?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Cortisol is the primary biomarker associated with the hypothalamic–pituitary–adrenal (HPA) axis. This research aimed at assessing the predictive potential and the relationship of cortisol concentrations in four media (blood, milk, whey and hair) with parity, lactation stage and productive classes. Multiparous (n = 30) and primiparous (n = 38) Italian Mediterranean buffaloes were used and assigned to four productive classes (based on percentiles of mature equivalent milk yield (EMY) and mature equivalent energy corrected milk (eECM)), and cortisol concentrations were measured using a in house radioimmunoassay (RIA) method. Parity did not show a significant effect on cortisol concentrations of the four media. The catabolic stage of lactation (up to 90 d in milk (DIM)) was characterised by higher cortisol concentrations compared to the second anabolic stage (beyond 150 DIM) in milk formulations. The plasmatic concentrations of cortisol were higher at the catabolic and the first anabolic stage (91–150 DIM) compared to the second anabolic (p = 0.022 and p = 0.009, respectively). Buffaloes beyond 150 DIM differed from those below 90 DIM (p < 0.001) and between 91 and 150 DIM (p < 0.05) in hair. Finally, hair cortisol concentrations were negatively correlated to EMY, mature equivalent protein content (EPC) (p < 0.01) and eECM (p < 0.05). The whey showed a potential to predict the concentrations of cortisol in whole extracted milk (R2 = 0.31). Hence the development of a real-time automated cortisol measurement in milk could be a valuable tool in Italian Mediterranean Buffalo farming.
HIGHLIGHTS
The lactation stage significantly influences plasma, hair and milk cortisol concentrations.
Hair cortisol concentrations are negatively correlated to milk quality parameters.
The whey is a potential predictor of the cortisol concentrations in whole extracted milk.
Introduction
Nowadays, animal welfare in dairy farms remains a major concern, in order to protect livestock from poor conditions and improve the quality of animal-derived food. Therefore, it is imperative to interpret their behaviour, as well as their cognitive needs and capacities (Nawroth et al. Citation2019) to both encounter their requirements and reduce stressful situations. Stress is an animal condition resulting from the action of one or more stressors of either external or internal origin (Bova et al. Citation2014). Several physiological and pathological conditions can be responsible for an alteration of the homeostasis in the animals. Parity and stage of lactation are physiological factors, as such they can influence metabolic profile (Kuczyńska et al. Citation2021), milk yield and somatic cell count (Sabek et al. Citation2021), and acid base balance (Walter et al. Citation2022) of dairy cattle. In particular, the physiological processes that cows enact to adapt to the stage of lactation involve the reduction of immune competence, negative energy balance, hypocalcaemia, inflammatory responses and oxidative stress (Trevisi and Minuti, Citation2018).
Similarly, milk yield may represent a physiological burden in dairy ruminants and may be associated with the hypothalamic–pituitary–adrenal (HPA) axis activation (Otten et al. Citation2023). As a matter of fact, milk production challenges the metabolism and health of dairy cows (Gross and Bruckmaier, Citation2019) since complex adaptation processes take place to enable the maintenance of the animals’ energy and nutrient homeostasis to meet the requirements for the metabolically prioritised mammary gland in early lactation (Drackley, Citation1999; Ingvartsen, Citation2006; van Knegsel et al. Citation2014). Consequently, metabolic stress arises with various effects on the immune system, reproductive performance, milk yield, product quality and the overall well-being of the dairy cow (Drackley et al. Citation2005; Bradford et al. Citation2015; Bruckmaier and Gross, Citation2017). The glucocorticoid hormone cortisol, due to its multifaceted role in the physiological stress response, is the primary physiological biomarker for many phenotypic and metabolic changes in animals associated with activation of the HPA axis (Hellhammer et al. Citation2009).
In dairy ruminants, conflicting results have been reported about the influence of parity and lactation stage on cortisol concentrations. Some authors (Wu et al. Citation2019) reported that days in milk (DIM) and parity do not affect serum cortisol concentrations in Holstein cows, whereas Saqib et al. (Citation2022) concluded that lactation numbers and DIM have an impact on blood cortisol in buffaloes. Other studies carried out in dairy cattle (Fukasawa et al. Citation2008; Gellrich et al. Citation2015) and goats (Díaz et al. Citation2013) demonstrated that both parity and DIM affect milk cortisol concentrations, but no clear effects are recorded on hair, since contrasting results have been reported (Burnett et al. Citation2014, Citation2015). In Italian Mediterranean buffalo parity and stage of lactation strongly affect milk yield and quality (Costa et al. Citation2020), response to temperature-humidity index (THI) (Matera, Di Vuolo, et al. Citation2022; Matera, Cotticelli, et al. Citation2022) and milk electrical conductivity (Matera, Di Vuolo, et al. Citation2022; Matera, Cotticelli, et al. Citation2022).
Plasma cortisol concentrations have been widely used to evaluate acute responses to stressful stimuli (Kovács et al. Citation2021). However, the process of blood sampling is accompanied by additional burden, which can affect the test results due to the stress response to immobilisation and handling. Therefore, milk has been suggested as an alternative to blood for dairy ruminants (Pošćić et al. Citation2017) and the protocol for assessing milk cortisol has been recently validated also in buffalo (Cotticelli et al. Citation2022). Both these biological fluids provide short-term evaluation about the HPA axis activity (in milk with a lag-time) (Sgorlon et al. Citation2015). On the other hand, hair analysis can be used for the assessment of long-term retrospective concentrations of cortisol, since it is non-invasive and has a long-time lag for changes (Sharma et al. Citation2019). The aim of this study was to evaluate the influence of parity, stage of lactation and productive levels on cortisol concentrations in several biological matrices sampled in Italian Mediterranean buffaloes and to study which one could show a promising predictive potential.
Materials and methods
All the experimental procedures and the care of the animals complied to the Italian legislation on animal care (DL n.116, 27/1/1992) and received the approval of the Ethical Committee of the University of Naples “Federico II” (Protocol number: 25539-2022).
Animals and sampling procedures
The Italian Mediterranean dairy buffaloes (Bubalus bubalis) used in this study were located in a commercial dairy farm in southern Italy (Campania region, 41°03′40.6″N − 14°02′16.5″E), where a total of 950 buffaloes were bred. Multiparous (n = 30) and primiparous (n = 38) buffaloes were kept in pens with concrete floor (straw was used for bedding and renewed every 2 days) and were routinely milked twice daily (morning and afternoon) in herringbone milking parlour (12 + 12) with low pipeline. An availability of space of 15 m2/head and 80 cm front manger were guaranteed throughout the study. Buffaloes received a total mixed ration administered twice daily. At beginning of the trial an area of 10 cm2 was shaved in all the animals on the left prescapular region. After 50 days three substrates (blood, milk and regrown hair) were concurrently collected on each animal in different lactation period and productive level (see statistical analysis section). Milk quality traits included milk yield (as daily yield (kg)) fat, protein, lactose content of milk (expressed as daily percentage), somatic cells count (SCC), mature equivalent milk yield (EMY), mature equivalent fat content (EFC) and mature equivalent protein content (EPC) (expressed as kg/lactation, Trus and Buttazzoni Citation1990). Milk yield was measured using milk metres according to ICAR procedures (Citation2022), while fat, protein, lactose contents of milk were determined by mid-infrared spectroscopy using a MilkoScan FT6000 (Foss Electric A/S, Hillerod, Denmark). SCC was assessed using the Fossomatic FC (Foss Electric A/S) and log-transformed into somatic cell score (SCS) using the following formula (Ali and Shook, Citation1980):
Energy-corrected milk (ECM = 740 kcal) was calculated according to the formula from Campanile et al. (Citation1998):
The same formula was adapted for the calculation of mature equivalent energy-corrected milk (eECM):
where:
EFC and EPC are percentages, calculated by dividing mature equivalent fat and protein contents (kg/lactation) by mature EMY (kg/lactation);
dEMY is the daily mature EMY, calculated by dividing mature EMY (kg/lactation) by 270 (standard lactation length (days) of buffalo cows).
Biological samples collection
Sterile falcon tubes (Falcon® 50 mL, Corning Science, Mexico) were used to collect individual milk samples from the at-line sampler (MM15 DeLaval). It automatically collects a representative sample of milk from an individual buffalo during milking. So, each individual specimen was representative of the whole milking as previously described (Cotticelli et al. Citation2022). After collection, samples were immediately placed into dry ice (−78 °C) and transported to the laboratory where they were stored at −20 °C until lab processing.
Blood (10 mL) was collected from the mammary vein into vacutainer tubes (lithium heparin anticoagulant) puncture. Samples were centrifuged at 1500 × g for 15 min and the plasma was aliquoted into Eppendorf tubes (1 mL) and stored at −20 °C.
For analyses only regrown hair was obtained from the scapular region of the buffalo using a razorblade given that the animals were shaved for the first time 50 days before the substrates collection, and the hair was discarded (Meyer and Novak Citation2012). Therefore, the samples collected for analyses represented the integrated circulating steroids concentrations over the previous 50 days (Meyer and Novak, Citation2012; Russell et al. Citation2012; Caslini et al. Citation2016).
Samples processing and analysis
Milk processing
After thawing, two aliquots of whole milk were constituted for each specimen. The first was extracted as per Cotticelli et al. (Citation2022). In brief, 5 mL of methanol (Sigma-Aldrich, St. Louis, MO, 99.8%) were added to 400 µL of sample, mixed for 5 min at room temperature and centrifuged for 15 min at 3500 RPM and 4 °C. The vials were frozen and the solvent was moved to a tube to dry at 37 °C under an airstream suction hood. The residue was dissolved in 0.5 mL of RIA buffer (0.05 M phosphate-buffered saline [PBS], pH 7.5, 0.1% BSA).
The second aliquot was processed to obtain the whey. The coagulation procedure was exactly as per Cotticelli et al. (Citation2022). Briefly, rennet (400 µL) was added to whole milk (5 mL), mixed and incubated at 37 °C for 30 min. Samples were then centrifuged at 3500 × g for 10 min at 4 °C and fat and curd phases were separated twice.
Plasma processing
Plasma samples were aliquoted (0.25 mL) in a glass vial, extracted with 5 mL of ≥99.8% diethyl ether (Sigma-Aldrich St. Louis, MO), centrifuged at 1500 × g for 5 min and incubated at −20 °C for 18 h. Next, the liquid in the vial was dried at 37 °C under an airstream suction hood. The remaining residue was dissolved in 0.5 mL of PBS, 0.05 M, pH 7.5.
Hair processing
Hair samples were prepared for cortisol assay as per Peric et al. (Citation2022). In brief, approximately 60 mg of trimmed hair was washed twice in 3 mL isopropanol (Sigma -Aldrich, St. Louis, MO) and extracted in a glass vial with 3 mL of methanol (Sigma-Aldrich, St. Louis, MO). The vials were incubated at 37 °C for 18 h and then evaporated to dryness at 37 °C under an airstream suction hood. The remaining residue was dissolved in 0.60 mL of PBS, 0.05 M, pH 7.5.
Cortisol radioimmunoassay
The cortisol concentrations were measured in whole extracted milk, whey, hair and plasma using the in-house radioimmunoassay (RIA) method (Cotticelli et al. Citation2022; Peric et al. Citation2022). The cross-reactivities of the anti-cortisol antibody with other steroids were as follows: cortisone 4.3%, corticosterone 2.8%, 11-deoxycorticosterone 0.7%, 17-hydroxyprogesterone 0.6%, dexamethasone 0.1%, progesterone, 17-hydroxypregnenolone, DHEA-S, androsterone sulphate and pregnenolone <0.01%. After washing the plate with RIA buffer, the standards (5–200 pg/well), the quality-control extract, the test sample or extract and the tracer (hydrocortisone {cortisol [1,2,6,7-3H (N)]-}, Perkin-Elmer Life Science, Boston, MA) were added, and the plate was incubated overnight at 4 °C. The bound hormones were separated from the free hormones by decanting and washing the wells in RIA buffer. After the addition of 200 µL of scintillation cocktail (MicroScint-20, Perkin-Elmer Life Science, Boston, MA, the plate was counted on a β-counter (Top-Count, Perkin-Elmer Life Science, Boston, MA). The sensitivity of the assay was 16.8 pg/mL. The samples showed inter- and intra-assay coefficients of variation in repeated determinations of duplicate samples of 7.6% and 12.7% (whey), 3.7% and 10.1% (plasma), 3.7% and 9.9% (hair).
Statistical analysis
Statistical analyses were carried out using SPSS (29.0.1.0) for Windows 10 (SPSS Inc., Chicago, IL). According to the lactation stage, buffaloes were assigned to three classes (Matera et al. Citation2021): class C (catabolic phase of lactation, between 60 and 90 DIM, n = 23), class A1 (first anabolic phase, from 91 to 150 DIM, n = 19), and class A2 (second anabolic phase, beyond 150 DIM; n = 26). Furthermore, animals were divided according to their productive levels (4 classes), based on percentiles of EMY (kg) and eECM ().
Table 1. Percentiles of EMY (mature equivalent milk yield) and eECM (mature equivalent energy corrected milk) used to allocate the buffaloes to the four classes of productive levels.
The normal distribution of data was verified using the Shapiro–Wilk test. Possible correlations between cortisol concentrations of the four matrices and mature equivalent milk parameters were studied using Spearman’s correlation. Cortisol concentrations between the two milk formulations were compared using Wilcoxon signed-ranks test for related samples The Kruskal–Wallis test was used to compare the cortisol concentrations of the four matrices between lactation stages, productive levels and parities. The predictive potential of the matrices was tested by including whey, whole extracted milk and plasmatic cortisol concentrations in a linear regression model (prior log-transformation). A statistically significant difference was accepted at p < 0.05 and tendency was discussed at p < 0.10. Unless otherwise stated data are medians ± standard deviations.
Results
The DIM and the age of the dairy buffaloes used in this study alongside the quality and quantity parameters of milk recorded are shown in .
Table 2. Descriptive statistic of the buffaloes (n = 68) enrolled in the trial, and quality and quantity parameters of milk. Data are means ± SE.
The distribution of the concentrations of cortisol in the four matrices analysed in the present study is presented in .
Table 3. Descriptive statistic of cortisol concentrations of the four matrices.
Parity did not show any significant effect on the cortisol concentrations for each of the four matrices, with similar values in whey, whole extracted milk, hair and plasma (p > 0.10).
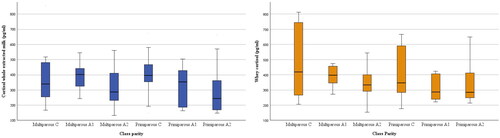
The analysis of the stage of lactation () on cortisol concentrations (pg/mL) in whole extracted milk revealed that significantly (p < 0.05) higher concentrations were recorded during the catabolic compared to second anabolic phase in whole extracted milk. A similar trend was also observed in whey, although in this case the values did not reach the statistical significance (p < 0.10).
Figure 1. Cortisol concentrations (pg/mL) of 68 milk samples according to parity and stage of lactation.
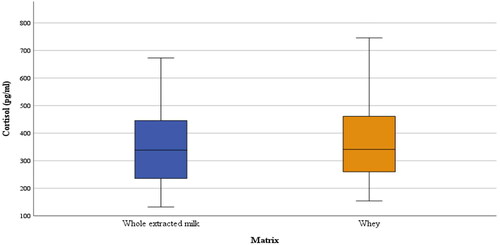
In general, cortisol concentrations (pg/mL) did not differ (p > 0.10) between whey and whole extracted milk () with median values of 340.65 and 338.53 pg/mL for whey and whole extracted milk, respectively.
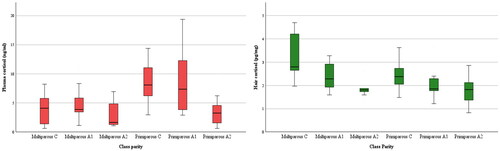
Plasma cortisol concentrations were higher during the catabolic and the first anabolic stage compared to the second anabolic (p = 0.022 and p = 0.009, respectively), independently of parity as reported previously. Similarly, the lactation stage had a significant effect on hair cortisol concentrations (). The three lactation phases differed significantly in hair, with the second anabolic stage that differed both from the catabolic (p < 0.001) and the first anabolic stage (p < 0.05), similarly these last two tended to differ (p = 0.051).
Figure 3. Cortisol concentrations in hair (pg/mg) and plasma (ng/mL) according to parity and stage of lactation.
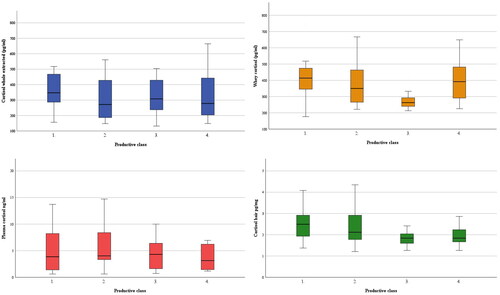
The concentrations of cortisol of the four matrices were analysed according to productive levels of buffaloes and are presented in . The medians showed a repeatable pattern among the four matrices and the effect of the productive level was significant in three matrices: productive class 3 differed to class 1 in whole extracted milk (p < 0.05), to class 1 (p < 0.01), 2 and 4 (p < 0.05) in whey, to class 1 and 4 in hair (p < 0.05).
Figure 4. Cortisol concentrations of whole extracted milk (pg/mL), whey (pg/mL), plasma (ng/ml) and hair (pg/mg) sorted by productive classes (1–4).
As reported in , the Spearman’s correlation showed that hair cortisol concentrations were negatively correlated with EMY (ρ = −0.358; p < 0.01), EPC (ρ = −0.347; p < 0.01) and eECM (ρ = −0.288; p < 0.05), and a tendency was observed with EFC (ρ = −0.247; p = 0.069). Similarly, a negative correlation tended to be significant between whey cortisol concentrations and EFC (ρ = −0.229; p < 0.10).
Table 4. Correlations between the cortisol concentrations of the four matrices and the mature equivalent milk parameters (mature equivalent milk yield, EMY; mature equivalent fat content, EFC; mature equivalent protein content, EPC; mature equivalent energy corrected milk, eECM).
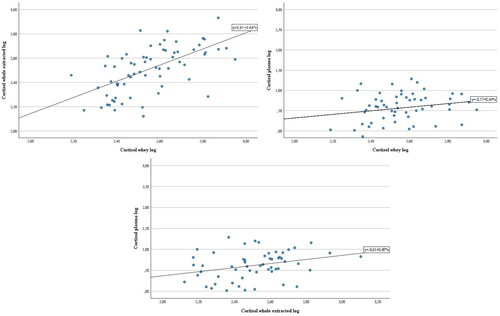
Furthermore, significant positive correlations were recorded between whey cortisol concentrations and both plasma cortisol (ρ = 0.229, p < 0.10) and whole extracted milk (ρ = 0.631, p < 0.001), and between these last two (ρ = 0.298, p < 0.05), suggesting the potential of the two milk formulations to predict plasmatic concentrations of cortisol in buffalo. Therefore, milk concentrations were tested in a linear regression model (Figure ).
Figure 5. Cortisol concentrations of whole extracted milk (pg/mL) (left panel) and plasma (ng/mL) (right panel) regressed on whey (pg/mL) and of plasma (ng/mL) regressed on whole extracted milk (below panel) (pg/mL) in a linear model. The whole extracted milk, whey and plasma cortisol concentrations were log-transformed and included as independent and dependent variables, respectively.
Discussion
The objective assessment of animal welfare is one of the challenges that livestock systems are actually obliged to face with. Cortisol evaluation may represent a simple, reliable and cheap parameter that could be considered in this sense, since this hormone may be assessed in several matrices with different significance: in fact, the measurement of cortisol concentration in milk and blood is an indicator of responses to acute stress, while its concentration in hair is used to evaluate the retrospective condition of the animals. In the present research, we aimed at studying both the relationship of concentrations of cortisol with parity, lactation stage and productive levels in Italian Mediterranean buffaloes and to evaluate its physiological concentrations in different matrices.
Few studies are reported in literature on cortisol concentrations in buffalo species. In particular, cortisol has been investigated in milk (Cotticelli et al. Citation2022), whereas the impact of parity and lactation stage on its concentrations was studied on plasma in Pakistani buffaloes (Saqib et al. Citation2022). However, to our knowledge, no information is present by using different matrices from the same animals. We used two formulas of buffalo milk that proved to be highly correlated in a previous study (Cotticelli et al. Citation2022). A significant positive correlation has been confirmed also in this study, and cortisol concentrations did not differ (p > 0.05) between the two formulations. It confirms the stability, the reliability and the reproducibility of whey and whole extracted milk for the measurement of cortisol by RIA.
As specified above, cortisol in hair samples reflects the hormone concentration over a prolonged period of time (Heimbürge et al. Citation2019). In particular, hair specimen reflects cortisol concentrations from a period of approximately three months and the retrospective value of hair has been already extensively exploited in previous research (Cotticelli et al. Citation2022; Otten et al. Citation2023; Peric et al. Citation2024).
The parity was expected to exert an effect on cortisol concentrations. As reported by Siewert et al. (Citation2019), primiparous animals must deal with the milking management practices for the first time and are less used to milking practices. In addition, it is largely known that primiparous cows may be more affected from stress than multiparous cows, especially during the transition period (Burnett et al. Citation2014). Nevertheless, previous studies reported conflicting results even within the same species, arguing that more physiological stress parameters are needed to evaluate the parity effect (Ferreira et al. Citation2021).
In this study, primiparous and multiparous buffaloes showed similar whole extracted and whey cortisol concentrations. Contrasting results are present in literature. In dairy cattle, some authors (Fukasawa et al. Citation2008) reported no differences according to parity, whereas others (Gellrich et al. Citation2015) highlighted that milk cortisol tends (p = 0.07) to be lower in second parity animals in comparison with those with higher parity. The concentrations of cortisol described in this study were higher than those reported for dairy cattle. Therefore, it’s possible to suggest that the values considered for the stress-response of the bovine species should not be directly transferred to buffaloes, and the analysis of the effect of parity should be adapted accordingly. It is worth pointing out that a higher variability was observed in the stress-response of primiparous buffaloes compared to multiparous, since they highlighted a more asymmetrical distribution of whey cortisol concentrations.
Our results are consistent with a recent study, that reported no significant effect of parity on plasma concentrations of cortisol in Nellore cows (Ferreira et al. Citation2021); on the contrary Saqib et al. (Citation2022) compared dairy buffaloes of first, second and third parity and reported a declining trend of serum cortisol concentrations with the increasing lactation number.
The parity effect was proved to be not significant in hair of Holstein dairy cows by some authors (Burnett et al. Citation2014), although the same authors in a subsequent study (Burnett et al. Citation2015) reported a more pronounced effect of parity by including only animals with absence of clinical diseases. In this case, multiparous cows showed greater concentrations of cortisol than primiparous counterparts. The effect of parity should be also discussed according to the metabolic and nutritional alterations, in particular primiparous cows are more sensitive than multiparous to negative energy balance. In this study, primiparous produced higher energy correct milk compared to multiparous (15.28 and 14.38 for primiparous and multiparous, respectively), it may confirm that metabolic status and energy utilisation differ among parities, due to fat deposits and energy requirements that are greater in primiparous (Yehia et al. Citation2020). In addition, it has been already reported that nulliparous display a higher metabolic effort than multiparous (Ferreira et al. Citation2021).
Since the dynamics of physiological processes associated with different calving numbers (namely parity) are different and they last long timeline, it is likely that they end up being dampened over the time and not even hair as the most retrospective matrix used in this study has been able to highlight differences between the two groups of buffaloes.
The stage of lactation largely influenced cortisol concentrations in all matrices. It is known that after calving buffaloes undergo several physiological changes, including, but not limiting, negative energy balance, uterine involution and ovarian cyclic activity resumption (Campanile et al. Citation2006, Citation2010). All these conditions represent stressful events for the animals that may respond to increasing cortisol concentrations. As expected, our results showed an increase of cortisol concentration in animals in the first stages of lactation in almost all matrices considered. Buffaloes were characterised by higher cortisol concentrations during the catabolic phase of lactation (within 90 DIM) compared to the second anabolic phase (beyond 150 DIM) in both whey (p < 0.10) and whole extracted milk (p < 0.05). Our results agree with both Fukasawa et al. (Citation2008) and Díaz et al. (Citation2013), who reported higher values of milk cortisol in early lactation than in mid and late lactation in Holstein cows and in Murciano-Granadina goats, respectively.
Plasma cortisol concentration was higher in buffaloes between 60 and 90 DIM compared to other groups, as reported by Saqib et al. (Citation2022) in dairy buffaloes. Also in this case, different plasma cortisol concentrations were recorded during post-partum period, although it must be underlined that this study was focused on the transition period and only animals within 56 d from calving were recruited.
Similarly, the lactation stage significantly affected hair cortisol concentrations. Our results are consistent with those reported by Burnett et al. (Citation2015) for Holstein dairy cows, even if their experimental design compared hair cortisol within 126 d from calving. Moreover, Endo et al. (Citation2017) reported higher concentrations of hair cortisol at 60–90 d after parturition and a subsequent decrement in Brown Swiss cross-bred and Holstein cows. It is conceivable that, unlike the effect of parity, the metabolic changes related to the lactation stage are effectively described by the hair, since they fall within the timeframes retrospectively covered by this matrix.
The interrelationships between productive levels and cortisol concentrations have been investigated in dairy cattle with contrasting results (Otten et al. Citation2023; Tallo-Parra et al. Citation2018). In our study, the relationship between cortisol concentrations and productive parameters were further examined by correlating the values of the four matrices to the mature equivalent milk parameters. Mature equivalent parameters are lactation records that have been adjusted for age at freshening, frequency of milking and season of the year at calving. They estimate how much a cow would have produced if she were of a mature age, calved during an average month, and were milked twice a day.
The negative correlations between the hair cortisol, EMY, EPC and eECM and between whey cortisol and EFC seem to reveal a detrimental effect of the cortisol on both quality and quantity parameters of buffalo milk. Our results are consistent with the higher hair cortisol observed in cows with lower milk yield reported by Burnett et al. (Citation2015) and Tallo-Parra et al. (Citation2018). The productive level represents mid-term physiological burden, so the hair may represent a matrix of choice to picture how it relates to the HPA axis activity. Still, it is suggested that more research is needed to deepen the effect of productive level on cortisol concentrations of dairy buffaloes, possibly enhancing the cause-effect relationship between HPA axis and milk yield.
To pursue the final aim of the study, the cortisol concentrations of the two milk formulas were included in a regression model, since they showed high correlation coefficients with plasma. Our results suggest that the whey and the whole extracted milk could be further investigated as potential predictor of the concentrations of cortisol of the other two matrices. If the predictive potential of the whey on the whole extracted milk would be confirmed, it could serve the purpose of avoiding the extraction process that is necessary for measuring cortisol concentrations in whole milk and is expensive, time-consuming and operator dependent. Also, predicting the plasma cortisol concentrations by milk could help sparing the animals the blood sampling, that is always invasive and stressful.
Conclusions
This study aimed to analyse for the first time the effect of parity, lactation stage and productive level on cortisol concentrations of dairy buffaloes in four matrices indicating short to mid-long term hormone concentrations. In fact, these factors are major concerns in animal welfare and occasionally can represent prolonged stressors throughout the productive career of dairy animals.
According to our results, it can be concluded that the lactation stage showed a comparable influence on cortisol concentrations across the four media, and it was also consistent with the results previously reported in other ruminants. On the contrary, further studies are needed to get conclusions about the effects of parity that seemed to overrun the period retrospectively covered by the biological matrices used, including hair. The influence of cortisol on productive level was only pictured by the hair being a retrospective matrix that include a broader timeframe compared to punctual matrices (plasma and milk) providing only physiological snapshots at a single point in time. Finally, milk seems to have predictive potential to estimate cortisol concentrations in buffalo and may be used as parameter for stress determination avoiding blood sampling. It is concluded that the development of a real-time automated cortisol measurement in milk could be a profitable tool in Italian Mediterranean Buffalo farming.
Ethical approval
All experimental procedures and the care of the animals complied to the Italian legislation on animal care (DL n.116, 27/1/1992) and were approved by the Ethical Committee of the University of Naples ‘Federico II’ (Protocol number: 25539-2022).
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Additional information
Funding
References
- Ali AKA, Shook GE. 1980. An optimum transformation for somatic cell concentration in milk. J Dairy Sci. 63(3):487–490. doi: 10.3168/jds.S0022-0302(80)82959-6.
- Bova TL, Chiavaccini L, Cline GF, Hart CG, Matheny K, Muth AM, Voelz BE, Kesler D, Memili E. 2014. Environmental stressors influencing hormones and systems physiology in cattle. Reprod Biol Endocrinol. 12(12):58. doi: 10.1186/1477-7827-12-58.
- Bradford BJ, Yuan K, Farney JK, Mamedova LK, Carpenter AJ. 2015. Invited review: inflammation during the transition to lactation: new adventures with an old flame. J Dairy Sci. 98(10):6631–6650. doi: 10.3168/jds.2015-9683.
- Bruckmaier RM, Gross JJ. 2017. Lactational challenges in transition dairy cows. Anim Prod Sci. 57(7):1471–1481. doi: 10.1071/AN16657.
- Burnett TA, Madureira AM, Silper BF, Nadalin A, Tahmasbi A, Veira DM, Cerri RL. 2014. Short communication: factors affecting hair cortisol concentrations in lactating dairy cows. J Dairy Sci. 97(12):7685–7690. doi: 10.3168/jds.2014-8444.
- Burnett TA, Madureira AM, Silper BF, Tahmasbi A, Nadalin A, Veira DM, Cerri RL. 2015. Relationship of concentrations of cortisol in hair with health, biomarkers in blood, and reproductive status in dairy cows. J Dairy Sci. 98(7):4414–4426. doi: 10.3168/jds.2014-8871.
- Campanile G, De Filippo C, Di Palo R, Taccone W, Zicarelli L. 1998. Influence of dietary protein on urea levels in blood and milk of buffalo cows. Livest Prod Sci. 55(2):135–143. doi: 10.1016/S0301-6226(98)00123-7.
- Campanile G, Neglia G, Di Palo R, Gasparrini B, Pacelli C, D'Occhio MJ, Zicarelli L. 2006. Relationship of body condition score and blood urea and ammonia to pregnancy in Italian Mediterranean buffaloes. Reprod Nutr Dev. 46(1):57–62. doi: 10.1051/rnd:2005066.
- Campanile G, Baruselli PS, Neglia G, Vecchio D, Gasparrini B, Gimenes LU, Zicarelli L, D'Occhio MJ. 2010. Ovarian function in the buffalo and implications for embryo development and assisted reproduction. Anim Reprod Sci. 121(1–2):1–11. doi: 10.1016/j.anireprosci.2010.03.012.
- Caslini C, Comin A, Peric T, Prandi A, Pedrotti L, Mattiello S. 2016. Use of hair cortisol analysis for comparing population status in wild red deer (Cervus elaphus) living in areas with different characteristics. Eur J Wildl Res. 62(6):713–723. doi: 10.1007/s10344-016-1049-2.
- Costa A, Negrini R, De Marchi M, Campanile G, Neglia G. 2020. Phenotypic characterization of milk yield and quality traits in a large population of water buffaloes. Animals (Basel). 10(2):327. doi: 10.3390/ani10020327.
- Cotticelli A, Verde MT, Matera R, Pividori I, Prandi A, Neglia G, Peric T. 2022. Validation of a radioimmunoassay method for cortisol in buffalo milk whey. A preparatory step for future sensor technology. Ital J Anim Sci. 21(1):1622–1631. doi: 10.1080/1828051X.2022.2147868.
- Díaz JR, Alejandro M, Romero G, Moya F, Peris C. 2013. Variation in milk cortisol during lactation in Murciano-Granadina goats. J Dairy Sci. 96(2):897–905. doi: 10.3168/jds.2012-5614.
- Drackley JK. 1999. ADSA Foundation Scholar Award. Biology of dairy cows during the transition period: the final frontier? J Dairy Sci. 82(11):2259–2273. doi: 10.3168/jds.s0022-0302(99)75474-3.
- Drackley JK, Dann HM, Douglas N, Guretzky NAJ, Litherland NB, Underwood JP, Loor JJ. 2005. Physiological and pathological adaptations in dairy cows that may increase susceptibility to periparturient diseases and disorders. Ital J Anim Sci. 4(4):323–344. doi: 10.4081/ijas.2005.323.
- Endo N, Kuroki R, Tanaka T. 2017. Comparison of productive and reproductive performance and hair cortisol levels between Brown Swiss cross-bred and Holstein cows housed in the same barn. Anim Sci J. 88(10):1506–1512. doi: 10.1111/asj.12828.
- Ferreira MFL, Rennó LN, Rodrigues II, Detmann E, Paulino MF, de Campos Valadares Filho S, Martins HC, Moreira SS, de Lana DS. 2021. Effects of parity order on performance, metabolic, and hormonal parameters of grazing beef cows during pre-calving and lactation periods. BMC Vet Res. 17(1):311. doi: 10.1186/s12917-021-03019-0.
- Fukasawa M, Tsukada H, Kosako T, Yamada A. 2008. Effect of lactation stage, season and parity on milk cortisol concentration in Holstein cows. Livest Sci. 113(2–3):280–284. doi: 10.1016/j.livsci.2007.05.020.
- Gellrich K, Sigl T, Meyer HH, Wiedemann S. 2015. Cortisol levels in skimmed milk during the first 22 weeks of lactation and response to short-term metabolic stress and lameness in dairy cows. J Anim Sci Biotechnol. 6(1):31.
- Gross JJ, Bruckmaier RM. 2019. Invited review: metabolic challenges and adaptation during different functional stages of the mammary gland in dairy cows: perspectives for sustainable milk production. J Dairy Sci. 102(4):2828–2843. doi: 10.3168/jds.2018-15713.
- Heimbürge S, Kanitz E, Otten W. 2019. The use of hair cortisol for the assessment of stress in animals. Gen Comp Endocrinol. 270:10–17. doi: 10.1016/j.ygcen.2018.09.016.
- Hellhammer DH, Wüst S, Kudielka BM. 2009. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 34(2):163–171. doi: 10.1016/j.psyneuen.2008.10.026.
- Global Standard for Livestock Data: Section 2-Guideline for Dairy Cattle Milk Recording, ICAR 2022. Available online: https://www.icar.org/index.php/icar-recording-guidelines/
- Ingvartsen KL. 2006. Feeding-and management-related diseases in the transition cow: physiological adaptations around calving and strategies to reduce feeding-related diseases. Anim Feed Sci Technol. 126(3–4):175–213. doi: 10.1016/j.anifeedsci.2005.08.003.
- Kovács L, Kézér FL, Bodó S, Ruff F, Palme R, Szenci O. 2021. Salivary cortisol as a non-invasive approach to assess stress in dystocic dairy calves. Sci Rep. 11(1):6200. doi: 10.1038/s41598-021-85666-9.
- Kuczyńska B, Puppel K, Gołębiewski M, Wisniewski K, Przysucha T. 2021. Metabolic profile according to the parity and stage of lactation of high-performance Holstein-Friesian cows. Anim Biosci. 34(4):575–583. doi: 10.5713/ajas.20.0018.
- Matera R, Cotticelli A, Salzano A, Piscopo N, Balestrieri A, Campanile G, Neglia G. 2021. Influence of days after calving and thermal stress on the efficacy of a progesterone-based treatment in acyclic Italian Mediterranean Buffalo. Animals (Basel). 11(11):3166. doi: 10.3390/ani11113166.
- Matera R, Di Vuolo G, Cotticelli A, Salzano A, Neglia G, Cimmino R, D’Angelo D, Biffani S. 2022. Relationship among milk conductivity, production traits, and somatic cell score in the Italian Mediterranean Buffalo. Animals. 12(17):2225. doi: 10.3390/ani12172225.
- Matera R, Cotticelli A, Gómez Carpio M, Biffani S, Iannacone F, Salzano A, Neglia G. 2022. Relationship among production traits, somatic cell score and temperature–humidity index in the Italian Mediterranean Buffalo. Ital J Anim Sci. 21(1):551–561. doi: 10.1080/1828051X.2022.2042407.
- Meyer JS, Novak MA. 2012. Minireview: hair cortisol: a novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology. 153(9):4120–4127. doi: 10.1210/en.2012-1226.
- Nawroth C, Langbein J, Coulon M, Gabor V, Oesterwind S, Benz-Schwarzburg J, von Borell E. 2019. Farm animal cognition-linking behavior, welfare and ethics. Front Vet Sci. 6:24. doi: 10.3389/fvets.2019.00024.
- Otten W, Heimbürge S, Tuchscherer A, Kanitz E. 2023. Hair cortisol concentration in postpartum dairy cows and its association with parameters of milk production. Domest Anim Endocrinol. 84–85:106792. doi: 10.1016/j.domaniend.2023.106792.
- Peric T, Comin A, Montillo M, Spigarelli C, Corazzin M, Cotticelli A, Prandi A. 2022. Postnatal and postweaning endocrine setting in dairy calves through hair cortisol, dehydroepiandrosterone and dehydroepiandrosterone sulphate. Agric Nat Resource. 56(5):867–876.
- Peric T, Veronesi MC, Prandi A, Fusi J, Faustini M, Probo M. 2024. Postpartum hair cortisol, dehydroepiandrosterone sulfate and their ratio in beef cows: exploring association with parity and conception outcome. Theriogenology. 214:352–359. doi: 10.1016/j.theriogenology.2023.11.008.
- Pošćić N, Gabai G, Stefanon B, Da Dalt L, Sgorlon S. 2017. Milk cortisol response to group relocation in lactating cows. J Dairy Res. 84(1):36–38. doi: 10.1017/S0022029916000790.
- Russell E, Koren G, Rieder M, Van Uum S. 2012. Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology. 37(5):589–601. doi: 10.1016/j.psyneuen.2011.09.009.
- Sabek A, Li C, Du C, Nan L, Ni J, Elgazzar E, Ma Y, Salem AZM, Zhang S. 2021. Effects of parity and days in milk on milk composition in correlation with β-hydroxybutyrate in tropic dairy cows. Trop Anim Health Prod. 53(2):270. doi: 10.1007/s11250-021-02690-7.
- Saqib MN, Qureshi MS, Suhail SM, Khan RU, Bozzo G, Ceci E, Laudadio V, Tufarelli V. 2022. Association among metabolic status, oxidative stress, milk yield, body condition score and reproductive cyclicity in dairy buffaloes. Reprod Domest Anim. 57(5):498–504. doi: 10.1111/rda.14086.
- Sgorlon S, Fanzago M, Guiatti D, Gabai G, Stradaioli G, Stefanon B. 2015. Factors affecting milk cortisol in mid lactating dairy cows. BMC Vet Res. 11(1):259. doi: 10.1186/s12917-015-0572-9.
- Sharma A, Umapathy G, Kumar V, Phillips CJC. 2019. Hair Cortisol in Sheltered Cows and Its Association with Other Welfare Indicators. Animals (Basel). 9(5):248. doi: 10.3390/ani9050248.
- Siewert JM, Salfer JA, Endres MI. 2019. Milk yield and milking station visits of primiparous versus multiparous cows on automatic milking system farms in the Upper Midwest United States. J Dairy Sci. 102(4):3523–3530. doi: 10.3168/jds.2018-15382.
- Tallo-Parra O, Carbajal A, Monclús L, Manteca X, Lopez-Bejar M. 2018. Hair cortisol and progesterone detection in dairy cattle: interrelation with physiological status and milk production. Domest Anim Endocrinol. 64:1–8. doi: 10.1016/j.domaniend.2018.02.001.
- Trevisi E, Minuti A. 2018. Assessment of the innate immune response in the periparturient cow. Res Vet Sci. 116:47–54. doi: 10.1016/j.rvsc.2017.12.001.
- Trus D, Buttazzoni LG. 1990. A multiple trait approach to modelling the lactation curve. Proceedings of the 4th World Congress on Genetics Applied to Livestock Production. Edinburgh, 23–27 July 1990. XIII. Plenary Lectures, Molecular Genetics and Mapping, Selection, Prediction and Estimation; p. 492–495.
- van Knegsel ATM, Hammon HM, Bernabucci U, Bertoni G, Bruckmaier RM, Goselink RMA, Gross JJ, Kuhla B, Metges CC, Parmentier HK, et al. 2014. Metabolic adaptation during early lactation: key to cow health, longevity and a sustainable dairy production chain. CAB Rev. 9:1–15. doi: 10.1079/PAVSNNR20149002.
- Walter LL, Gärtner T, Gernand E, Wehrend A, Donat K. 2022. Effects of parity and stage of lactation on trend and variability of metabolic markers in dairy cows. Animals (Basel). 12(8):1008. doi: 10.3390/ani12081008.
- Wu X, Sun HZ, Xue M, Wang D, Guan L, Liu J. 2019. Days-in-milk and parity affected serum biochemical parameters and hormone profiles in mid-lactation holstein cows. Animals. 9(5):230. doi: 10.3390/ani9050230.
- Yehia SG, Ramadan ES, Megahed EA, Salem NY. 2020. Effect of parity on metabolic and oxidative stress profiles in Holstein dairy cows. Vet World. 13(12):2780–2786. doi: 10.14202/vetworld.2020.2780-2786.