 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Current experiment was performed in order to investigate the effects of Bacillus subtilis-based probiotic supplementation and fermented soybean meal (FSBM) on gut health, immune response to Newcastle disease virus (NDV), dry matter digestibility and growth performance in broiler chickens. Three hundred 1-day-old male broiler chicks (Ross 308) were allocated to five dietary treatments using a completely randomised design. Each treatment was replicated six times with 10 birds/replicate. The dietary treatments were (1) control group [basal diet (BD), without probiotic and FSBM], (2) BD + 0.02% probiotic (0.8 × 105 CFU of spores/gr feed), (3) BD + 0.04% probiotic (1.6 × 106 CFU of spores/gr feed), (4) 10% replacement of soybean meal (SBM) with FSBM in BD and (5) 20% replacement of SBM with FSBM in BD. Soybean fermentation was carried out under solid fermentation condition by B. subtilis spore. FSBM replacement at 20% improved weight gain and feed conversion ratio compared with the control group (p < 0.05). FSBM replacement at the level of 20% led to increase in villi height (p < 0.05). Villus height in the ileum was increased by 0.04% probiotic supplementation. FSBM replacement improved chicks’ immune response against NDV vaccine on day 42 (p < 0.05), but the effect was not significant for probiotic supplementation. Coliforms count in ileum was reduced by probiotic and FSBM supplementation (p < 0.05). Dry matter digestibility was improved by probiotic supplementation at 0.04%. According to the results of the current study, replacing SBM with FSBM at 20% and dietary supplementation of B. subtilis spores improved growth performance, intestinal health, immune system and dry matter digestibility of broilers.
Fermentation is one of the most effective methods which can be used to decrease the feed anti-nutritional factors and to improve feed nutritional properties.
Both the direct fed microbe via Bacillus subtilis spores supplementation and replacement of fermented soybean meal (FSBM) instead of soybean meal improved the chicks’ performance which mostly can be related to the beneficial effects of B. subtilis and FSBM on the gut health.
Highlights
Introduction
Growth-promoting antibiotics have long been used to increase feed efficiency and bird health conditions in the poultry industry. However, biosafety risks to human and animal health, including antibiotic resistance in bacteria and the accumulation and persistence of antibiotics in poultry products, have led to the elimination of growth-promoting antibiotics (Toghyani et al. Citation2010). Therefore, probiotics, prebiotics, organic acids and fermented feed have been used as alternatives to in feed antibiotics to alter poultry feed and improve bird performance and health (Nava et al. Citation2009). The beneficial effects of probiotics on performance parameters and general health of poultry has been documented (Levy et al. Citation2015; Seifi et al. Citation2017). According to the currently adopted definition by FAO and WHO, probiotics are ‘live microorganisms which when administered in adequate amounts confer a healthy benefit on the host’. Although use of probiotics in poultry diet were resulted in healthy gastrointestinal with an improved intestinal function, feed conversion ratio (FCR), body weight and consequently birds performance, these effects have not always been consistent (Lee et al. Citation2010; Nosrati et al. Citation2017). The use of fermented products in animal feed leads to beneficial effects on the function and health of the gastrointestinal tract by promoting probiotic effects, and therefore, can act as an alternative to antibiotics to stimulate growth. Soybean meal (SBM) is one of the most common sources of plant protein, widely used in poultry nutrition (Chiang et al. Citation2009). However, its use in poultry feed is often limited due to anti-nutritional factors (ANFs), including oligosaccharides, trypsin inhibitors, allergenic proteins and phytic acid, resulting in reduced digestion, absorption and utilisation of nutrients (Feng, Liu, Xu, Wang, et al. Citation2007; Li et al. Citation2014). Microbial fermentation is an effective method to eliminate or reduce ANFs and improve the nutritional value of plant-based protein meals (Ashayerizadeh et al. Citation2017; Jazi et al. Citation2017). Previous studies have shown a reduction in trypsin and other ANFs in fermented soybean meals (FSBM; Wang et al. Citation2014; Sharawy et al. Citation2016). The aim of the present study was to evaluate the impact of antimicrobial properties of FSBM, which may act similar to probiotics, on improving growth performance of broiler chicken through affecting gut health, immune response and nutrition digestibility.
Materials and methods
Fermented Soybean Meal preparation
Anaerobic solid-state fermentation with Bacillus subtilis (Gallipro® 200, Biochem, Germany) was used to FSBM. The Gallipro® 200 contains 4 × 109 spores of B. subtilis spores per gram with registration number DSM17299 in the European Union. Ninhydrin (Sarin et al. Citation1981), Bradford (Bradford Citation1976) and SDS-PAGE test were used to determine the highest amount of released peptides (Table ), the lowest amount of trypsin inhibitor and the index urease activity (Table ) as well as the lowest amount of glycinin and β-conglycinins (Figure ). Based on the analysis results obtained with FSBM, solid fermentation process in tray with a ratio of 30% water to 70% soybean (with a depth of 5–10 cm) for a duration of 72 h was the best one. For this purpose, 2 × 109 spores of Bacillus subtilis per kg of SBM were inoculated directly in the medium. To get rid of fungal and bacterial contaminants, a heat treatment at 85 °C for 30 min was used. Determination of urease and trypsin inhibitor activity index in SBM and FSBM was performed by AOAC (Citation2005) and Liu (Citation2019) method, respectively.
Figure 1. Glycinin and β-conglycinins content of soybean meal fermented by platter, aerobic and flooding methods using Bacillus subtilis spores at 0, 24, 48 and 72 h incubation.
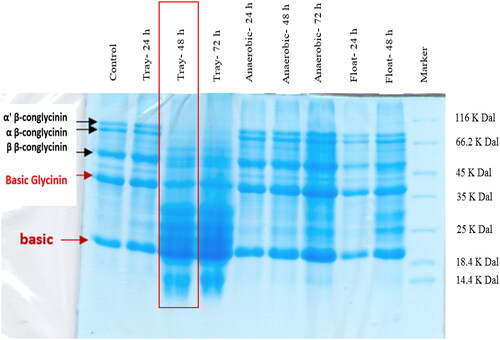
Table 1. Peptide content of soybean meal fermented by Bacillus subtilis based on standard glycine diagram.
Table 2. Chemical analysis of common soybean meal (SBM) and fermented soybean meal (FSBM).
Experimental design and diets
All care of animal, samples collection and animal procedures were approved by Institutional Animal Care and Use Committee of the Guilan University (110,051/p15). A total of 300 Ross 308 one day-old broiler chickens was weighed, and randomly allocated into five treatment groups with six replicate pens (10 birds per pen) based on a completely randomised design. The treatments consisted of (1) control diet (without probiotic and FSBM), (2) control diet + 0.02% probiotic (0.8 × 105 CFU of spores/gr feed), (3) control diet + 0.04% probiotic (1.6 × 106 CFU of spores/gr feed), (4) 10% replacement of SBM with FSBM and (5) 20% replacement SBM with FSBM. All diets were formulated based on nutrient requirement recommendation of Ross 308 strain catalogue (Aviagen Citation2019). The chick’s vaccination schedules, and ingredients and chemical compositions of the experimental diets are shown in Tables and , respectively.
Table 3. The vaccination program used in the experiment.
Table 4. Ingredients and chemical composition of the diets.
Sampling and measurements
For growth performance assessment, body weight gain (BWG) and feed intake (FI) were measured at 10 days of age, and then FCR was calculated accordingly. At day 28, 2 birds per replicate (12 birds per treatment) were randomly selected to count coliform population in the ileum and to investigate the morphological development of the small intestine in duodenum, jejunum and ileum.
Blood samples were collected from the wing veins of 12 birds/treatment (2 birds/replicate) at 28, 42 days of age. The serum was then separated and stored at −20 °C for antibody titre against Newcastle Disease Virus (NDV) analysis.
Humoral immune response against NDV
After vaccination in 6 and 18 days, sixty birds (1 chicks/replicate) in 28 and 42 days of age were randomly selected and blood samples were taken from the wing vein. Serum was separated and processed for hemagglutination inhibition (HI) test for NDV (Thayer and Beard Citation1998).
The antigen titre for running the HI test was determined by standard HA technique using NDV vaccine as antigen. The reciprocal of the highest dilution of the NDV antigen causing 100% agglutination of an equal volume of standardised RBCs was taken as the HA titre of the antigen (Alexander and Senne Citation2003).
Coliforms count
Digesta from the ileum were collected separately and immediately stored at −80 °C until needed for bacteriological analysis. Serial dilutions of the rinse diluent were prepared in sterile physiological saline. Total aerobic bacterial populations were enumerated on plate count agar. 100 µL from a serial dilution of the rinse diluent was plated on the surface of the MacConkey agar and incubated at 37 °C for 24 h to counting the colony-forming unit (Berrang and Dickens Citation2000).
Intestine morphology
In order to intestine morphology measurements, tissue samples were collected from duodenum, jejunum and ileum and flushed with buffered saline and fixed in 10% neutral buffered formalin for histo-morphological analysis. Samples were embedded in paraffin wax, sectioned and stained with haematoxylin and eosin. Sample sections were captured using a Leica DM LB microscope (Leica Microscope GmbH, 218 Wetzlar, Germany) in 10×, and morphometric indices were determined as described by Olnood et al. (Citation2015).
Dry matter digestibility
Titanium dioxide as a nutrient marker was added to finisher diets at 5 g/kg diet from 24 to 28 days of age for determination of dry matter digestibility. When the birds were 28-day-old, two birds from each cage were randomly selected and intestinal contents of them were collected from the ileum (between Meckel’s diverticulum and the ileo-cecal junction) by gently finger-stripping the intestinal segment. The digesta contents collected from the birds of two cages were pooled to represent one replicate (3 replicates per treatment). The digesta samples were kept at −20 °C until analysis. Representative samples of diets and ileal digesta were analysed for DM using atomic absorption (Guzman-Cedillo et al. Citation2017). The digestibility for DM was calculated as follow:
Statistical analysis
All statistical analyses were carried out using GLM procedures of SAS. Significant differences among individual group means were determined with Tukey (HSD) test (SAS Institute Citation2000). Test for the response of BWG in regard to doses of probiotic and FSBM was conducted by Sigmaplot software (Systat Software Inc Citation2017).
Results
The effects of probiotic and FSBM replacement on growth performance parameters of the chicks are shown in Table . The effect of probiotic and FSBM replacement was significant and improved BWG and FI (p < 0.05) but were not effect on FCR (p > 0.05) compared with the control group in 10 days old. Both of probiotic and FSBM replacement led to significant increase in BWG and decrease FCR compared with the control group in grower phase (p < 0.05). In finisher phase, 20% FSBM replacement increased and decreased BWG and FCR compared with the control group, respectively (p < 0.05). Probiotic supplementation and FSBM led to improve in BWG and FCR in comparison with the control during the whole period of the experiment (p < 0.05). Test for the response of BWG in regard to doses of probiotic and FSBM are depicted in Figure . The BWG response to both probiotic and FSBM was quadratic (Figure ).
Figure 2. Test for the response of body weight gain (BWG) to doses of probiotic (a) and fermented soybean meal (FSBM, b).
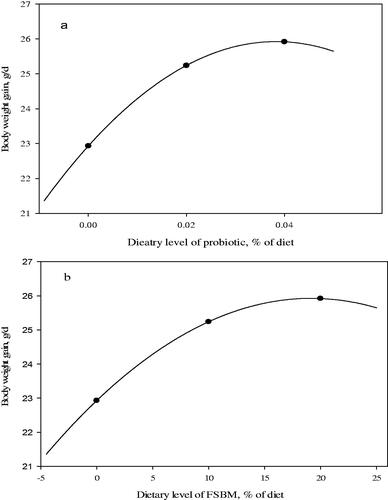
Table 5. The effect of probiotic and fermented soybean meal (FSBM) on growth performance of broiler chicks at 10 days of age.
The effects of probiotic and FSBM replacement on small intestine histo-morphometric parameters in 28 days are shown in Table . Replacement of FSBM increased the duodenal villi height (p < 0.05). There was a tendency for an increase in both duodenal villi height with FSBM replacement and ileal villi height/crypt depth ratio in probiotic supplemented diet, but these differences were not significant (p > 0.05). A significant increase in ileal villi height was observed in the 0.04% probiotic supplemented group compared with the control groups (p < 0.05).
Table 6. The effect of probiotic and fermented soybean meal (FSBM) on villi height (VH), crypt depth (CD) and the VH/CD ratio in different segments of small intestine in 28 days old chicks.
The effects of probiotic and FSBM replacement on antibody titre against NDV and ileal coliform population are shown in Table . Concerning immune response to NDV, the probiotic supplementation and FSBM replacement had no effect on the antibody titres against NDV in 28 days but 20% FSBM replacement enhanced significantly the antibody titres against NDV at 42 days of age (p < 0.05). As presented in Table , dietary supplementation of probiotic and 20% FSBM replacement reduced coliform counts in the ileum in compared with the control groups (p < 0.05). The effect of probiotic and FSBM replacement on dry matter digestibility is displayed in Table . The digestibility was significantly higher in chicks supplemented with 0.04% probiotic (p < 0.05). FSBM replacement at 20% showed an insignificant increase in dry matter digestibility (p > 0.05).
Table 7. The effect of probiotic and fermented soybean meal (FSBM) on antibody titre against NDV, coliforms count and dry mater digestibility (DMD) in broiler chicks.
Discussion
It has been reported that the ANFs in SBM, including trypsin inhibitors, β-conglycinin, glycinin and phytate, interrupt nutrient digestion and absorption (Yan et al. Citation2022). The results regarding to the reduced ANFs and increased small-size peptides by SBM fermentation meet the results of Feng, Liu, Xu, Wang, et al. (Citation2007). Several studies have demonstrated that partial replacement of SBM with FSBM with elimination or reducing of ANFs improves digestive enzyme activity, and gut morphology and consequently the growth performance of broiler chicks (Jazi et al. Citation2019; Soumeh et al. Citation2019). Based on the pre-experiment results (shown in Figure and Tables and ) the aerobic fermentation method of SBM in platter for 3 days was the best one. According to the results of the current study, both probiotic supplementation and SBM replacement with FSBM improved the growth performance of the chicks. In consistent with our results, in previous studies partial inclusion of FSBM in broiler diets improved weight gain and feed efficiency (Ashayerizadeh et al. Citation2017; Jazi et al. Citation2017). Feeding FSBM significantly increases the activities of digestive enzymes in intestinal contents of broiler chickens (Feng, Liu, Xu, Liu, et al. Citation2007). As reported by Floch and Seve (Citation2000), the greater duodenal villus height of birds on FSBM treatments is one of the most important factors responsible for the superior productive performance of the FSBM-fed birds. Increased villus height could be resulted in a greater absorptive capability for available nutrients, which in turn reflected its effect in lower FCR observed in the current study. FSBM reduces β-conglycinin and glycine levels which cause intestinal villi degeneration in the small intestine (Wang et al. Citation2014). Increased levels of small peptides (Tang et al. Citation2012) and reduced trypsin inhibitors (Tang et al. Citation2012) in FSBM appeared to be responsible for improved small intestinal structure and function. Wang et al. (Citation2003) in a study with pigs reported that dietary addition of small peptides improved intestinal morphology as determined by greater villus heights and lower crypt depths in the duodenum, jejunum and ileum. Feng, Liu, Xu, Wang, et al. (Citation2007) also reported reduced gut inflammation and improved nutrient bioavailability and digestibility using FSBM-based diets. Consistent with our findings, previous study in broiler chicks by Feng, Liu, Xu, Wang, et al. (Citation2007) on fermented protein meals have shown that inclusion of FSBM improved the small intestine morphological parameters. However, the improvement of growth may also be attributed to beneficial changes that occurred in microbial profiles due to dietary inclusion of FSBM and probiotics. Recently, feed processing (Yan et al. Citation2022) and feed additives (Abd El-Hack et al. Citation2020) are attaining importance in the poultry industry, as well as in health-care systems, because of their wide spectrum of beneficial impacts, such as promoting growth and production, immune enhancement and health protection. The results of this study indicated that probiotic and FSBM inclusion in broilers diets manipulated the gastrointestinal microbiota, especially coliforms, which are in line with the finding of Jazi et al. (Citation2018). Microbial activity in the gastrointestinal tract has been shown to have a significant effect on bird performance and health (Niba et al. Citation2008). Probiotics prevent the colonisation of pathogenic bacteria by producing antibodies, competing for attachment to the intestinal epithelium, competing for nutrients between microorganisms and bactericidal effects (Zhang and Kim Citation2014). Concerning this issue, it was concluded that probiotics based on Bacillus subtilis produced superior results in term of growth performance and intestinal health in broiler chicks (Abudabos et al. Citation2020). In addition, subtle changes to microbiota profile introduced by probiotic supplement were beneficial with reduction of the major pathogen, disease and dysbiosis (Aljumaah et al. Citation2020).
According to our results, despite an increase in the antibody titres against NDV in response to Bacillus subtilis-based probiotic, its effect was not significant which is in line with the findings of Seifert et al. (Citation2011). In the current study, antibody titre against NDV in FSBM-fed chicks was significantly higher than the control group that is in agreement with the results of Li et al. (Citation2020). Formation of small-sized peptides during fermentation are believed to be associated with an increase in immunoglobulin levels in birds (Tang et al. Citation2012; Xu et al. Citation2012). Balanced microbiota and modulation of the host immune system are also potential health-promoting effects of probiotics. Many factors influencing the condition of the gastrointestinal tract and the microbial population affect the digestibility of food. In this regard, many probiotic species produce enzymes that help food digestion (Zhang and Kim Citation2014; Hossain et al. Citation2016; Reis et al. Citation2017). The production of enzymes, reducing the population of pathogenic bacteria, improve in gastrointestinal health and immunity status of the chicks are the ways which a probiotics could affect the birds’ performance (Reis et al. Citation2017). Based on our finding, a significant increase was observed in dry mater digestibility in chicks supplemented with 4% probiotic. On day 28, in spite of a numerical increase in dry mater digestibility in FSBM-fed chicks, the effect was not significant. Yan et al. (Citation2022) reported that FSBM diets improved nutritional digestibility in the pigs, which may be derived from its beneficial effects on intestinal integrity, anti-oxidative capacity and immune function. However, a variety of ANFs present in SBM, such as antigenic proteins, trypsin inhibitors and oligosaccharides, interfere with digestion and absorption and have negative effects on animal health (Soumeh et al. Citation2019). The intestinal microbiota of animals plays an important role in maintaining intestinal homeostasis and animal health by modulating nutrient digestion, protecting against enteric pathogens, enhancing intestinal immunity and performing other physiological functions.
Conclusion
The results obtained in the current study indicated that microbial fermentation significantly reduced the content of ANFs in SBM and effectively improved its nutritional properties as a potential probiotic source. Both replacing SBM with FSBM and Bacillus subtilis supplementation improved growth performance, intestinal health, immune system status and dry matter digestibility in broiler chicks.
Acknowledgment
We are thankful to the Poultry Research Station in the University of Guilan for sample collection and Tarbiat Modares University (Tehran) for data analysis. The authors are grateful to Biochem Company for providing probiotic (Gallipro® 200) in the present study.
Disclosure statement
No potential conflict of interest was reported by the author(s). There has been no significant financial support for this work that could have influenced its outcome.
References
- Abd El-Hack ME, El-Saadony MT, Shafi ME, Shaza Y, Qattan A, Batiha GE, Asmaa FK, Abdel-Moneim AME, Alagawany M. 2020. Probiotics in poultry feed: a comprehensive review. J Anim Physiol Anim Nutr. 104(6):1835–1850.
- Abudabos AM, Abudabos MR, Aljumaah MM, Alkhlifi AA, Alabdullatif GM, Suliman Ali R, Sulaiman AL. 2020. Comparative effects of Bacillus subtilis and Bacillus licheniformis on live performance, blood metabolites and intestinal features in broileri with Salmonella infection during the finisher phase. Microb Pathog. 139:103870.
- Alexander DJ, Senne D. 2003. Newcastle disease. Dis Poultry. 11:64–87.
- Aljumaah MR, Alkhulaifi M, Abudabos AM, Aljumaah RS, Alsaleh A, Stanley D. 2020. Bacillus subtilis PB6 based probiotic supplementation prevents dysbiosis in Clostridium perfringens challenge. PLOS One. 15(6):e0232781.
- AOAC. 2005. Association of official analytical chemists. 21th ed. Gaithersburg, MD: AOAC International.
- Ashayerizadeh A, Dastar B, Shams Shargh M, Sadeghi Mahoonak AR, Zerehdaran S. 2017. Fermented rapeseed meal is effective in controlling Salmonella enterica serovar Typhimurium infection and improving growth performance in broiler chicks. Vet Microbiol. 201:93–102.
- Aviagen. 2019. Aviagen Ross 308 broiler nutrition guide. WebMD. http://en.aviagen.com/.
- Berrang ME, Dickens JA. 2000. Presence and level of Campylobacter spp. on broiler carcasses throughout the processing plant. J Appl Poult Res. 9(1):43–47.
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254.
- Chiang G, Lu WQ, Piao XS, Hu JK, Gong L, Thacker PA. 2009. Effects of feeding solid-state fermented rapeseed meal on performance, nutrient digestibility, intestinal ecology and intestinal morphology of broiler chickens. Asian Australas J Anim Sci. 23(2):263–271.
- Feng J, Liu X, Xu ZR, Liu YY, Lu YP. 2007. Effects of Aspergillus oryzae 3.042 fermented soybean meal on growth performance and plasma biochemical parameters in broilers. Anim Feed Sci Technol. 134(3–4):235–242.
- Feng J, Liu X, Xu ZR, Wang YZ, Liu JX. 2007. Effects of fermented soybean meal on digestive enzyme activities and intestinal morphology in broilers. Poult Sci. 86(6):1149–1154.
- Floch NL, Seve B. 2000. Protein and amino acid metabolism in the intestine of the pig: from digestion to appearance in the portal vein. Prod. Anim. 13:303–314.
- Guzman-Cedillo AE, Corona L, Castrejon-Pineda F, Rosiles-Martínez R, Gonzalez-Ronquillo M. 2017. Evaluation of chromium oxide and titanium dioxide as inert markers for calculating apparent digestibility in sheep. J. Appl. Anim. Res. 45(1):275–279.
- Hossain MM, Begum M, Kim IH. 2016. Effect of Bacillus subtilis, Clostridium butyricum and Lactobacillus acidophilus endospores on growth performance, nutrient digestibility, meat quality, relative organ weight, microbial shedding and excreta noxious gas emission in broilers. Veterinarni Medicina. 60(2):77–86.
- Jazi V, Ashayerizadeh A, Toghyani M, Shabani A, Tellez G, Toghyani M. 2018. Fermented soybean meal exhibits probiotic properties when included in Japanese quail diet in replacement of soybean meal. Poultr. Sci. 97(6):2113–2122. DOI: 10.3382/ps/pey071.
- Jazi V, Boldaji F, Dastar B, Hashemi SR, Ashayerizadeh A. 2017. Effects of fermented cottonseed meal on the growth performance, gastrointestinal microflora population and small intestinal morphology in broiler chickens. Br Poult Sci. 58(4):402–408.
- Jazi V, Mohebodini H, Ashayerizadeh A, Shabani A, Barekatain R. 2019. Fermented soybean meal ameliorates Salmonella Typhimurium infection in young broiler chickens. Poult Sci. 98(11):5648–5660.
- Lee KW, Lee SH, Lillehoj HS, Li GX, Jang SI, Babu US, Park MS, Kim DK, Lillehoj EP, Neumann AP, et al. 2010. Effects of direct fed microbials on growth performance, gut morphometric, and immune characteristics in broiler chickens. Poult Sci. 89(2):203–216.
- Levy AW, Kessler JW, Fuller L, Williams S, Mathis GF, Lumpkins B, Valdez F. 2015. Effect of feeding an encapsulated source of butyric acid (ButiPEARL) on the performance of male Cobb broilers reared to 42 d of age. Poult Sci. 94(8):1864–1870.
- Li Y, Guo B, Wu Z, Wang W, Li C, Liu G, Cai H. 2020. Effects of fermented soybean meal supplementation on the growth performance and cecal microbiota community of broiler chickens. Animals. 10(6):1098–1019.
- Li CY, Lu JJ, Wu CP, Lien TF. 2014. Effects of probiotics and Bremelain fermented soybean meal replacing fish meal on growth performance, nutrient retention and carcass traits of broilers. Livestock Science. 163:94–101.
- Liu K. 2019. Soybean trypsin inhibitor assay: Further improvement of the standard method approved and reapproved by American Oil Chemists’ Society and American Association of Cereal Chemists International. J Am Oil Chem Soc. 96(6):635–645. DOI: 10.1002/aocs.12205.
- Nava GM, Attene-Ramos MS, Gaskins HR, Richard JD. 2009. Molecular analysis of microbial community structure in the chicken ileum following organic acid supplementation. Vet Microbiol. 137(3–4):345–353.
- Niba AT, Yajima K, Kudi AC, Beal JD, Brooks PH, BSAS. 2008. Effect of concentration of phenolic compounds of two sorghum varieties on fermentation of sorghum with lactic acid bacteria for inclusion in poultry diets. In Proceedings of the British Society of Animal Science; , Scarborough, UK: Cambridge University Press; p. 80.
- Nosrati M, Javandel F, Camacho LM, Khusro A, Cipriano M, Seidavi A, Salem AZM. 2017. The effects of antibiotic, probiotic, organic acid, vitamin C, and Echinacea purpurea extract on performance, carcass characteristics, blood chemistry, microbiota, and immunity of broiler chickens. J Appl Poultry Res. 26(2):295–306.
- Olnood CG, Beski SS, Iji PA, Choct M. 2015. Delivery routes for probiotics effects on broiler performance, intestinal morphology and gut microflora. Anim Nutr. 1(3):192–202.
- Reis MP, Fassani EJ, Júnior AG, Rodrigues PB, Bertechini AG, Barrett N, Persia ME, Schmidt CJ. 2017. Effect of Bacillus subtilis (DSM 17299) on performance, digestibility, intestine morphology, fdand pH in broiler chickens. J Appl Poult Res. 26(4):573–583.
- Sarin VK, Kent SB, Tam JP, Merrifield RB. 1981. Quantitative monitoring of solid-phase peptide synthesis by the ninhydrin reaction. Anal Biochem. 117(1):147–157.
- SAS Institute. 2000. SAS users guide: statistics, version 9.12. Cary, NC: SAS Institute Inc.; p. 126–78.
- Seifert S, Fritz C, Carlini N, Barth S, Franz C, Watzl B. 2011. Modulation of innate and adaptive immunity by the probiotic Bifidobacterium longum PCB133 in turkeys. Poult Sci. 90(10):2275–2280.
- Seifi K, Karimi Torshizi MA, Rahimi S, Kazemifard M. 2017. Efficiency of early, single-dose probiotic administration methods on performance, small intestinal morphology, blood biochemistry, and immune response of Japanese quail. Poult Sci. 96(7):2151–2158.
- Sharawy Z, Goda AMA-S, Hassaan MS. 2016. Partial or total replacement of fish meal by solid state fermented soybean meal with Saccharomyces cerevisiae in diets for Indian prawn shrimp, Fenneropenaeus indicus, postlarvae. Anim Feed Sci Technol. 212:90–99.
- Soumeh EA, Mohebodini H, Toghyani M, Shabani A, Ashayerizadeh A, Jazi V. 2019. Synergistic effects of fermented soybean meal and mannan-oligosaccharide on growth performance, digestive functions, and hepatic gene expression in broiler chickens. Poult Sci. 98(12):6797–6807.
- Systat Software Inc. 2017. Sigma plot for windows, version 14. San Jose, CA, USA: SS Inc.
- Tang JW, Sun H, Yao XH, Wu YF, Wang X, Feng J. 2012. Effects of replacement of soybean meal by fermented cottonseed meal on growth performance, serum biochemical parameters and immune function of yellow-feathered broilers. Asian Australas J Anim Sci. 25(3):393–400.
- Thayer SG, Beard CW. 1998. Serological procedures. In: Swayne DE, Glisson JR, Jackwood MW, Pearson JE, Reed WM editors. A Laboratory Manual for the Isolation and Identification of Avian Pathogens. 4th ed. Am. Assoc. Avian Pathologists, Kennett Square, PA; p. 255–266.
- Toghyani M, Toghyani M, Gheisari A, Ghalamkari G, Mohammadrezaei M. 2010. Growth performance, serum biochemistry and blood hematology of broiler chicks fed different levels of black seed (Nigella sativa) and peppermint (Mentha piperita). Livestock Science. 129(1–3):173–178.
- Wang T, Fu YM, Lev JL, Jiang HS, Li YP, Chen CY. 2003. Effects of mini-peptides on the growth performance and the development of small intestines in weaning piglets. Anim Husb Vet Med. 35:4–e8.
- Wang Y, Liu XT, Wang HL, Li DF, Piao XS, Lu WQ. 2014. Optimization of processing conditions for solid-state fermented soybean meal and its effects on growth performance and nutrient digestibility of weanling pigs. Livestock Sci. 170:91–99.
- Xu FZ, Zeng XG, Ding X. 2012. Effects of replacing soybean meal with fermented rapeseed meal on performance, serum biochemical variables and intestinal morphology of broilers. Asian-Australas J Anim Sci. 25(12):1734–1741.
- Yan H, Jin JQ, Yang P, Yu B, He J, Mao XB, Yu J, Chen DW. 2022. Fermented soybean meal increases nutrient digestibility via the improvement of intestinal function, anti-oxidative capacity and immune function of weaned pigs. Animal. 16(6):100557–100510.
- Zhang ZF, Kim IH. 2014. Effects of multistrain probiotics on growth performance, apparent ileal nutrient digestibility, blood characteristics, cecal microbial shedding, and excreta odor contents in broilers. Poult Sci. 93(2):364–370.
