Abstract
This experiment was conducted to investigate the effects of palygorskite and probiotics complex (Pal–Pro) supplementation on laying performance, the microflora structure and biodiversity of Xuefeng black-bone chicken. A total of 432 Xuefeng black-bone chickens were randomly assigned into 4 groups with 6 replicates of 18 hens. The control group (group A) was fed the basal diet, and the experimental groups (groups B, C and D) were fed basal diet supplemented with 250, 500 and 750 mg/kg Pal–Pro for 8 weeks. The results were as follows: (1) The Pal–Pro supplementation linearly increased (p < .05) laying rate, and linearly decreased the feed conversion ratio (p < .05). (2) At the phylum level, compared with the group A, the abundance of Bacteroidetes was increased (p < .05) and the abundance of Proteobacteria was decreased (p < .05) in group D, and the abundance of Fusobacteria was increased in group C (p < .05). (3) At the genus level, the abundance of Bacteroides, Lachnospiraceae_unclassified and Ruminococcaceae_UCG-014 of group D were higher (p < .05) than those in group A, the abundance of CHKCI001 of group B was higher than that in group A, and compared with group A, the abundance of Fusobacterium was increased (p < .05) and the abundance of Pseudomonas was decreased in groups C and D (p < .05). (4)The Spearman correlation analysis showed that Aeriscardovia was negatively correlated with laying rate (p < .01), and Enterococcus and Ruminococcaceae_UCG-014 were positively correlated with egg weight (p < .05). In conclusion, dietary Pal–Pro may improve laying performance by adjusting the flora structure. The recommended dosage is 750 mg/kg.
Dietary supplementation of Pal–Pro improved the laying performance of Xuefeng black-bone chicken.
Dietary supplementation of Pal–Pro could alter the faecal microbial community of Xuefeng black-bone chicken.
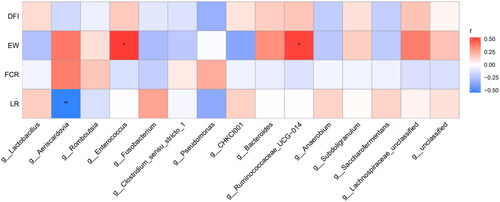
Aeriscardovia was negatively correlated with laying rate, Enterococcus and Ruminococcaceae_UCG∼014 were positively correlated with egg weight.
HIGHLIGHTS
Introduction
Poultry gut is the main way for pathogens to invade the body, and gut health is closely related to the healthy growth of poultry. A large number of microorganisms are colonised in the gut, and the host and the gut microbes coexist mutually beneficially. On the one hand, the host provides the growth environment and conditions for the gut microbes, and on the other hand, the gut microbes play a crucial role in resisting the pathogen invasion, immune regulation and nutrient digestion (Sergeant et al. Citation2014; Roberts et al. Citation2015; Clavijo and Florez Citation2018). A stable microflora is conducive to healthy growth and production of poultry, while gut microbial imbalance can lead to intestinal infections, decreased production performance and increased mortality (Kogut Citation2019).
In recent years, studies have revealed that mineral feed additives, such as montmorillonite (Chen et al. Citation2019), bentonite (Lee et al. Citation2018) and zeolite (Prasai et al. Citation2016), can affect poultry gut microbes. Palygorskite (Pal) has a strong adsorption capacity and antibacterial function (Wang et al. Citation2015; Zhang et al. Citation2016; Ding et al. Citation2021). It has been reported that palygorskite could also improve intestinal antioxidant and immune capacity, improve intestinal morphology and enhance intestinal barrier function of laying hens or broilers (Su, Chen, Chen, et al. Citation2018; Su, Chen, Cheng, et al. Citation2018). Probiotics (Pro) mainly promote the healthy growth of animals by inhibiting the growth of harmful bacteria in the gut and creating a suitable environment for beneficial bacteria growth (Collado et al. Citation2007; Bailey et al. Citation2017). Numerous studies have demonstrated that probiotics could promote performance of animals by altering gut flora (Chen, Li et al. Citation2020; Tian et al. Citation2020; Zhang et al. Citation2021).
The combined use of multiple additives has become a tendency in the animal husbandry industry. Some studies have demonstrated that the combination of additives could increase productivity, such as palygorskite and ginger essential oil complex (Lei et al. Citation2017; Xu et al. Citation2020), palygorskite combined with trace elements (Ding et al. Citation2021; Wang et al. Citation2021) and probiotic blend fermented herbal (Wang, Li et al. Citation2021). And our former studies have shown similar results on production performance of chickens, including mixture of Bacillus subtilis and essential oils (Liu et al. Citation2020), the combination of Bacillus subtilis and montmorillonite (Chen et al. Citation2020) and essential oil/palygorskite composite (Cheng et al. Citation2022). Pro usually rely on mineral carriers to be added to the diet, and Pal not only have similar biological functions with Pro, but could also act as carriers for Pro. And there are several reports about the effect of Pal and Pro complex (Pal–Pro) on faecal microbial community. Therefore, we suspect that Pal–Pro may have a synergistic effect on poultry gut health. Xuefeng black-bone chicken, a valuable native breed, is the only black-bone chicken in Hunan Province of China. Black-bone chicken has medicinal value due to the amounts of melanin that are deposited in body, which could scavenge free radicals (Bustamante et al. Citation1993) and improve antioxidant capacity (Blarzino et al. Citation1999). The black-bone chicken is usually a source of tonic food in China. Consequently, we used 16S rDNA sequencing to compare the effect of different levels of Pal–Pro on faecal flora of Xuefeng black-bone chicken.
Materials and methods
All the birds and the experimental protocols were approved by the Institutional Animal Care and Use Committee of Hunan Agricultural University, Hunan, China.
The Pal–Pro
The Pal–Pro final product, SINIPRO MAX, was provided by Jiangsu Sinitic Biological Technology Co., Ltd. (Jiangsu, China) and its main components are as follows: palygorskite >95%, Bacillus subtilis (≥0.5 × 109 CFU/kg) and Bacillus licheniformis (≥0.5 × 109 CFU/kg) <5%.
Animals, experimental design and management
Xuefeng black-bone chicken was obtained from Hunan Yunfeifeng Agricultural Commercial Company (Hunan, China). A total of 432 healthy Xuefeng black-bone chickens at 42 weeks of age with similar weight were randomly and equally divided into 4 treatments with 6 replicates of 18 hens. The dietary treatments were as follows: (1) basal diet; (2) basal diet + 250 mg/kg Pal–Pro; (3) basal diet + 500 mg/kg Pal–Pro; (4) basal diet + 750 mg/kg Pal–Pro. The composition and ingredients of the diets are shown in Table . The experimental diets were formulated to meet the nutrient requirement according to the China Agricultural Standard (NY/T 33-2004).
Table 1. Composition and nutrient levels of the experimental diets (dry matter basis).
Each replicate with 12 cages and 3 hens were raised in a cage (38 × 28 × 36 cm; length × width × height). After 7 days of adaptation, all hens were fed the assigned experimental diets for 56 days. The hens were fed twice a day (06.30 am and 2.30 pm) and given ad libitum access to water throughout the experiment. The temperature and relative humidity in the chicken coop was 27.55 ± 2.35 °C and 78.65 ± 3.68% (mean ± SD), respectively. The lighting regimen used was a 16 h light and 8 h darkness cycle throughout the entire experimental period.
Laying performance
During the experiment period, the number of eggs, egg weight and the feed intake were recorded weekly for each replicate to calculate the laying rate and feed conversion ratio. To ensure data credibility, mortality and health status were visually recorded daily.
Sample collection
At the end of the test period, dung board was immediately arranged to collect fresh greenish-yellow faeces samples after feeding and to ensure sample contact with air for <15 min. And each replicate take four different position fecal samples (total 4–6 g) into the microtubes and stored that at −80 °C for further analysis.
DNA extraction
DNA from different samples was extracted using the E.Z.N.A. ®Stool DNA Kit (D4015, Omega, Inc., Norwalk, CT, USA) according to manufacturer’s instructions. The total DNA was eluted in 50 μL of Elution buffer and stored at −80 °C until measurement in the PCR by LC-Bio Technology Co., Ltd, Hang Zhou, Zhejiang Province, China.
PCR amplification and 16S rDNA sequencing
DNA amplicons were amplified using sequencing universal primers for the V3–V4 domain of bacterial 16S rRNA gene by PCR. The information of primers is presented in Table (Logue et al. Citation2016) and the PCR products were confirmed with 2% agarose gel electrophoresis. The PCR products were purified by AMPure XT beads (Beckman Coulter Genomics, Danvers, MA, USA) and quantified by Qubit (Invitrogen, Waltham, MA, USA). The amplicon pools were prepared for sequencing, and the size and quantity of the amplicon library were assessed on Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA) and with the Library Quantification Kit for Illumina (Kapa Biosciences, Woburn, MA, USA), respectively. The libraries were sequenced on NovaSeq PE250 platform.
Table 2. 16S rDNA sequencing primer sequences.
Sequence data analysis
Samples were sequenced on an Illumina NovaSeq platform according to the manufacturer’s recommendations, provided by LC-Bio. Paired-end reads were assigned to samples based on their unique barcode and truncated by cutting off the barcode and primer sequence. Paired-end reads were merged using FLASH. Quality filtering on the raw reads was performed under specific filtering conditions to obtain the high-quality clean tags according to the fqtrim (v0.94). Chimeric sequences were filtered using Vsearch software (v2.3.4). After dereplication using DADA2, we obtained feature table and feature sequence. Alpha diversity and beta diversity were calculated by normalising to the same sequences randomly. Then according to SILVA (release 132) classifier, feature abundance was normalised using relative abundance of each sample. Alpha diversity is applied in analysing complexity of species diversity for a sample through five indices, including Chao1, Observed species, Goods coverage, Shannon, Simpson, and all these indices in our samples were calculated with QIIME2. Blast was used for sequence alignment, and the feature sequences were annotated with SILVA database for each representative sequence. Other diagrams were implemented using the R package (v3.5.2).
Statistical analysis
Laying performance and alpha diversity data were analysed by one-way analysis of variance (ANOVA) and orthogonal polynomial comparisons were used to test the linear and quadratic responses to the Pal–Pro levels. The abundance of flora was analysed by Kruskal–Wallis tests. p < .05 was considered statistically significant. And the correlation between the laying performance and microbiome was assessed by Spearman correlation. All data were analysed by SPSS 22.0 statistical software (SPSS Institute Inc., Chicago, IL, USA).
Results
Laying performance
The effects of Pal–Pro in diets on laying performance of Xuefeng black-bone chicken are shown in Table . The Pal–Pro treatments increased (linear, p < .05) the laying rate and decreased (linear, p < .05) feed conversion ratio. However, there were no differences on egg weight and daily feed intake among the four groups in this experiment (p > .05).
Table 3. Effects of palygorskite and probiotics complex (Pal–Pro) on laying performance of Xuefeng black-bone chicken.a
Diversity of faecal microbiota
Alpha diversity is presented in Table . The sequencing coverage of each group was above 99.9%, which indicated that the sequencing depth has covered all the species in the sample. However, there were no differences on Observed_species, Chao1, Shannon and Simpson (p > .05).
Table 4. Effects of palygorskite and probiotics complex (Pal–Pro) on Alpha diversity of faecal flora of Xuefeng black-bone chicken.a
Variation in faecal microbiota composition at phylum level
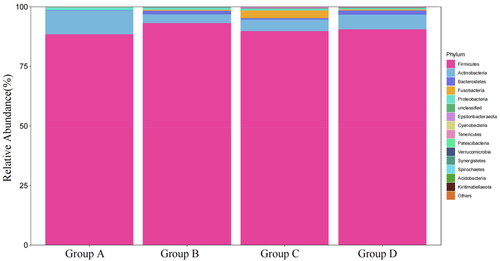
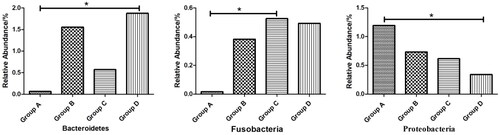
Figure shows that the abundance of the top 15 microorganisms at phylum level. The faecal microbiota of each group was dominated by Firmicutes, Actinobacteria, Bacteroidetes, Fusobacteria, Proteobacteria, and the Firmicutes was the most dominant bacterial group which accounted for more than 85% of the total microbial community detected. Meanwhile, the statistical results shown in Figure , compared with group A, shows that the abundance of Fusobacteria of group C and the abundance of Bacteroidetes of group D were significantly increased (p < .05), and the abundance of Proteobacteria of group D was significantly decreased (p < .05).
Variation in faecal microbiota composition at genus level
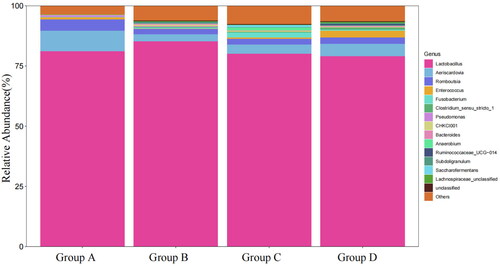
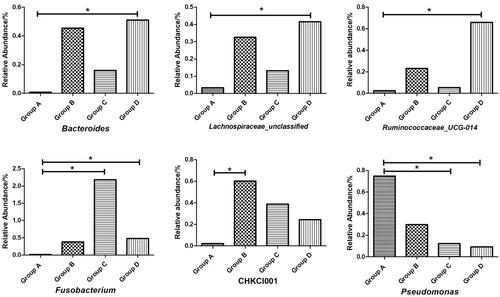
Figure presents the abundance of the top 15 microorganisms at genus level. The Lactobacillus was the primary dominant bacterial group that accounted for more than 80% of the total microbial community. And compared with group A (Figure ), the abundance of Bacteroidetes, Lachnospiraceae_unclassified and Ruminococcaceae_UCG-014 of group D were significantly higher (p < .05). Compared with the group A, the abundance of Fusobacterium of groups C and D and the abundance of CHKCI001 of group B were significantly increased (p < .05). In addition, the abundance of Pseudomonas of groups C and D in Proteobacteria was significantly lower than in group A (p < .05).
Correlation between microbiome and laying performance
We performed Spearman correlation to analyse the top 15 faecal microorganisms at genus level and the laying performance of broiler chicken (Figure ). Aeriscardovia was negatively correlated with laying rate (p < .01), Enterococcus and Ruminococcaceae_UCG∼014 were positively correlated with egg weight (p < .05) and other microbiome were not correlated with laying performance (p > .05).
Discussion
The poor laying performance of local chickens in China has been one of the reasons to limit the development of chickens local industry. Xuefeng black-bone chicken has a similar situation (Liu et al. Citation2020). Therefore, it’s imperative to improve the laying performance through nutritional regulation. Our previous studies have found that the combination of Bacillus subtilis and montmorillonite (Chen et al. Citation2020), and essential oil/palygorskite composite (Cheng et al. Citation2022) can significantly increase the laying performance of laying hens, and this study shows similar results. We speculate the cause may be that the Pal–Pro not only improves gut health by reducing harmful substances and bacteria in gut (Zhao et al. Citation2012; Meyer et al. Citation2020), but also improves animal health by increased antioxidant and immune capability (Cheng et al. Citation2018; Deng et al. Citation2021).
The diversity of gut microbes plays an important role in maintaining the gastrointestinal homeostasis, and faecal microbes could also partly reflect the state of gut health, and it is more convenient and efficient to obtain (Zhang et al. Citation2018). Alpha diversity refers to the species richness and diversity of microbial communities within a specific environment or ecosystem, and a metric commonly used to reflect the alpha diversity include Observed_species, Chao1, Simpson and Shannon (Grice et al. Citation2009; Zhou et al. Citation2016). Chalvatzi et al. (Citation2016) demonstrated that dietary 0.5% Pal could promote the early growth and development of laying hens by improving the structure of caecal microbial colonies. And many studies proved that Pro could increase intestinal microbial diversity (Kristensen et al. Citation2016; Hu et al. Citation2017; Wang et al. Citation2021). In this study, Goods_coverage were above 99% indicated that the results could fully reflect the species and diversity of microorganism; however, we found that dietary Pro–Pal had no effect on alpha diversity. We speculated that all the groups have similar feeding environment where internal microbial species are limited, so it is difficult to increase the intestinal diversity (Yu et al. Citation2021). On the other hand, short time and small amount of exogenous additives may hardly change microbial diversity. Also there are some studies that showed similar results (Rodrigues et al. Citation2020; Wang et al. Citation2021; Zhang et al. Citation2021).
The faecal microbial composition is complex and large, and its changes could affect the health and product efficiency of animals. We tried to interpret the changes of laying performance by analysing the changes of microorganisms at the phylum and genus levels. At the phylum levels, Huang et al. (Citation2018) established that the first chicken gut microbiome dataset reveals that Firmicutes, Actinobacteria, Proteobacteria and Bacteroidetes were the dominant bacterial in chicken gut and account for 88% of the total microbial. In this paper, Firmicutes and Actinobacteria were the dominant bacterial and account for 90% of the total microbial, which is similar to some previous studies. At the same time, we found that the abundance of Bacteroides in group D and the abundance of Fusobacterium in group C were significantly higher than those observed in group A. Bacteroidetes are involved in metabolism by decomposing polysaccharides and regulating fat accumulation, and its relative abundance increased is conducive to the absorption of nutrients, which may be related to the increase of laying rate in this experiment (Lapebie et al. Citation2019). It has been proven that Fusobacterium could improve animal health by influencing the content of short-chain fatty acids in gut and against pathogens which could promote tumour growth (Kelly et al. Citation2018; Craig et al. Citation2020). Proteobacteria is harmful bacteria in the gut that could cause intestinal inflammation or death in chickens (Maharshak et al. Citation2013; Flanagan et al. Citation2014; Selvanantham et al. Citation2016). Our study found that dietary Pal–Pro could decrease the abundance of Proteobacteria. And a lot of studies have proved that Pal or Pro could prevent the growth of harmful bacteria, which were consistent with our results (Tejada-Simon and Pestka Citation1999; Dumitru et al. Citation2018; Wang et al. Citation2019; Ding et al. Citation2021).
In the genus level, the abundance of Ruminococcaceae_UCG∼014, Bacteroides and Lachnospiraceae_unclassified observed in the group A was significantly lower than those observed in group D, and the abundance of Fusobacterium observed in the group A was significantly lower than that observed in groups C and D. They are beneficial in the chicken gut. Bacteroides and Ruminococcaceae_UCG∼014 are associated with the absorption and digestion of nutrients, like decompose polysaccharides, degrade cellulose, and regulate fat accumulation (Thoetkiattikul et al. Citation2013; Patra and Yu Citation2015; Lapebie et al. Citation2019). Ruminococcaceae_UCG∼014, Lachnospiraceae_unclassified and Fusobacterium are related to the content of short-chain fatty acids in the intestine. The short-chain fatty acids have the functions of anti-inflammation, anti-cancer, scavenging drug-resistant pathogenic bacteria (Smith et al. Citation2013; Fernández et al. Citation2016; Craig et al. Citation2020). Meanwhile, Ruminococcaceae_UCG∼014 and Lachnospiraceae_unclassified have inhibitory effect on pathogenic bacteria (Surana and Kasper Citation2017; Chiumento et al. Citation2019). Pseudomonas is universal pathogenic bacteria in Proteobacteria, which could reduce body immune response, and its reduction in abundance is conducive to body health of animals (VanDevanter et al. Citation2021; Vallabhaneni et al. Citation2021). There are few studies about CHKCI001, and the results indicated that the abundance of CHKCI001 in group B was significantly higher than in group A, and a paper speculated that Pro could reduce its abundance in the gut, but the exact mechanism is unknown (Emami et al. Citation2021).
In this study, Spearman correlation analysis showed that Aeriscardovia was negatively correlated with laying rate, and the increase in the laying rate in the Pal–Pro group may be owing to the decreased abundance of Aeriscardovia. However, Aeriscardovia belongs to the Bifidobacterium family of Actinobacteria. Bifidobacteria could ferment carbohydrates and convert them into organic acids to absorb nutrients better, so does Aeriscardovia (Simpson et al. Citation2004; Lugli et al. Citation2017). There are few studies on Aeriscardovia, and the specific mechanism of action needs further study. And both Ruminococcaceae_UCG∼014 and Enterococcus could promote the growth of the animals. Ruminococcaceae_UCG∼014 is closely related to the cellulose in the rumen and could promote the degradation of cellulose (Patra and Yu Citation2015; Shang et al. Citation2018). Some studies demonstrated that Enterococcus could increase the growth performance by improving the intestinal environment of chicken (Krauze et al. Citation2020; He et al. Citation2021). In our study, Ruminococcaceae_UCG∼014 and Enterococcus were positively correlated with egg weight. However, there is no significant difference in egg weight in the four groups. The reason may be that Xuefeng black-bone chicken, as a local breed, has a small variation in egg weight, meanwhile egg weight was affected by many factors.
In conclusion, dietary Pal–Pro could increase the laying performance of broiler chicken, which may correlate with the faecal microbial community alter. This study provides a theoretical basis for the combined use of Pal and Pro and the recommended dose is 750 mg/kg.
Ethical approval
All the birds and the experimental protocols were approved by the Institutional Animal Care and Use Committee of Hunan Agricultural University, Hunan, China.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bailey JR, Vince V, Williams NA, Cogan TA. 2017. Streptococcus thermophilus NCIMB 41856 ameliorates signs of colitis in an animal model of inflammatory bowel disease. Benef Microbes. 8(4):605–614.
- Blarzino C, Mosca L, Foppoli C, Coccia R, De Marco C, Rosei MA. 1999. Lipoxygenase/H2O2-catalyzed oxidation of dihdroxyindoles: synthesis of melanin pigments and study of their antioxidant properties. Free Radic Biol Med. 26(3–4):446–453.
- Bustamante J, Bredeston L, Malanga G, Mordoh J. 1993. Role of melanin as a scavenger of active oxygen species. Pigment Cell Res. 6(5):348–353.
- Chalvatzi S, Kalamaki MS, Arsenos G, Fortomaris P. 2016. Dietary supplementation with the clay mineral palygorskite affects performance and beneficially modulates caecal microbiota in laying pullets. J Appl Microbiol. 120(4):1033–1040.
- Chen C, Li J, Zhang H, Xie Y, Xiong L, Liu H, Wang F. 2020. Effects of a probiotic on the growth performance, intestinal flora, and immune function of chicks infected with Salmonella pullorum. Poult Sci. 99(11):5316–5323.
- Chen JF, Liu X, Qu X, Guo S. 2019. Montmorillonite improved the intestinal mucosal barrier functions of laying hens in late production. J Anim Physiol Anim Nutr. 103(4):1081–1089.
- Chen JF, Xu MM, Kang KL, Tang SG, He CQ, Qu XY, Guo SC. 2020. The effects and combinational effects of Bacillus subtilis and montmorillonite on the intestinal health status in laying hens. Poult Sci. 99(3):1311–1319.
- Cheng H, Chen JF, Tang SG, Guo SC, He CQ, Qu XY. 2022. Effects of essential oil/palygorskite composite on performance, egg quality, plasma biochemistry, oxidation status, immune response and intestinal morphology of laying hens. Poult Sci. 101(4):101632.
- Cheng Y, Xu Q, Chen Y, Su Y, Wen C, Zhou Y. 2018. Modified palygorskite improves immunity, antioxidant ability, intestinal morphology, and barrier function in broiler chickens fed naturally contaminated diet with permitted feed concentrations of Fusarium mycotoxins. Toxins. 10(11):482.
- Chiumento S, Roblin C, Kieffer-Jaquinod S, Tachon S, Leprètre C, Basset C, Aditiyarini D, Olleik H, Nicoletti C, Bornet O, et al. 2019. Ruminococcin C, a promising antibiotic produced by a human gut symbiont. Sci Adv. 5(9):eaaw9969.
- Clavijo V, Florez MJV. 2018. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: a review. Poult Sci. 97(3):1006–1021.
- Collado MC, Meriluoto J, Salminen S. 2007. Role of commercial probiotic strains against human pathogen adhesion to intestinal mucus. Lett Appl Microbiol. 45(4):454–460.
- Craig AD, Khattak F, Hastie P, Bedford MR, Olukosi OA. 2020. Xylanase and xylo-oligosaccharide prebiotic improve the growth performance and concentration of potentially prebiotic oligosaccharides in the ileum of broiler chickens. Br Poult Sci. 61(1):70–78.
- Deng YY, Xiong XW, Liu X, He CQ, Guo SC, Tang SG, Qu XY. 2021. Palygorskite combined probiotics improve the laying performance, hatching performance, egg quality, plasma antioxidative status, and immune response of broiler breeders. Ital J Anim Sci. 20(1):1292–1301.
- Ding J, Hui A, Wang W, Yang F, Kang Y, Wang A. 2021. Multifunctional palygorskite@ZnO nanorods enhance simultaneously mechanical strength and antibacterial properties of chitosan-based film. Int J Biol Macromol. 189:668–677.
- Dumitru M, Sorescu I, Habeanu M, Tabuc C, Idriceanu L, Jurcoane S. 2018. Preliminary characterisation of Bacillus subtilis strain use as a dietary probiotic bio-additive in weaning piglet. Food Feed Res. 45(7):203–211.
- Emami NK, White MB, Calik A, Kimminau EA, Dalloul RA. 2021. Managing broilers gut health with antibiotic-free diets during subclinical necrotic enteritis. Poult Sci. 100(5):101055.
- Fernández J, Redondo-Blanco S, Gutiérrez-del-Río I, Miguélez EM, Villar CJ, Lombo F. 2016. Colon microbiota fermentation of dietary prebiotics towards short-chain fatty acids and their roles as anti-inflammatory and antitumour agents: a review. J Funct Foods. 25:511–522.
- Flanagan L, Schmid J, Ebert M, Soucek P, Kunicka T, Liska V, Bruha J, Neary P, Dezeeuw N, Tommasino M, et al. 2014. Fusobacterium nucleatum associates with stages of colorectal neoplasia development, colorectal cancer and disease outcome. Eur J Clin Microbiol Infect Dis. 33(8):1381–1390.
- Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, Bouffard GG, Blakesley RW, Murray PR, Green ED, NISC Comparative Sequencing Program, et al. 2009. Topographical and temporal diversity of the human skin microbiome. Science. 324(5931):1190–1192.
- He Y, Liu X, Dong Y, Lei J, Ito K, Zhang B. 2021. Enterococcus faecium PNC01 isolated from the intestinal mucosa of chicken as an alternative for antibiotics to reduce feed conversion rate in broiler chickens. Microb Cell Fact. 20(1):122.
- Hu S, Wang L, Jiang Z. 2017. Dietary additive probiotics modulation of the intestinal microbiota. Protein Pept Lett. 24(5):382–387.
- Huang P, Zhang Y, Xiao K, Jiang F, Wang H, Tang D, Liu D, Liu B, Liu Y, He X, et al. 2018. The chicken gut metagenome and the modulatory effects of plant-derived benzylisoquinoline alkaloids. Microbiome. 6(1):211.
- Kelly D, Yang L, Pei Z. 2018. Gut microbiota, fusobacteria, and colorectal cancer. Diseases. 6(4):109.
- Kogut MH. 2019. The effect of microbiome modulation on the intestinal health of poultry. Anim Feed Sci Technol. 250:32–40.
- Krauze M, Abramowicz K, Ognik K. 2020. The effect of addition of probiotic bacteria (Bacillus subtilis or Enterococcus faecium) or phytobiotic containing cinnamon oil to drinking water on the health and performance of broiler chickens. Ann Anim Sci. 20(1):191–205.
- Kristensen NB, Bryrup T, Allin KH, Nielsen T, Hansen TH, Pedersen O. 2016. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med. 8(1):52.
- Lapebie P, Lombard V, Drula E, Terrapon N, Henrissat B. 2019. Bacteroidetes use thousands of enzyme combinations to break down glycans. Nat Commun. 10(1):2043.
- Lee ES, Song EJ, Lee SY, Park SL, Kim D, Kim D, Kim JH, Lim SI, Nam YD. 2018. Effects of bentonite Bgp35b-p on the gut microbiota of mice fed a high-fat diet. J Sci Food Agric. 98(11):4369–4373.
- Lei H, Wei Q, Wang Q, Su A, Xue M, Liu Q, Hu Q. 2017. Characterization of ginger essential oil/palygorskite composite (GEO-PGS) and its anti-bacteria activity. Mater Sci Eng C Mater Biol Appl. 73:381–387.
- Liu X, Liu W, Deng Y, He C, Xiao B, Guo S, Zhou X, Tang S, Qu X. 2020. Use of encapsulated Bacillus subtilis and essential oils to improve antioxidant and immune status of blood and production and hatching performance of laying hens. Ital J Anim Sci. 19(1):1583–1591.
- Logue JB, Stedmon CA, Kellerman AM, Nielsen NJ, Andersson AF, Laudon H, Lindström ES, Kritzberg ES. 2016. Experimental insights into the importance of aquatic bacterial community composition to the degradation of dissolved organic matter. ISME J. 10(3):533–545.
- Lugli GA, Milani C, Turroni F, Duranti S, Mancabelli L, Mangifesta M, Ferrario C, Modesto M, Mattarelli P, Jiří K, et al. 2017. Comparative genomic and phylogenomic analyses of the Bifidobacteriaceae family. BMC Genomics. 18(1):568.
- Maharshak N, Packey CD, Ellermann M, Manick S, Siddle JP, Huh EY, Plevy S, Sartor RB, Carroll IM. 2013. Altered enteric microbiota ecology in interleukin 10-deficient mice during development and progression of intestinal inflammation. Gut Microbes. 4(4):316–324.
- Meyer MM, Fries-Craft KA, Bobeck EA. 2020. Composition and inclusion of probiotics in broiler diets alter intestinal permeability and spleen immune cell profiles without negatively affecting performance1. J Anim Sci. 98:skz383.
- Patra AK, Yu Z. 2015. Essential oils affect populations of some rumen bacteria in vitro as revealed by microarray (RumenBactArray) analysis. Front Microbiol. 6:297.
- Prasai TP, Walsh KB, Bhattarai SP, Midmore DJ, Van TT, Moore RJ, Stanley D. 2016. Biochar, bentonite and zeolite supplemented feeding of layer chickens alters intestinal microbiota and reduces campylobacter load. PLoS ONE. 11(4):e0154061.
- Roberts T, Wilson J, Guthrie A, Cookson K, Vancraeynest D, Schaeffer J, Moody R, Clark S. 2015. New issues and science in broiler chicken intestinal health: emerging technology and alternative interventions. J Appl Poult Res. 24(2):257–266.
- Rodrigues DR, Briggs W, Duff A, Chasser K, Murugesan R, Pender C, Ramirez S, Valenzuela L, Bielke LR. 2020. Comparative effectiveness of probiotic-based formulations on cecal microbiota modulation in broilers. PLoS One. 15(5):e0225871.
- Selvanantham T, Lin Q, Guo CX, Surendra A, Fieve S, Escalante NK, Guttman DS, Streutker CJ, Robertson SJ, Philpott DJ, et al. 2016. NKT cell-deficient mice harbor an altered microbiota that fuels intestinal inflammation during chemically induced colitis. J Immunol. 197(11):4464–4472.
- Sergeant MJ, Constantinidou C, Cogan TA, Bedford MR, Penn CW, Pallen MJ. 2014. Extensive microbial and functional diversity within the chicken cecal microbiome. PLoS One. 9(3):e91941.
- Shang Q, Shan X, Cai C, Hao J, Li G, Yu G. 2018. Correction: dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 9(1):655.
- Simpson PJ, Ross RP, Fitzgerald GF, Stanton C. 2004. Bifidobacterium psychraerophilum sp. nov. and Aeriscardovia aeriphila gen. nov., sp. nov., isolated from a porcine caecum. Int J Syst Evol Microbiol. 54(Pt 2):401–406.
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. 2013. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 341(6145):569–573.
- Su Y, Chen Y, Chen L, Xu Q, Kang Y, Wang W, Wang A, Wen C, Zhou Y. 2018. Effects of different levels of modified palygorskite supplementation on the growth performance, immunity, oxidative status and intestinal integrity and barrier function of broilers. J Anim Physiol Anim Nutr. 102(6):1574–1584.
- Su Y, Chen YP, Cheng YF, Wen C, Zhou YM. 2018. Effects of modified palygorskite supplementation on egg quality and mineral element content, and intestinal integrity and barrier function of laying hens. Biol Trace Elem Res. 186(2):529–537.
- Surana NK, Kasper DL. 2017. Moving beyond microbiome-wide associations to causal microbe identification. Nature. 552(7684):244–247.
- Tejada-Simon MV, Pestka JJ. 1999. Proinflammatory cytokine and nitric oxide induction in murine macrophages by cell wall and cytoplasmic extracts of lactic acid bacteria. J Food Prot. 62(12):1435–1444.
- Thoetkiattikul H, Mhuantong W, Laothanachareon T, Tangphatsornruang S, Pattarajinda V, Eurwilaichitr L, Champreda V. 2013. Comparative analysis of microbial profiles in cow rumen fed with different dietary fiber by tagged 16S rRNA gene pyrosequencing. Curr Microbiol. 67(2):130–137.
- Tian Z, Wang X, Duan Y, Zhao Y, Zhang W, Azad MAK, Wang Z, Blachier F, Kong X. 2020. Dietary supplementation with Bacillus subtilis promotes growth and gut health of weaned piglets. Front Vet Sci. 7:600772.
- Vallabhaneni S, Huang JY, Grass JE, Bhatnagar A, Sabour S, Lutgring JD, Campbell D, Karlsson M, Kallen AJ, Nazarian E, EIP Work Group, et al. 2021. Antimicrobial susceptibility profiles to predict the presence of carbapenemase genes among carbapenem-resistant Pseudomonas aeruginosa. J Clin Microbiol. 59(6):e02874-20.
- VanDevanter DR, Heltshe SL, Hilliard JB, Konstan MW. 2021. Pseudomonas aeruginosa antimicrobial susceptibility test (AST) results and pulmonary exacerbation treatment responses in cystic fibrosis. J Cyst Fibros. 20(2):257–263.
- Wang W, Li Y, Wang W, Gao B, Wang Z. 2019. Palygorskite/silver nanoparticles incorporated polyamide thin film nanocomposite membranes with enhanced water permeating, antifouling and antimicrobial performance. Chemosphere. 236:124396.
- Wang W, Wang F, Kang Y, Wang A. 2015. Nanoscale dispersion crystal bundles of palygorskite by associated modification with phytic acid and high-pressure homogenization for enhanced colloidal properties. Powder Technol. 269:85–92.
- Wang Y, Heng C, Zhou X, Cao G, Jiang L, Wang J, Li K, Wang D, Zhan X. 2021. Supplemental Bacillus subtilis DSM 29784 and enzymes, alone or in combination, as alternatives for antibiotics to improve growth performance, digestive enzyme activity, anti-oxidative status, immune response and the intestinal barrier of broiler chickens. Br J Nutr. 125(5):494–507.
- Wang Y, Li J, Xie Y, Zhang H, Jin J, Xiong L, Liu H. 2021. Effects of a probiotic-fermented herbal blend on the growth performance, intestinal flora and immune function of chicks infected with Salmonella pullorum. Poult Sci. 100(7):101196.
- Xu N, Zhou R, Jiang Q, Kong L, Lei H. 2020. GEO-PGS composite shows synergistic and complementary effect on Escherichia coli and improvement of intestinal dysfunction. Food Chem Toxicol. 135:110936.
- Yu C, Zhou C, Tan Z, Cai X, Wang S. 2021. Effects of Enteromorpha polysaccharide dietary addition on the diversity and relative abundance of ileum flora in laying hens. Microb Pathog. 158:105004.
- Zhang L, Wu W, Lee Y, Xie J, Zhang H. 2018. Spatial heterogeneity and co-occurrence of mucosal and luminal microbiome across swine intestinal tract. Front Microbiol. 9:48.
- Zhang S, Zhong G, Shao D, Wang Q, Hu Y, Wu T, Ji C, Shi S. 2021. Dietary supplementation with Bacillus subtilis promotes growth performance of broilers by altering the dominant microbial community. Poult Sci. 100(3):100935.
- Zhang Z, Wang W, Kang Y, Zong L, Wang A. 2016. Tailoring the properties of palygorskite by various organic acids via a one-pot hydrothermal process: a comparative study for removal of toxic dyes. Appl Clay Sci. 120:28–39.
- Zhao W, Liu X, Huang Q, Walker SL, Cai P. 2012. Interactions of pathogens Escherichia coli and Streptococcus suis with clay minerals. Appl Clay Sci. 69:37–42.
- Zhou X, Jiang X, Yang C, Ma B, Lei C, Xu C, Zhang A, Yang X, Xiong Q, Zhang P, et al. 2016. Cecal microbiota of Tibetan Chickens from five geographic regions were determined by 16S rRNA sequencing. Microbiologyopen. 5(5):753–762.