kg significantly increased the BW at 21 and 42 days (p < 0.05). During the starter period (days 1–21), dietary 500 and 1,000 mg rutin/kg increased the ADFI and ADG (p < 0.05), while the FCR showed improvement in a dose-dependent manner (P-quadratic <0.05) with the increasing levels of dietary rutin. During the grower period (days 22–42), in comparison with the control group, dietary 500 mg rutin/kg significantly increased the ADG (p < 0.05). Over the whole trial period (days 1–42), 500 and 1,000 mg rutin/kg significantly increased the ADFI and ADG of broilers (p < 0.05), and 500 mg rutin/kg significantly improved the FCR of the broilers (p < 0.05).
Table 3. Effects of dietary rutin on growth performance of broilers.
As indicated in , compared with the control group, dietary 500 and 1,000 mg rutin/kg significantly increased the serum GH level (p < 0.05), but no significant difference in serum IGF-1 concentration was observed among the 4 groups (p > 0.05).
Table 4. Effects of dietary rutin on serum GH/IGF-1 concentration of broilers (day 42).
indicates that the VH, VH:CD and VA values in jejunal mucosa were increased by 500 mg rutin/kg as compared with the control group (p < 0.05). However, there was no significant difference in jejunal VW or CD values among the 4 groups (p > 0.05).
Table 5. Effects of dietary rutin on jejunal tissue morphology of broilers (day 42).
The TEM Images (Figure ) show that TJs of intestinal epithelial cells in the control group and the 500 mg rutin/kg group were both normal, and there was no obviously damaged TJ structure. However, the intervals of cells seemed to be narrower in the jejunum of broilers from the 500 mg rutin/kg group.
Figure 1. Effects of dietary rutin on the tight junctions (TJs) of the jejunal mucosa of 42-day-old broilers (representative images of jejunum using transmission electron microscopy). Note: red arrows, the TJs; A, control group, basal diet supplemented 0 mg rutin/kg; B, 500 mg rutin/kg group, basal diet supplemented 500 mg rutin/kg; Scale bar, 1.0 μm.
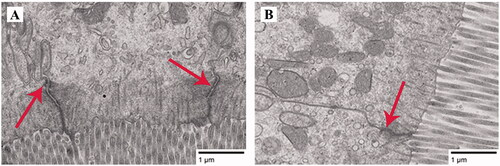
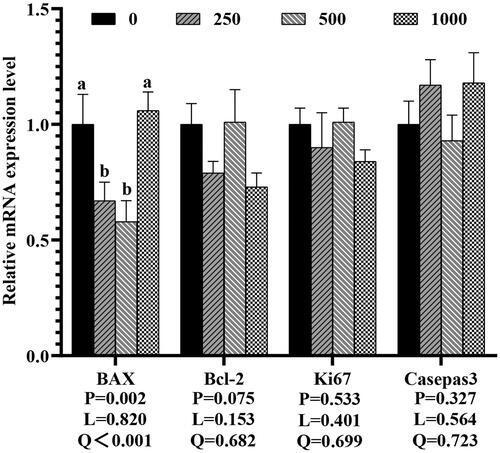
Figure shows that dietary 250, 500 and 1,000 mg rutin/kg had no significant effect on Bcl-2, Ki67, or Caspase3 mRNA expression in the broilers’ jejunal mucosa compared to the control group (p > 0.05), whereas dietary 250 and 500 mg rutin/kg dramatically lowered BAX mRNA expression in the broilers’ jejunal mucosa (p < 0.05).
Figure 2. Effects of dietary rutin on the mRNA expressions of proliferation and apoptosis related genes in jejunum mucosa. Note: a, b a, ba,b a, ba,b Means within the same gene of the histogram with different superscript differ significantly (P < 0.05). BAX, B-cell lymphoma 2 associated X; Bcl-2, B-cell lymphoma 2. 0, basal diet (control group); 250, 500 and 1,000, basal diet further supplemented with 250, 500 and 1,000 mg rutin/kg, respectively. Q and L are the quadratic and linear responses, respectively, to the levels of dietary supplementation with rutin.
As indicated in , in comparison to the control group, dietary 250, 500 and 1,000 mg rutin/kg did not significantly alter the thymus, spleen or bursa indexes of broilers (p > 0.05). Compared to the control group, dietary 500 and 1,000 mg rutin/kg significantly increased serum IgA level (p < 0.05). In addition, dietary supplementation with increasing levels of rutin linearly and quadratically decreased serum TNF- α concentration (P-linear and quadratic <0.05), but had no effect on serum IgM and IgG concentrations (p > 0.05).
Table 6. Effects of dietary rutin on immune organ indexes and serum immunoglobulin and inflammatory factors of broilers (day 42).
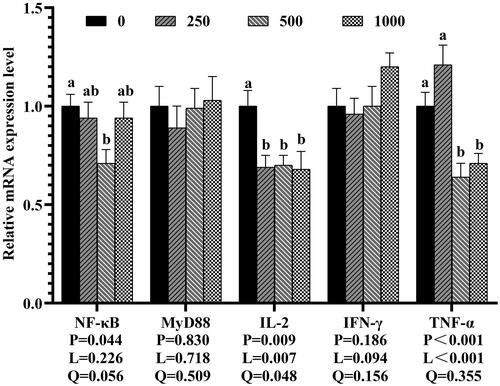
As revealed in Figure , dietary 500 mg rutin/kg significantly decreased the mRNA expression of NF-κB, and dietary 500 and 1,000 mg rutin/kg significantly down-regulated TNF-α mRNA expression in jejunal mucosa as compared to the control group (p < 0.05). The mRNA expression of IL-2 dose-dependently decreased with the increasing levels of rutin (P-linear and quadratic <0.01). However, dietary 250, 500 and 1,000 mg rutin/kg did not significantly effect on the mRNA expressions of Myd88 and IFN-γ in the jejunal mucosa (p > 0.05).
Figure 3. Effects of dietary rutin on the mRNA expressions of immune-related genes in jejunal mucosa of 42-day-old broilers. Note: a, bMeans within the same gene of the histogram with different superscript differ significantly (P < 0.05). NF-κB, nuclear factor kappa-B; MyD88, myeloid differentiation factor 88; IL-2, interferon-2; IFN-γ, interferon -γ; TNF-α, tumor necrosis factor-α. 0, basal diet (control group); 250, 500 and 1,000, basal diet further supplemented with 250, 500 and 1,000 mg rutin/kg, respectively. Q and L are the quadratic and linear responses, respectively, to the levels of dietary supplementation with rutin.
As summarised in , dietary 500 and 1,000 mg rutin/kg significantly reduced D-LA and LPS concentration and DAO activity in serum compared to the control group (p < 0.05). Meanwhile, there was no significant difference in LPS concentration or DAO activity across the 3 rutin groups (p > 0.05). However, serum D-LA content was significantly lower in the 500 mg rutin/kg group than that in the 1,000 mg rutin/kg group (p < 0.05).
Table 7. Effects of dietary rutin on serum intestinal barrier indicators of broilers (day 42).
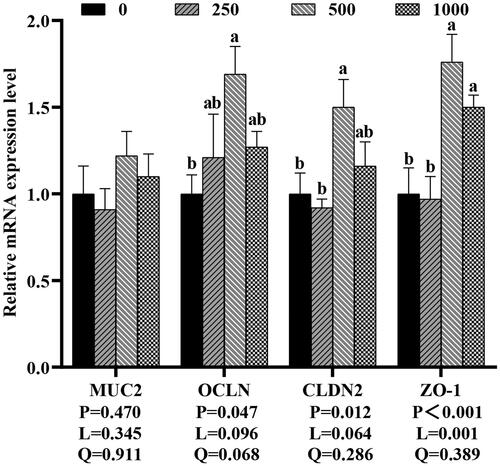
In Figure , compared to the control group, dietary 250, 500 and 1,000 mg rutin/kg did not significantly change the mRNA expression of MUC2 (p > 0.05), while 500 and 1,000 mg rutin/kg raised ZO-1 mRNA expression (p < 0.05), and 500 mg rutin/kg significantly enhanced the OCLN and CLDN2 mRNA expression in the broilers’ jejunal mucosa (p < 0.05).
Figure 4. Effects of dietary rutin on the mRNA expressions of intestinal barrier-related genes in jejunal mucosa of 42-day-old broilers. Note: a, bmeans within the same gene of the histogram with different superscript differ significantly (P < 0.05). MUC2, mucin2; OCLN, occludin; CLDN2, claudin-2; ZO-1, zonula occludens-1; 0, basal diet (control group); 250, 500 and 1,000, basal diet further supplemented with 250, 500 and 1,000 mg rutin/kg, respectively. Q and L are the quadratic and linear responses, respectively, to the levels of dietary supplementation with rutin.
Compared to the control group, dietary 500 mg rutin/kg increased T-SOD activity and decreased MDA content in the jejunal mucosa (p < 0.05, ). Broilers fed 500 and 1,000 mg rutin/kg exhibited higher T-AOC activity in the jejunal mucosa (p < 0.05). However, no significant variations in CAT and GSH-Px activity were found among the 4 groups (p > 0.05).
Table 8. Effects of dietary rutin on antioxidant capacity of jejunal mucosa of broilers (day 42).
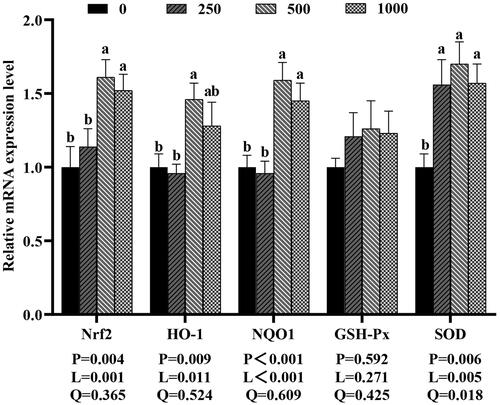
As shown in Figure , compared to the control group, dietary 500 and 1,000 mg rutin/kg increased the Nrf2 and NQO1 mRNA expression (p < 0.05), and dietary 500 mg rutin/kg significantly increased the mRNA expression of HO-1 (p < 0.05). The mRNA expression of SOD increased with the increasing levels of dietary rutin (P-linear and quadratic <0.01). However, dietary 250, 500 and 1,000 mg rutin/kg did not significantly affect the mRNA expression of GSH-Px in the jejunal mucosa of the broilers (p > 0.05).
Figure 5. Effects of dietary rutin on the mRNA expressions of antioxidant-related genes in jejunal mucosa of 42-day-old broilers. Note: a, b means within the same gene of the histogram with different superscript differ significantly (P < 0.05). Nrf2, nuclear factor erythroid-2-related factor 2; HO-1, heme oxygenase-1; NQO1, NAD(P)H quinone oxidoreductase 1; GSH-Px, glutathione peroxidase; SOD, superoxide dismutase. 0, basal diet (control group); 250, 500 and 1,000, basal diet further supplemented with 250, 500 and 1,000 mg rutin/kg, respectively. Q and L are the quadratic and linear responses, respectively, to the levels of dietary supplementation with rutin.
Discussion
It has become a new trend to use plant extracts or phytochemicals to improve growth or meat quality in poultry nutrition (Qi et al. Citation2017). Rutin is one of the cheapest natural plant extracts (flavonoids) and performs multiple biological activities, such as anti-oxidation and enhancing immunity, which shows great potential as feed additive in the poultry industry (Russo et al. Citation2000; Caglayan et al. Citation2019; Awad et al. Citation2019). Therefore, the effects of dietary supplementation of 250, 500 and 1,000 mg rutin/kg on broilers were investigated in the present study. Our results indicate that dietary supplementation of 500 and 1,000 mg rutin/kg diets significantly increased ADG and BW, which were similar to those reported by Hassan et al. (Citation2018), who found that broilers receiving 1 g rutin/kg diet had higher BW and protein efficiency ratio. Zhang and Kim (Citation2020) reported that dietary supplementation of 250, 500 and 1,000 mg quercetin/kg in the diet, one kind of flavonoid, had a quadratic effect on the body weight gain of broilers. Moreover, our results indicate broilers receiving 250, 500 and 1,000 mg rutin/kg showed increased ADFI and improved FCR in the starter period (days 1–21) and the whole trial period (days 1–42). However, dietary supplementation of 250 mg rutin/kg failed to induce marked effects on growth performance in the grower period (days 22–42), suggesting that different levels of rutin might be added to diets during different stages of growth in broilers. The levels of several hormones, such as GH and IGF-1, are closely related to the growth performance of animals. The GH can act on growth hormone receptor of skeletal muscle to produce paracrine or autocrine IGF-1, which can further promote protein synthesis through the IGF-1 receptor to upregulate skeletal muscle growth and development, and thus improve the growth performance of animals (Sotiropoulos et al. Citation2006; Ouyang et al. Citation2016). Similarly, our results show that broilers receiving 500 and 1,000 mg rutin/kg had a higher serum GH concentration, but the serum IGF-1 concentration was not affected, suggesting that the beneficial effects of rutin on growth may not be related to serum IGF-1 concentration, although the serum GH concentration was increased.
The small intestine is one of the most important organs for nutrient absorption. The VH, VW, CD, VH:CD, and VA are important indicators of intestinal morphology. Improvements in these parameters are closely related to the enhanced absorption of nutrients (Barzegar et al. Citation2021). Broilers receiving 500 mg rutin/kg showed higher VH, VH:CD, and VA values of the jejunal mucosa, suggesting that dietary supplementation of rutin could improve the jejunal morphology and might further improve the nutrient absorption of broilers. In agreement with our results, it has been verified that chickens treated with flavonoid compounds had greater VA, VH:CD, or VH values in the duodenum (Setiawan et al. Citation2018; Prihambodo et al. Citation2021). Moreover, our results showed that dietary supplementation of 500 mg rutin/kg inhibited the mRNA expression of BAX, which is one of the most important proapoptotic genes (Jiang et al. Citation2014; Campbell and Tait Citation2018). Once BAX activity is inhibited, apoptosis is hindered (Sakuragi et al. Citation2002). Our results suggest that the improved intestinal morphology might be related to decreased BAX expression in the mucosa of broilers.
Our results show that dietary treatments had no effect on thymus, bursa or spleen indexes of broilers, which is consistent with the results of Yang et al. (Citation2019). However, serum IgA concentration linearly dose-dependently increased with the increasing rutin levels. The IgA, as one of the main immunoglobulins in serum, is an important indicator of humoral immunity (Brandtzaeg Citation2009; Li et al. Citation2020). These results indicate that supplementing broiler diet with rutin may enhance the humoral immunity without affecting the development of the immune organs of broilers (Xing et al, Citation2020). Yang et al. (Citation2020) also found that dietary quercetin (0.02–0.06%) improved the serum IgA concentration of broilers in a dose-dependent manner. Moreover, our results show that dietary supplementation of 250, 500 and 1,000 mg rutin/kg decreased serum TNF-α content and IL-2 mRNA expression, and 500 mg rutin/kg diet decreased the mRNA expression of NF-κB and TNF-α in jejunal mucosa. TNF-α and IL-2 are cytokines that promote inflammation, which participate in the important inflammatory processes (Kammoun et al. Citation2014). NF-κB is a transcription factor that controls immunological response, especially for inflammation, by inducing the transcription and synthesis of pro-inflammatory factor TNF-α (Xu et al. Citation1998; Karin and Lin Citation2002; Kim et al. Citation2007). In agreement with our results, Su et al. (Citation2019) reported that rutin showed strong anti-inflammatory effects on inhibiting the synthesis of NF-κB and reducing pro-inflammatory factors, such as IL-1β, TNF-α, and IL-6 content. These findings, taken together, suggest that rutin might enhance immune response and reduce inflammation via inhibiting NF-κB signalling pathway in broilers.
D-LA, a product of intestinal bacterial metabolism, can be transferred to portal circulation when the permeability of the intestinal mucosa and capillaries increases (Yin et al. Citation2021). The LPS, mainly originating from intestinal gram-negative bacteria (such as Escherichia coli), can induce systemic inflammation (Rietschel et al. Citation1994). In the upper section of the intestinal mucosa, DAO, a highly active intracellular enzyme, is widely expressed (Li et al. Citation2015), and enhanced serum DAO activity is frequently linked to damaged intestinal mucosa and increased intestinal permeability. Thus, serum D-LA and LPS concentration, and DAO activity can be considered as important indicators reflecting the permeability of jejunal mucosal epithelial cells. In this study, broilers receiving 500 and 1,000 mg rutin/kg diets had lower serum D-LA and LPS concentration and DAO activity, suggesting that dietary supplementation of rutin (500 and 1,000 mg/kg) improved the intestinal barrier function of broilers. These results were in line with the report by Song et al. (Citation2017), who found that supplementing Artemisia annua (containing flavonoids) to the diet decreased plasma DAO activity in broilers. Moreover, in the present study, broilers receiving 500 mg rutin/kg diet showed up-regulated mRNA expression of genes related TJ (including ZO-1, OCLN and CLDN2) expression, and improved the TJ structure in the jejunal mucosa of broilers. TJs, which are composed of a large number of functional and structural proteins, are the main connections that constitute the mechanical barrier structure and play an important role in intestinal barrier function (Bazzoni et al. Citation2000; Suzuki Citation2013). Taken together, our results suggest that supplementing broiler with rutin could improve the intestinal barrier of broilers via enhancing expression of TJs, which is consistent with the previous reports by Noda et al. (Citation2012) and Gil-Cardoso et al. (Citation2016), who found that dietary flavonoids can protect ZO-1 delocalisation, and inhibit the decrease in ZO-1 and OCLN expression, increase transepithelial electrical resistance and improve the intestinal integrity.
An imbalance in the production and clearance of reactive oxygen species, such as hydroxyl radical and superoxide anion, leads to oxidative stress in farm animals (Lykkesfeldt and Svendsen, Citation2007; Mu et al. Citation2021). The concentration of MDA has been recognised as a marker that reflects the degree of lipid peroxidation (Del et al. Citation2005). Enzymatic and non-enzymatic systems cooperatively regulate antioxidant capacity and alleviate the oxidative stress of tissues. The T-AOC and enzymes of SOD, GSH-Px and CAT are common indicators for non-enzymic and enzymic antioxidant systems, respectively (Bakar et al. Citation2019). In the current study, broilers receiving 500 mg rutin/kg diet had higher T-AOC and T-SOD activity, and lower MDA content in the jejunal mucosa, indicating that supplementing broiler diet with rutin could improve the antioxidant capacity and relieve oxidative stress in broilers by enhancing both enzymic and non-enzymic antioxidant systems. In line with our present results, it has been proven that flavonoids are effective antioxidants, which can enhance the T-SOD and CAT activity, and decrease the MDA concentration (Gautam et al. Citation2016; Saleh et al. Citation2019). To further explore the possible molecular mechanism for the beneficial effects of dietary rutin, the mRNA expressions of genes related to the Nrf2 signal pathway were evaluated in the present study. As a critical transcription factor, Nrf2 connects with the antioxidant responsive element and controls a series of cytoprotective genes, such as NQO1 and HO-1, to regulate the antioxidant system (Kensler et al. Citation2007). The results of our present study show that broilers receiving 500 mg rutin/kg diet showed up-regulated mRNA expressions of HO-1, Nrf2, NQO1 and SOD in the jejunal mucosa, which are consistent with the increased T-AOC and T-SOD activity and decreased MDA content. Similar to our results, Pan et al. (Citation2014) reported that Nrf2 and HO-1 protein expression was higher in the livers of rats treated with rutin. It has also been proven that dietary supplementation of plant extracts with high levels of flavonoids could enhance the expression and activities of antioxidant enzymes via activating the Nrf2 signalling pathway (Gessner et al. Citation2013; Shen, et al. Citation2019; Guo et al. Citation2020). Therefore, it is reasonable to believe that rutin may enhance the antioxidant capacity of broilers by regulating the related gene expression in Nrf2 signalling pathway.
Conclusion
In conclusion, our results indicate that dietary supplementation of rutin improves the growth performance (especially for the starter period), jejunal morphology and enhances the intestinal barrier function of broilers. The beneficial effects of rutin on broilers may be related to the enhanced immunity by inhibiting the NF-κB signalling pathway, and the improved antioxidant capacity of intestine by activating the Nrf2/HO-1 pathway. The optimal dose of rutin is 500 mg/kg in broiler diet.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abo-Al-Ela HG, El-Kassas S, El-Naggar K, Abdo SE, Jahejo AR, Al Wakeel RA. 2021. Stress and immunity in poultry: Light management and nanotechnology as effective immune enhancers to fight stress. Cell Stress Chaperones. 26(3):457–472.
- Alonso-Castro AJ, Domínguez F, García-Carrancá A. 2013. Rutin exerts antitumor effects on nude mice bearing SW480 tumor. Arch Med Res. 44(5):346–351.
- Awad A, Zaglool AW, Khalil SR. 2019. Immunohaematological status and mRNA expression of the genes encoding interleukin-6, nuclear-factor kappa B, and tumor-necrosis factor-α in the spleen of broilers supplemented with dietary rutin. Anim Prod Sci. 59(8):1454–1461.
- Bakar E, Ulucam E, Cerkezkayabekir A, Sanal F, Inan M. 2019. Investigation of the effects of naringin on intestinal ischemia reperfusion model at the ultrastructural and biochemical level. Biomed Pharmacother. 109:345–350.
- Barzegar M, Zaghari M, Zhandi M, Sadeghi M. 2021. Effects of zinc dosage and particle size on gut morphology, tight junctions and TNF-alpha expression in broiler breeder hens. J Anim Physiol Anim Nutr. 0:1–11.
- Bazzoni G, Martinez-Estrada OM, Orsenigo F, Cordenonsi M, Citi S, Dejana E. 2000. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J Biol Chem. 275(27):20520–20526.
- Brandtzaeg P. 2009. Mucosal immunity: induction, dissemination, and effector functions. Scand J Immunol. 70(6):505–515.
- Caglayan C, Kandemir FM, Darendelioğlu E, Yıldırım S, Kucukler S, Dortbudak MB. 2019. Rutin ameliorates mercuric chloride-induced hepatotoxicity in rats via interfering with oxidative stress, inflammation and apoptosis. J Trace Elem Med Biol. 56:60–68.
- Campbell KJ, Tait SWG. 2018. Targeting BCL-2 regulated apoptosis in cancer. Open Biol. 8(5):180002.
- Casa CL, Villegas I, Lastra C, Motilva V, Calero M. 2000. Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J Ethnopharmacol. 71(1–2):45–53.
- Del RD, Stewart AJ, Pellegrini N. 2005. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 15(4):316–328.
- Dobson VL, Duthie SJ, Hinselwood DC, Kyle J, Collins AR, Boyle SP. 2000. Bioavailability and efficiency of rutin as an antioxidant: a human supplementation study. Eur J Clin Nutr. 54(10):774–782.
- Dong L, Zhong X, He J, Zhang L, Bai K, Xu W, Wang T, Huang X. 2016. Supplementation of tributyrin improves the growth and intestinal digestive and barrier functions in intrauterine growth-restricted piglets. Clin Nutr. 35(2):399–407.
- Gautam R, Singh M, Gautam S, Rawat JK, Saraf SA, Kaithwas G. 2016. Rutin attenuates intestinal toxicity induced by methotrexate linked with anti-oxidative and anti-inflammatory effects. BMC Complement Altern Med. 16:99.
- Gessner DK, Fiesel A, Most E, Dinges J, Wen G, Ringseis R, Eder K. 2013. Supplementation of a grape seed and grape marc meal extract decreases activities of the oxidative stress-responsive transcription factors NF-κB and Nrf2 in the duodenal mucosa of pigs. Acta Vet Scand. 55(1):18.
- Gil-Cardoso K, Ginés I, Pinent M, Ardévol A, Blay M, Terra X. 2016. Effects of flavonoids on intestinal inflammation, barrier integrity and changes in gut microbiota during diet-induced obesity. Nutr Res Rev. 29(2):234–248.
- Guo SW, Ma JX, Xing YY, Xu YQ, Jin X, Yan SM, Shi BL. 2020. Artemisia annua L. aqueous extract as an alternative to antibiotics improving growth performance and antioxidant function in broilers. Ital J Anim Sci. 19(1):399–409.
- Hassan FAM, Roushdy EM, Kishawy ATY, Zaglool AW, Tukur HA, Saadeldin IM. 2018. Growth performance, antioxidant capacity, lipid-related transcript expression and the economics of broiler chickens fed different levels of rutin. Animals. 9(1):7.
- Hosseinzadeh H, Nassiri-Asl M. 2014. Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. J Endocrinol Invest. 37(9):783–788.
- Jiang H, Zhao PJ, Su D, Feng J, Ma SL. 2014. Paris saponin I induces apoptosis via increasing the Bax/Bcl-2 ratio and Caspase3 expression in gefitinib-resistant non-small cell lung cancer in vitro and in vivo. Mol Med Rep. 9(6):2265–2272.
- Kammoun HL, Kraakman MJ, Febbraio MA. 2014. Adipose tissue inflammation in glucose metabolism. Rev Endocr Metab Disord. 15(1):31–44.
- Karin M, Lin A. 2002. NF-kappaB at the crossroads of life and death. Nat Immunol. 3(3):221–227.
- Kensler TW, Wakabayashi N, Biswal S. 2007. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 47:89–116.
- Kim JB, Han AR, Park EY, Kim JY, Cho W, Lee J, Seo EK, Lee KT. 2007. Inhibition of LPS-induced iNOS, COX-2 and cytokines expression by poncirin through the NF-kappaB inactivation in RAW 264.7 macrophage cells. Biol Pharm Bull. 30(12):2345–2351.
- Kumar P, Kumar S, KTripathi M, Mehta N, Ranjan R, Bhat Z, Singh P. 2013. Flavonoids in the development of functional meat products: A review. Vet World. 6(8):573.
- Landy N, Ghalamkari GH, Toghyani M. 2011. Performance, carcass characteristics, and immunity in broiler chickens fed dietary neem (Azadirachta indica) as alternative for an antibiotic growth promoter. Livest Sci. 142(1–3):305–309.
- Li Q, Wan G, Peng C, Xu L, Yu Y, Li L, Li G. 2020. Effect of probiotic supplementation on growth performance, intestinal morphology, barrier integrity, and inflammatory response in broilers subjected to cyclic heat stress. Anim Sci J. 91(1):e13433.
- Li Y, Zhang H, Chen YP, Yang MX, Zhang LL, Lu ZX, Zhou YM, Wang T. 2015. Bacillus amyloliquefaciens supplementation alleviates immunological stress and intestinal damage in lipopolysaccharide-challenged broilers. Anim Feed Sci Technol. 208:119–131.
- Lin J, Hunkapiller AA, Layton AC, Chang YJ, Robbins KR. 2013. Response of intestinal microbiota to antibiotic growth promoters in chickens. Foodborne Pathog Dis. 10(4):331–337.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR. Methods. 25(4):402–408.
- Long SF, He TF, Wu D, Yang M, Piao XS. 2020. Forsythia suspensa extract enhances performance via the improvement of nutrient digestibility, antioxidant status, anti-inflammatory function and gut morphology in broilers. Poult Sci. 99(9):4217–4226.
- Lykkesfeldt J, Svendsen O. 2007. Oxidants and antioxidants in disease: oxidative stress in farm animals. Vet J. 173(3):502–511.
- Min XH, Yu T, Qing Q, Yuan YH, Zhong W, Chen GC, Zhao LN, Deng N, Zhang LF, Chen QK. 2014. Abnormal differentiation of intestinal epithelium and intestinal barrier dysfunction in diabetic mice associated with depressed Notch/NICD transduction in Notch/Hes1 signal pathway. Cell Biol Int. 38(10):1194–1204.
- Mishra B, Jha R. 2019. Oxidative stress in the poultry gut: potential challenges and interventions. Front Vet Sci. 6:60.
- Mu S, Yang W, Huang G. 2021. Antioxidant activities and mechanisms of polysaccharides. Chem Biol Drug Des. 97(3):628–632.
- Noda S, Tanabe S, Suzuki T. 2012. Differential effects of flavonoids on barrier integrity in human intestinal Caco-2 cells. J Agric Food Chem. 60(18):4628–4633.
- NRC (National Research Council). 1994. Nutrient requirements of poultry: 1994. Washington, DC: National Academies Press.
- Olaleye MT, Akinmoladun AC. 2013. Comparative gastroprotective effect of post-treatment with low doses of rutin and cimetidine in rats. Fundam Clin Pharmacol. 27(2):138–145.
- Ouyang K, Xu M, Yan J, Wang W. 2016. Effects of alfalfa flavonoids on broiler performance, meat quality and gene expression. Can J Anim Sci. 96:331–340.
- Pan PH, Lin SY, Wang YY, Chen WY, Chuang YH, Wu CC, Chen CJ. 2014. Protective effects of rutin on liver injury induced by biliary obstruction in rats. Free Radic Biol Med. 73:106–116.
- Prihambodo TR, Sholikin MM, Qomariyah N, Jayanegara A, Batubara I, Utomo DB, Nahrowi N. 2021. Effects of dietary flavonoids on performance, blood constituents, carcass composition and small intestinal morphology of broilers: a meta-analysis. Anim Biosci. 34(3):434–442.
- Qi S, Wang T, Chen R, Wang C, Ao C. 2017. Effects of flavonoids from Allium mongolicum Regel on growth performance and growth-related hormones in meat sheep. Anim Nutr. 3(1):33–38.
- Rajput DS, Zeng D, Khalique A, Rajput SS, Wang H, Zhao Y, Sun N, Ni X. 2020. Pretreatment with probiotics ameliorate gut health and necrotic enteritis in broiler chickens, a substitute to antibiotics. AMB Expr. 10(1):220.
- Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, Ulmer AJ, Hringer UZ, Seydel U, Di PF. 1994. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 8(2):217–225.
- Russo A, Acquaviva R, Campisi A, Sorrenti V, Di Giacomo C, Virgata G, Barcellona ML, Vanella A. 2000. Bioflavonoids as antiradicals, antioxidants and DNA cleavage protectors. Cell Biol Toxicol. 16(2):91–98.
- Sakuragi N, Salah-Eldin AE, Watari H, Itoh T, Inoue S, Moriuchi T, Fujimoto S. 2002. Bax, Bcl-2, and p53 expression in endometrial cancer. Gynecol Oncol. 86(3):288–296.
- Saleh A, ElFayoumi HM, Youns M, Barakat W. 2019. Rutin and orlistat produce antitumor effects via antioxidant and apoptotic actions. Naunyn Schmiedebergs Arch Pharmacol. 392(2):165–175.
- Setiawan H, Jingga ME, Saragih HT. 2018. The effect of cashew leaf extract on small intestine morphology and growth performance of Jawa Super chicken. Vet World. 11(8):1047–1054.
- Shen MM, Zhang LL, Chen YN, Zhang YY, Han HL, Niu Y, He JT, Zhang YL, Cheng YF, Wang T. 2019. Effects of bamboo leaf extract on growth performance, meat quality, and meat oxidative stability in broiler chickens. Poult Sci. 98(12):6787–6796.
- Song Z, Cheng K, Zhang L, Wang T. 2017. Dietary supplementation of enzymatically treated Artemisia annua could alleviate the intestinal inflammatory response in heat-stressed broilers. J Therm Biol. 69:184–190.
- Sotiropoulos A, Ohanna M, Kedzia C, Menon R, Kopchick J, Kelly P, Pende M. 2006. Growth hormone promotes skeletal muscle cell fusion independent of insulin-like growth factor 1 up-regulation. Proc Natl Acad Sci USA. 103(19):7315–7320.
- Su S, Li X, Li S, Ming P, Huang Y, Dong Y, Ding H, Feng S, Li J, Wang X, et al. 2019. Rutin protects against lipopolysaccharide-induced mastitis by inhibiting the activation of the NF-κB signaling pathway and attenuating endoplasmic reticulum stress. Inflammopharmacol. 27(1):77–88.
- Surai PF. 2014. Polyphenol compounds in the chicken/animal diet: from the past to the future. J Anim Physiol Anim Nutr. 98(1):19–31.
- Suzuki T. 2013. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 70(4):631–659.
- Wang C, Zhang L, Ying Z, He J, Zhou L, Zhang L, Zhong X, Wang T. 2018. Effects of dietary zinc oxide nanoparticles on growth, diarrhea, mineral deposition, intestinal morphology, and barrier of weaned piglets. Biol Trace Elem Res. 185(2):364–374.
- Wang T, Cheng K, Yu CY, Li QM, Tong YC, Wang C, Yang ZB, Wang T. 2021. Effects of a yeast-derived product on growth performance, antioxidant capacity, and immune function of broilers. Poult Sci. 100(9):101343.
- Xing R, Yang H, Wang X, Yu H, Liu S, Li P. 2020. Effects of calcium source and calcium level on growth performance, immune organ indexes, serum components, intestinal microbiota, and intestinal morphology of broiler chickens. J Appl Poult Res. 29(1):106–120.
- Xu J, Fan G, Chen S, Wu Y, Xu XM, Hsu CY. 1998. Methylprednisolone inhibition of TNF-α expression and NF-κB activation after spinal cord injury in rats. Mol Brain Res. 59(2):135–142.
- Yang JX, Guo J, Yuan JF. 2008. In vitro antioxidant properties of rutin. LWT Food Sci Technol. 41(6):1060–1066.
- Yang JX, Maria TC, Zhou B, Xiao FL, Wang M, Mao YJ, Li Y. 2020. Quercetin improves immune function in Arbor Acre broilers through activation of NF-kappa B signaling pathway. Poult Sci. 99(2):906–913.
- Yang S, Zhang J, Jiang Y, Xu Y, Jin X, Yan S, Shi B. 2022. Effects of dietary supplementation with Artemisia argyi alcohol extract on growth performance, blood biochemical properties and small intestinal immune markers of broilers challenged with lipopolysaccharide. Anim Prod Sci. 62(3):234–247.
- Yang X, Liu Y, Yan F, Yang C, Yang X. 2019. Effects of encapsulated organic acids and essential oils on intestinal barrier, microbial count, and bacterial metabolites in broiler chickens. Poult Sci. 98(7):2858–2865.
- Yang YF, Zhao LL, Shao YX, Liao XD, Zhang LY, Lin LU, Luo XG. 2019. Effects of dietary graded levels of cinnamon essential oil and its combination with bamboo leaf flavonoid on immune function, antioxidative ability and intestinal microbiota of broilers. J Int Agri. 18:2123–2132.
- Yin J, Wang S, Qiu Y, Jiang E, Du G, Wang W, Xu P, Yang H, Hu M, Xiao W. 2021. Screening for and combining serum intestinal barrier-related biomarkers to predict the disease severity of AECOPD. Ann Palliat Med. 10(2):1548–1559.
- Yoo H, Ku SK, Baek YD, Bae JS. 2014. Anti-inflammatory effects of rutin on HMGB1-induced inflammatory responses in vitro and in vivo. Inflamm Res. 63(3):197–206.
- Zhang S, Kim IH. 2020. Effect of quercetin (flavonoid) supplementation on growth performance, meat stability, and immunological response in broiler chickens. Livest Sci. 242(104286):104286.
