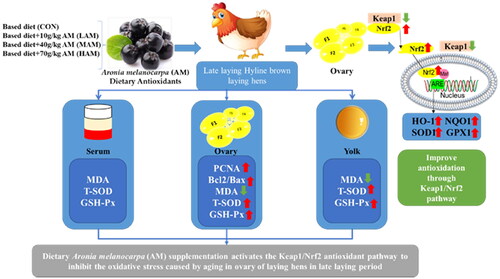
Abstract
The ageing of laying hens’ ovaries in the late laying period leads to oxidative stress, which ultimately results in the decrease of laying performance. Thus, we investigated Aronia melanocarpa (AM) as a potent antioxidant to improve ovarian senescence in the late laying hens through antioxidant pathway, thereby improving egg production performance. A total of 480 Hy-line brown laying hens aged 59 wks (60–67 wks) were randomly divided into CON, LAM, MAM and HAM groups and fed 0, 1, 4 and 7% AM, respectively. The results showed that compared with the CON group, the MAM group could significantly improve the laying rate and reduce the feed egg ratio at 64–67 weeks of age (p < 0.05). The AM groups increased the eggshell thickness, while HAM group significantly reduced yolk colour (p < 0.05). The MAM and HAM groups significantly increased egg white weight (p < 0.05). Meanwhile, the ovarian index in MAM group was increased, and the proteins related to proliferation and anti-apoptosis of ovarian cells (PCNA and Bcl2/Bax) were enhanced in all AM groups (p < 0.05). Compared with the CON group, all AM groups could significantly reduce the malondialdehyde (MDA) content of ovary and yolk, and increase the activities of glutathione peroxidase (GSH-Px) and total superoxide dismutase (T-SOD) in yolk. LAM and MAM groups significantly increased ovarian GSH-Px activity, but only the MAM group significantly increased ovarian T-SOD activity. The expression levels of Nuclear factor E2-related factor 2 (Nrf2), haem oxygenase 1 (HO-1) and glutathione peroxidase 1 (GPX1) genes and proteins were significantly increased in all AM groups, while the expression levels of kelch ECH – associated protein 1 (Keap1) genes and proteins were decreased. The expressions of NAD(P)H: quinone oxidoreductase 1 (NQO1) and superoxide dismutase 1 (SOD1) genes were significantly increased in all AM groups, but the expression of SOD1 protein was significantly increased in MAM group and NQO1 protein was significantly increased in HAM group. Antioxidant enzyme protein expressions in the ovary showed positive associations with antioxidant enzyme activity. Furthermore, the expression of Nrf2 protein in the ovary was inversely connected with Keap1 protein expression and favourably correlated with antioxidant protein expressions. In summary, dietary AM supplementation activates the Keap1/Nrf2 antioxidant pathway to inhibit the oxidative stress caused by ageing in ovary of late laying hens. Taken together, the results of the study suggest that the inclusion of 4% AM was able to better improve the laying performance and antioxidant capacity of laying hens in the late laying period.
HIGHLIGHTS
Aronia melanocarpa can improve the performance and egg quality of laying hens in the late laying period.
Aronia melanocarpa can alleviate oxidative stress caused by ovarian ageing by activating the Keap1/Nrf2 pathway in later laying hens
Introduction
The laying chicken industry is one of the key components in promoting sustainable food sources around the world (Al-Shammari et al. Citation2023). The later stage of production (defined as a 90% lower egg yield) accounts for a significant portion of the overall laying cycle (Cui et al. Citation2017). The main factors affecting laying performance of laying hens include physiological ageing, inappropriate feeding and nutritional management, environmental factors such as high temperature, humidity, odour and air quality, diseases and infections, and genetic factors (Krysiak et al. Citation2021; Silveira et al. Citation2021). The decline in egg production performance of laying hens in the later stages of egg production limits their economic benefits (Wen et al. Citation2019). Most of the studies indicate that the gradual decline of ovarian function, the inhibition of follicle development and the increase of oxidative stress level in late laying hens further promote the rapid decline of laying rate, which leads to the shortening of the utilisation period and the decrease of economic value of late laying hens (Wu et al. Citation2023). Therefore, our experiment mainly focuses on how to delay the ageing of laying hens’ ovaries and improve egg production performance, which is of great significance to the sustainable development of laying hens’ industry (Sharma et al. Citation2023).
Numerous investigations have revealed that ageing of laying hens can lead to de-creased ovarian function and egg production performance, and thus oxidative stress (Guo et al. Citation2021). With the increase of age, physiological ageing of laying hens leads to oxidative stress, the main mechanism of which is reactive oxygen species (ROS) production (Radak et al. Citation2024). Normally, complex antioxidant defense mechanisms (composed of multiple antioxidant related enzymes) eliminate ROS and keep various cells in a REDOX state (Kabir et al. Citation2023). However, with the increase of the age of laying hens, the activity of antioxidant enzymes is reduced, resulting in the destruction of the dynamic balance of the antioxidant system, resulting in oxidative stress (Miao et al. Citation2023).
The transcription factor Nrf2 is a key molecule that regulates the degree of cellular oxidative stress, responsible for activating antioxidant and stress defense mechanisms (Ma et al. Citation2021). The oxidation and anti-oxidation in dynamic equilibrium state, the Nrf2 sample with kelch ECH – associated protein 1 (Keap1) combined with each other, Keap1 is mainly located in cytoplasm (Yang and Smith Citation2023). When the cell is subjected to oxidative stress or electrophilic attack, Nrf2 dissociates from Keap1 and rapidly translocations into the nucleus (Baumel-Alterzon et al. Citation2021). It first forms a heterodimer with small Maf protein and binds to antioxidant response elements (ARE) to regulate antioxidant oxidase gene expression (Zou et al. Citation2023). The cell protection genes then further regulate the activity of downstream antioxidant enzymes. The ovary’s expression of Nrf2 and its downstream antioxidant related enzymes as a reflection of tissue antioxidant status is unknown. Most of the current research focuses on the effects of ageing on the production of laying hens and how to improve the laying performance (Dai et al. Citation2023). However, there are few researches on oxidative stress caused by ageing of laying hens and how to relieve oxidative stress.
In recent years, plant-derived antioxidants such as polyphenols (Abd El Hack et al. Citation2023), carotenoids (Dansou et al. Citation2023) and flavonoids (Dai et al. Citation2021) can improve the resistance of hens to oxidative stress and prolong the laying cycle of hens. Aronia melanocarpa (AM) is also known as the wild berry or ageless berry, Rosaceae, and is native to North America (Zhu et al. Citation2023). Due to the sour, astringent, and bitter almond tastes of raw fruit, it has limited application as a fruit juice or in the juice industry and is only used in mixed juices. China’s current national food safety standards revealed that AM has become a food-grade product after a safety risk assessment. In recent years, the planting area for AM has increased every year, and most of the planting areas are in the northeast of China, which is convenient for obtaining AM. The National Health Commission of China authorised AM as a new food in September 2018. It is mainly used in the production of fruit juice, fruit wine, jam, fruit tea and dietary supplements (Negreanu-Pirjol et al. Citation2023). Studies have shown that AM has antioxidant capacity, which can protect organs and reduce damage (Jurendić and Ščetar Citation2021). Currently, the majority of antioxidants are incorporated into feed in the form of extracts. In this study, AM was directly ground into fruit powder and added to the feed following the principles of isoenergy and isonitrogen as raw materials, in order to investigate the impact of AM on oxidative stress induced by ageing.
This study was to investigate the protective effect of AM on ovaries of laying hens in late laying period and its mechanism. To further explore the molecular mechanism of Keap1/Nrf2 pathway in ovaries of late laying hens. These results provide some experimental basis and theoretical basis for delaying ovarian senescence in poultry.
Materials and methods
Animal ethics
The Ethics committee of Jilin Agricultural University’s College of Life Sciences approved the study (AW02501202-2-1). Furthermore, the research design and reporting conformed to the principles of the ARRIVE 2.0 guidelines, ensuring comprehensive and transparent reporting of experimental procedures and results.
Birds and experimental design
AM fruit powder is generously provided by the Gogogo Egg Products Cooperative in Liaoyuan City, Jilin Province. Prior to feeding, the animal room underwent thorough fumigation and sterilisation using formaldehyde and potassium permanganate. A total of 480 healthy 59-wk-old Hy-line brown laying hens with same weight (1.90 ± 0.05 kg) were randomly divided into four groups with six replicates in each group, twenty in each replicate. The breeding unit is Liaoyuan Gogogo Egg Products Cooperative. The diets fed in each group were shown in Table S1. The proximate nutrient component of AM was Table S2. The experimental groups were supplemented with 0, 1, 4 and 7% AM (CON, LAM, MAM and HAM). The hens were accommodated in wire cages measuring 45 × 45 × 50 cm, with three hens allocated per cage. These cages were arranged in a three-tier ladder-type configuration within an environmentally controlled facility. All hens were fed twice a day at 8:00 am and 2:00 pm Laying hens had ad libitum access to drinking water during the whole study period. Manure removal is carried out daily at 7:30 am by an automated manure removal machine. During the whole test, the light was 17 h and the darkness was 7 h. At 67 wks of age, hens were weighed and anaesthetised with pentobarbital sodium after fasting for 24 h. All laying hens were humanely euthanized, and all samples (ovary) were rapidly frozen in liquid nitrogen and then stored at −80 °C.
Laying performance
In the process of raising hens, the weekly egg number and egg weight of each group were recorded, average daily feed intake (ADFI), feed conversion ratio (FCR) and egg production rate of each group were calculated, and the body weight of laying hens aged 63 and 67 wks were recorded.
Egg quality
After 8 wks of testing, fifteen eggs were selected for each group, Haugh unit, the egg shape index, albumen height, eggshell strength and eggshell thickness were respectively detected by a multifunctional egg quality detector (Japan Robotmation EMT-5200). Determine the colour of egg yolk with a colorimetric card. We use an egg yolk separator to separate egg white and egg yolk, and weigh them using an electronic balance (Accuab ACL, Seedox, Germany).
Sample collection
At the end of the experiment, six fasting hens were taken for weight measurement. Immediately after euthanasia, blood samples were collected via exsanguination of the left jugular vein with scalpels and centrifuged at 4,000 x g for 30 min at 4 °C to separate the serum. Then, the serum samples were frozen at −80 °C until analysis. The diameter of pregrade follicles (those exceeding 12 mm in diameter) was measured. The ovaries were immediately removed and quickly frozen at −80 °C for further analysis. Egg yolks were collected in sterile dishes and mixed. We freeze the egg yolks in a −20 °C freezer for 24 h. Frozen egg yolks were lyophilised for 96 h, with the vacuum level reaching 133 × 10−3 mbar and a condenser temperature of at least −60 °C in a Freeze Dry System (VFD-1000, Beijing, China).
Morphological observation of the ovary
Six hens were chosen from each experimental group at the 67 wk of the trial. After removing the ovaries, we clean and weigh them. Ovarian index = ovarian weight/body weight. Additionally, ovarian tissues were fixed in a paraformaldehyde solution with a concentration of more than 4%. Ovarian tissue is first dehydrated with graded ethanol, then transparent in xylene, and then embedded in paraffin. The tissue sections were cut into 5 μm according to the routine procedure, and the complete sections were transferred to a preheated 42 °C film spreading machine for spreading, and then quickly attached to the adhesive slide after unfolding. The resulting slices were stained with haematoxylin and eosin, and sealed with neutral gum. The observation and analysis were carried out by fluorescence orthoscopic microscope (DM4B, Leicas, Germany).
Serum, ovarian and yolk antioxidant capacity determination
Concentrations of malondialdehyde (MDA, A003-1), glutathione peroxidase (GSH-Px, A005-1) and total superoxide dismutase (T-SOD, A001-1) in serum, ovarian and yolk samples were determined following the manufacturer’s instructions and using kits using biochemical methods.
Quantitative real-time PCR analysis
Total RNA was isolated from ovarian tissue using pre-cooled Trizol reagents, chloroform, isopropyl alcohol, and ethanol (Takara, China). The concentration and purity of RNA were determined (Micro-Drop, Bio-DL Corporation, Shanghai, China) (Jing et al. Citation2022). We first used a reverse transcription kit to reverse trantranscript cDNA from total RNA. Then, according to gene sequence information in Genbank database, gene soft-ware Primer 5.0 was used to design Keap1, Nrf2, HO-1, NQO1, SOD1 and GPX1 gene-specific primers (Table S3), and primer synthesis was performed by relying on Shanghai Shengong Bioengineering Co., LTD. Total cDNA was quantitatively amplified using the SYBR Premix Ex TaqTM II fluorescence quantification kit and fluorescence quantitative PCR (Stratagene MX3000P, USA). The steward gene (β-actin) was used to standardise the relative expression level of mRNA, and the appropriate amount of mRNA was measured using the 2−ΔΔCT method.
Immunohistochemistry
We started with formalin to collect and immobilise ovarian tissue samples. The fixed tissue was embedded in paraffin wax for sectioning (thickness 5 μm). The slices were then soaked in xylene for dewaxing and rehydrated by a fractional alcohol series. Next, the staining rack with the slices was transferred to a staining tank containing a peroxidase inhibitor (3% hydrogen peroxide/methanol solution) to inhibit endogenous peroxidase activity, and incubate at room temperature in the dark for 5 min. Subsequently, they were placed in a dyeing tank and washed at room temperature 3 times for 5 min each time with PBS buffer by gently shaken on a horizontal shaker. Then, the slices were put into the citric acid antigen repair solution, and heated with the microwave oven for boiling, cooling for 2 min, medium and small heat heated for 5 min, cooling for 2 min, heating for 5 min, cooling to room temperature for antigen repair. Then the slices were soaked in PBS for 3 min, sealed with 1% BSA and closed at room temperature for 1 h. Goat anti-rabbit Nrf2 antibodies were applied to fully cover the tissue sections, and the slides were sealed and incubated at 4 °C overnight. As a negative control, PBS was used in place of the primary antibody. On the following day, the tissue sections were washed three times with PBS for 5 min each. Subsequently, the corresponding secondary antibodies were applied to fully cover the tissue sections, followed by an incubation at 37 °C for 30 min. Afterward, the sections were thoroughly rinsed with PBS. DAB colour development solution was added, and the light was closed for 3 min. Then the slides were rinsed with running water for 3 min, restained with haematoxylin, dehydrated following gradient alcohol combined with the xylene transparent, and sealed by using the neutral gum. They were finally observed under the microscope linked to a camera to take pictures.
Protein extraction and Western blot analysis
Total protein was extracted from ovarian and liver lysates from each hen by T-PER tissue protein extraction reagent (Thermo Pierce, novobiotec, Bejing, China) containing a protease inhibitor cocktail (Thermo Pierce, novobiotec, Bejing, China). Protein concentration was assessed by BCA protein assay. Ten microlitres of each sample at the same concentration were added to the wells of SDS-acrylamide gels of different concentrations. All proteins were separated and transferred to PVDF membranes and blocked with 5% skimmed milk diluted in TBS-0.1% v/v Tween 20. The membrane was incubated with an appropriate primary antibody for 24 h at 4 °C. After the incubation, each membrane was incubated with the corresponding anti-rabbit IgG antibody for 1 h. Specific bands were visualised using Spark ECL Western blotting substrate and quantified using ImageJ software. Primary and secondary antibodies utilised in this study were goat anti-rabbit PCNA, Bcl2, Bax, Keap1, Nrf2, HO-1, NQO1, SOD1, and GPX1 (Wanleibio, Liaoning, China; 1:2000), goat anti-rabbit secondary antibodies (CST, Shanghai, China; 1:1000), and β-actin (CST, Shanghai, China; 1:4000).
Statistical analysis
The data were presented as Mean ± SD using IBM SPSS statistics 26 software, while Graphpad Prism 9 was used to draw the column charts. One-way analysis of variance (ANOVA) was conducted to assess significant differences, followed by post hoc testing using the Duncan method. Spearman correlation coefficient was used to perform the correlation statistics on the Keap1/Nrf2 pathway and downstream antioxidant enzyme proteins, with the significant differences declared at p < 0.05.
Results
Body weight and laying rate
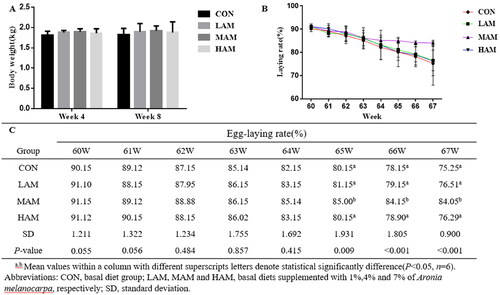
The body weight of laying hens was not different between the experimental groups (Figure ). The MAM group’s laying rate increased considerably when compared to the CON group (p < 0.05). Meanwhile, the egg laying rate in the MAM group was much greater than in the LAM and HAM groups (p < 0.05; Figure ).
Figure 1. Effects of AM supplementation on body weight and laying rate of late laying hens. a,bDifferent superscripts have different means (p < 0.05). (A) The body weight (kg) of layers in each group after 4 and 8 wks of feeding (n = 6). (B, C) the egg laying rate (%) of all groups of 60–67 wk old laying hens (n = 6). Abbreviations: CON, LAM, MAM and HAM, basal diets supplemented with 0, 1, 4 and 7% of Aronia melanocarpa (AM), respectively.
Laying performance
Table shows that there was no difference in average egg weight and ADFI between the groups. At the 64th–67th week, the egg production rate of the MAM group was significantly higher than that of the other groups (p < 0.05).
Table 1. Effects of aronia melanocarpa (AM) supplementation on laying performance of laying hens in late laying periodTable Footnotec.
Egg quality
Table shows that there was no significant effect on egg quality indicators (egg shape index, eggshell strength, albumen height, egg yolk weight and Haugh unit) at 63 and 67 wks. Compared with the CON group, all AM groups significantly increased eggshell thickness at 67 weeks of age (p < 0.05). Compared with the CON group, the egg white weight of MAM group and HAM group was significantly increased at 67 wks (p < 0.05), and egg yolk colour of HAM group was significantly decreased (p < 0.05).
Table 2. Effects of aronia melanocarpa (AM) supplementation on egg quality of laying hens in late laying periodTable Footnotec.
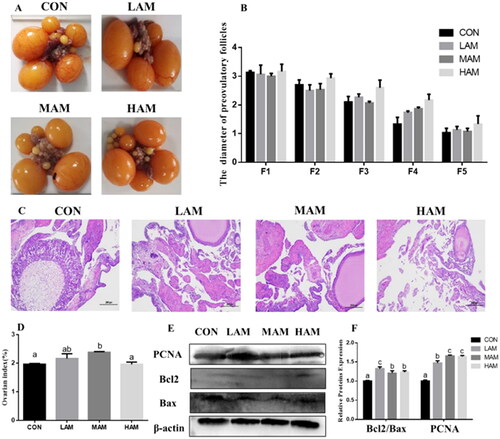
Ovarian morphology, index, cell proliferation and apoptosis
The diameter of the grade follicle is an intuitive indication of yolk deposition. There was no change in follicle diameter in each group (Figure ). The histomorphology of ovarian tissues revealed no significant variations in follicle diameter before grade in all AM groups compared to the CON group (Figure ). The ovarian index of MAM group was higher than that of the other three groups (p < 0.05, Figure ). There was no significant difference in ovarian index among the other three groups. Meanwhile, all AM groups had higher levels of expression of cell proliferation and apoptosis-related proteins PCNA and Bcl2/Bax in ovarian tissues than the CON group (Figure ) (p < 0.05).
Figure 2. Ovarian morphology, ovarian index, cell proliferation and apoptosis. a–cDifferent superscripts have different means (p < 0.05). (A) Ovary morphology. (B) The diameter of preovulatory follicles (n = 6). (C) Ovarian histomorphology, scale bar: 200 μm. (D) Ovarian index (n = 6). (E, F) Western blot analysis and protein expression levels of PCNA, Bcl2 and bax in ovary. Abbreviations: CON, LAM, MAM and HAM, basal diets supplemented with 0, 1, 4 and 7% of Aronia melanocarpa (AM), respectively.
Serum, ovarian and yolk antioxidant capacity
As shown in Table , in serum, the antioxidant and oxidation indexes did not differ significantly among the groups. In ovary, the MDA content in the AM groups exhibited a significant decrease compared to that of the control group (p < 0.05). GSH-Px activity was significantly increased in both the LAM and MAM groups, however T-SOD activity was only significantly elevated in the LAM group (p < 0.05). Compared to the CON group, all AM groups increased in the activities of GSH-Px and T-SOD of yolk tissues, and decreased in the concentration of MDA (p < 0.05).
Table 3. Effects of aronia melanocarpa (AM) supplementation on serum, ovarian and yolk antioxidant capacity of laying hens in late laying periodTable Footnotec.
Keap1/Nrf2 pathway gene and protein expression
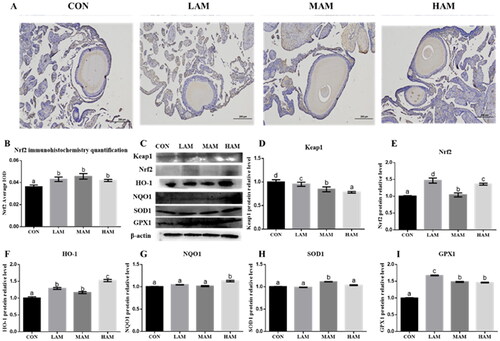
In three treatment groups, gene expression levels of Nrf2, HO-1, NQO1, SOD1 and GPX1 were significantly increased than in the CON group, but Keap1 gene expression was significantly decreased (p < 0.05, Figure ). Immunohistochemical data demonstrated that all AM groups had significantly higher levels of Nrf2 protein ex-pression as compared to the CON group (p < 0.05, Figure ). In three treatment groups, there was a substantial rise in the protein expression levels of Nrf2, HO-1, and GPX1 compared to the CON group, and a significant decrease in the protein expression level of Keap1. When compared to the CON group, the NQO1 protein expression level could only be considerably increased by the HAM group, while the SOD1 protein expression level could only be significantly increased by the MAM group (p < 0.05, Figure ).
Figure 3. Expression levels of antioxidant genes in ovarian tissue of hens. a–cDifferent superscripts have different means (p < 0.05, n = 6). Abbreviations: HO-1: haem oxygenase 1; NQO1, NAD(P)H: quinone oxidoreductase 1; SOD1: superoxide dismutase 1; GPX1: glutathione peroxidase 1; Keap1: kelch ECH – associated protein 1; Nrf2: nuclear factor E2-related factor 2. CON, LAM, MAM and HAM, basal diets supplemented with 0, 1, 4 and 7% of Aronia melanocarpa (AM), respectively.
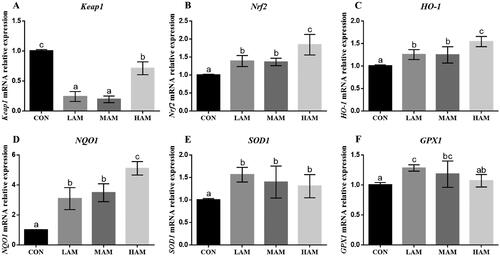
Figure 4. Expression levels of Nrf2 protein immunohistochemistry and antioxidant protein in ovarian tissue of hens. a–dDifferent superscripts have different means (p < 0.05). (A) Nrf2 protein immunohistochemistry, scale bar: 200 μm; (B) immunohistochemical quantification of Nrf2 protein (n = 6); (C–I) Western blot analysis and protein expressions of Keap1, Nrf2, HO-1, NQO1, SOD1 and GPX1 in ovary. Abbreviations: HO-1: haem oxygenase 1; NQO1, NAD(P)H: quinone oxidoreductase 1; SOD1: superoxide dismutase 1; GPX1: glutathione peroxidase 1; Keap1: kelch ECH – associated protein 1; Nrf2: nuclear factor E2-related factor 2. CON, LAM, MAM and HAM, basal diets supplemented with 0, 1, 4 and 7% of Aronia melanocarpa (AM), respectively.
Correlation analyses related to the Keap1/Nrf2 pathway
It can be seen from Table that the expression of SOD1 (r = 0.56, p < 0.05) and GPX1 (r = 0.75, p < 0.01) proteins in the ovary is positively correlated with their corresponding antioxidant enzyme activities. Nrf2 protein expression was positively correlated with HO-1 (r = 0.76, p < 0.01), NQO1 (r = 0.73, p < 0.01) and GPX1 (r = 0.52, p < 0.05) protein expression. It was negatively correlated with Keap1 protein expression (r = 0.55, p < 0.05). There was no correlation between HO-1 and NQO1 protein expression and enzyme activity, while there was no correlation between SOD1 and Nrf2 protein expression.
Table 4. Correlation analysis between expression of antioxidant enzyme protein and Nrf2 protein in ovary of laying hensTable Footnotea.
Discussion
Ovary senescence in the late laying period leads to a sharp decline in production performance and egg quality (Mahfuz and Piao Citation2019). In recent years, adding plant-derived antioxidants to the diet can improve the antioxidant capacity of laying hens, thereby improving the laying performance and prolonging the laying cycle (Selim et al. Citation2023). AM is a natural fruit with a high polyphenol content than most berries, such as blueberries (Wójtowicz et al. Citation2023). It has been discovered that the antioxidant function of plant polyphenols lies in their ability to serve as suppliers of hydrogen protons or electrons, scavenge free radicals, and ultimately terminate free radical chain reactions (Seidavi et al. Citation2022). Flavonoid compounds react with phenolic hydroxyl groups and free radicals to generate semiquinone radicals, thereby interrupting the chain reaction (Dai et al. Citation2021). Additionally, polyphenols can facilitate Keap1 protein degradation, promote Nrf2 nuclear translocation, enhance ARE binding capacity, activate the Keap1-NRF2-ARE signalling pathway, and subsequently regulate downstream antioxidant enzyme expression (Seidavi et al. Citation2022). Meanwhile, AM can effectively improve animal immunity (Jeon et al. Citation2018), protect the animal from disease provide the necessary nutrients (Kang et al. Citation2017), anti-ageing (Olechno et al. Citation2022), improve health (Sidor and Gramza-Michałowska Citation2019). This study was to investigate the protective effect of AM on ovaries of late laying hens and its mechanism of action.
The ovarian function of laying hens is tightly linked to the level of laying rate. It has been shown that when the ovaries are subjected to oxidative stress, the laying rate of laying hens decreases (Bao et al. Citation2022). Therefore, the potential impact of adding antioxidants to the diet on reducing age-related oxidative stress, and subsequently enhancing ovarian function and laying rate, warrants further investigation. Studies have shown that dietary supplementation of antioxidants (tea polyphenols, flavonoids) can reduce oxidative stress and improve production performance of laying hens through Nrf2 pathway (Zhou et al. 2021; Seidavi et al. Citation2022). Li et al. (Citation2022) found that anthocyanin-rich purple corn extract (360 mg/kg) increased the laying rate and feed to egg ratio of laying hens at 88 wks of age, but had no significant effect on body weight. Saberifar et al. (Citation2021) showed that adding antioxidant soybean isoflavone (20 mg/kg) in the production of laying hens could significantly increase the egg production and daily egg weight of Hy-line brown laying hens, and significantly reduce the feed to egg ratio. Our previous study found that the adding of AM increased the laying rate of Roman brown laying hens in the first wk of peak laying period, and had a certain increase in body weight. In this study, it was found that AM supplementation had no significant effect on body weight. However, laying rate was significantly higher in the MAM group than in the CON group.
It has been established that dietary supplements containing plant-derived antioxidants can alter egg laying performance and quality (Shi et al. Citation2023). Zhou et al. (2021) found that adding 600 mg/kg tea polyphenols to the diet increased the egg production of 63-wk-old Roman laying hens. E et al. (Citation2023) found that dietary supplementation of genistein (120 mg/kg) for 8 wks could increase the egg production rate and reduce the feed to egg ratio and egg breaking rate of 66-wk-old Hy-line brown laying hens. Chen et al. (Citation2022) found that dietary supplementation of 300–600 mg/kg Magnolol could increase laying rate and decrease feed to egg ratio of 50-wk-old fine powder and powder shell laying hens. We found that 4% AM supplementation in the feed of 60-wk-old Hy-line brown laying hens enhanced laying rate. Eggshell strength and eggshell thickness are closely related to egg breakage rate (Janmohammadi et al. Citation2023). The ageing of laying hens will cause the eggshell strength of eggs to decrease and increase the breakage rate, which will affect its commercial value. However, adding plant-derived antioxidants to the diet can slow down the ageing time of laying hens. The inclusion of vitamin C and polyphenols can enhance the mineral metabolism and collagen synthesis in eggshells, thereby playing a crucial role in promoting the increase of calcium levels in laying hens’ blood, consequently impacting the quality of eggshells (Shi et al. Citation2023). Huang et al. (Citation2022) found that dietary addition of mulberry leaf flavonoids could improve the eggshell thickness and strength of Qiling breeder hens at 60 wks of age. This study also found that dietary AM supplementation can significantly improve eggshell thickness and eggshell strength of laying hens in later stage. A previous study found that dietary AM supplementation increased eggshell thickness and decreased yolk colour in Roman brown laying hens (25 wks of age) at peak laying period (Jing et al. Citation2022). The colour of the yolk typically mirrors the nutrient composition of the egg and the additives incorporated into the feed. Variances in yolk colour arise from different feed ingredients and additives. However, it’s crucial to note that this divergence in colour doesn’t necessarily imply a direct impact on other quality attributes of the egg due to feed ingredients. It emphasises the complexity of assessing egg quality, where factors like shell hardness, protein content, and overall nutritional value should be comprehensively considered alongside yolk colour (Wang et al. Citation2017). In this study, it was discovered that dietary AM supplementation might increase eggshell strength. The egg white weight of MAM group and HAM group was significantly increased at 67 wks, and egg yolk colour of HAM group was significantly decreased. This may be due to the substitution of AM for corn and soybean meal, which results in a decrease in lutein content, which in turn affects the yolk colour (Wang et al. Citation2017).
The proliferation and apoptosis of ovarian cells are important biological processes that regulate the laying performance of poultry. They play an important role in maintaining the normal function of the ovary and reproductive health. Yang et al. (Citation2018) found that dietary quercetin can increase the laying rate of Hessian laying hens by increasing grade follicle diameter and ovarian cell proliferation, and reducing cell apoptosis. Dai et al. (Citation2021) showed that dietary supplementation of total flavonoids of hawthorn leaves (60 mg/kg) could increase the relative ovarian weight and egg production rate of Qiling breeder chickens. Previous research has found that oxidative stress causes ovarian granulosa cell apoptosis, which leads to decreased ovarian function (Bai et al. Citation2023). In this experiment, the addition of 1 and 4% AM can improve the ovarian index and ovarian cell proliferation and reduce the apoptosis of Hy-line laying hens, but has no significant effect on the grade follicle diameter. We speculated that AM can improve the antioxidant capacity of Hy-line brown late laying hens, promote the proliferation of ovarian somatic cells and reduce cell apoptosis, thereby alleviating the follicular atresia caused by oxidative stress, and ultimately affecting ovarian function and egg production performance. However, further experiments are needed to confirm our conjecture.
Oxidative stress disrupts the REDOX balance in laying hens, leading to reduced ovarian function and ultimately impacting egg-laying efficiency (Wan et al. Citation2022). The content of MDA in cells can reflect the production of oxygen free radicals (ROS) and the degree of lipid peroxidation (Ma et al. Citation2021). The intracellular antioxidant enzyme system, including SOD, CAT and GSH-Px, protects the ovarian function of laying hens by oxidative stress (Yang and Smith Citation2023). Huang et al. (Citation2022) found that flavonoids (60 mg/kg) significantly increased T-SOD content and decreased MDA content in ovarian tissues of 60-wk-old Qiling breeder hens. Gao et al. (Citation2020) found that natural astaxanthin significantly decreased MDA content in plasma, liver and yolk of 50-wk-old Hy-line Brown laying hens, while increasing the activities of T-SOD and GSH-Px. In the present study, dietary AM supplementation could significantly increase the contents of T-SOD and GSH-Px in egg yolk and decrease the contents of MDA in egg yolk and ovary. The LAM and MAM groups significantly increased the content of GSH-Px in the ovary, and only the LAM group significantly increased the content of T-SOD. Xing et al. (Citation2020) found that dietary supplementation of 400 mg/kg resveratrol can significantly increase the gene expression of SOD1 and GPX1 in ovarian tissue of 90-day-old fatty liver laying hens, and enhance the activities of T-SOD and GSH-Px. In this study, AM supplementation significantly increased the gene and protein expressions of SOD1 and GPX1. The expression of SOD1 and GPX1 was positively correlated with the activity of antioxidant enzymes, indicating that AM increased the activity of antioxidant enzymes by activating protein expression. Our previous study also found that the addition of AM in the diet can improve the activity of antioxidant enzymes and genes in laying hens during the peak laying period (Jing et al. Citation2022).
The transcription factor Nrf2 is a key molecule that regulates the degree of cellular oxidative stress, responsible for activating antioxidant and stress defense mechanisms (Mu and Kitts Citation2023). The oxidation and antioxidation in dynamic equilibrium state, the Nrf2 sample with Keap1 combined with each other, Keap1 is mainly located in cytoplasm (Liu et al. Citation2023). Keap1 and Nrf2 molecules are separated by oxidative stress and antioxidant substances (polyphenol compounds, lycopene, tea polyphenols and anthocyanins) (Seidavi et al. Citation2022). In this case, Nrf2 separates and enters the nucleus, activating the transcription of its target genes (Sha et al. Citation2019) and thus promoting the expression of antioxidant enzymes (HO-1, T-SOD, GPX, NQO1) and prevent cell apoptosis (Sajadi et al. Citation2023). Liu et al. (Citation2018) found that lycopene alleviates the D-Gal-induced ovarian tissue ageing model by activating Nrf2/HO-1 signalling pathway, thereby improving the activity and protein ex-pression of antioxidant enzymes T-SOD, CAT and GPX. Wang et al. (Citation2020) found that tea polyphenols can improve the gene and activity of antioxidant enzymes by activating Nrf2/HO-1 signalling pathway, thus alleviating oxidative stress in ovarian tissue of 67-wk-old Hy-line brown laying hens. We found that the addition of AM significantly increased the expression of Nrf2 antioxidant pathway and downstream antioxidase-related genes and proteins. Dietary AM supplementation can up-regulate Nrf2 and downstream antioxidant enzyme genes, suggesting that AM activates antioxidant enzyme gene and protein expression by activating Nrf2 gene and protein expression in the ovary. In addition, decreased Keap1 protein expression was negatively correlated with Nrf2 and antioxidant enzyme protein expression, suggesting that downregulation of cytoplasmic Keap1 promotes the translocation of Nrf2 from cytoplasm to nucleus. However, an earlier investigation also discovered that feed supplemented with AM enhanced the Keap1/Nrf2 pathway to prevent oxidative damage (Jing et al. Citation2022). The protective effect of Keap1/Nrf2 may be activated to prevent oxidative stress caused by ageing in laying hens. This study provides new evidence for the enhancement of ovarian antioxidant defense of laying hens with AM supplementation, and for the first time elucidates the important role of AM supplementation in activating Keap1/Nrf2 pathway in alleviating the oxidative stress caused by ageing in late laying hens.
Conclusion
In conclusion, dietary AM supplementation has the potential to activate the Keap1/Nrf2 antioxidant pathway in the ovaries of late laying hens, thereby inhibiting oxidative stress caused by ovarian ageing and exerting positive effects on eggshell strength, ovarian cell proliferation, and antioxidant capacity (Figure ). 4%AM could significantly increase the laying rate, egg white weight, ovarian index and reduce the feed egg ratio at 64–67 weeks of age. 7%AM could significantly increase egg white weight and reduce the yolk colour. Taken together, the results of the study suggest that the inclusion of 4% AM was able to better improve the laying performance and antioxidant capacity of laying hens in the late laying period.
Author contributions
Bo Jing: Data curation, Writing - original draft, Writing – review & editing, Investigation, Resources, Data curation. Hui Song: Conceptualisation, Methodology, Supervision. Haoyuan Wu: Software. Zhouyu Jin: Funding acquisition.
Supplemental Material
Download MS Word (22.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The results and analyses presented in this paper are freely available upon request.
Additional information
Funding
References
- Abd El Hack ME, Salem HM, Khafaga AF, Soliman SM, El Saadony MT. 2023. Impacts of polyphenols on laying hens’ productivity and egg quality: a review. J Anim Physiol An N. 107(3):928–947. doi: 10.1111/jpn.13758.
- Al-Shammari K, Zamil SJ, Batkowska J. 2023. The antioxidative influence of dietary creatine monohydrate and L-carnitine on laying performance, egg quality, ileal microbiota, blood biochemistry, and redox status of stressed laying quails. Poult Sci. 103(1):103166. doi: 10.1016/j.psj.2023.103166.
- Bai K, Hao E, Huang C, Yue Q, Wang D, Shi L, Chen Y, Chen H, Huang R, Sveriges L. 2023. Melatonin alleviates ovarian function damage and oxidative stress induced by dexamethasone in the laying hens through FOXO1 signaling pathway. Poultry Sci. 102(8):102745. doi: 10.1016/j.psj.2023.102745.
- Bao T, Yao J, Zhou S, Ma Y, Dong J, Zhang C, Mi Y. 2022. Naringin prevents follicular atresia by inhibiting oxidative stress in the aging chicken. Poult Sci. 101(7):101891. doi: 10.1016/j.psj.2022.101891.
- Baumel-Alterzon S, Katz LS, Brill G, Garcia-Ocaña A, Scott DK. 2021. Nrf2: the master and captain of beta cell fate. Trends Endocrinol Metab. 32(1):7–19. doi: 10.1016/j.tem.2020.11.002.
- Chen F, Zhang H, Zhao N, Du E, Jin F, Fan Q, Guo W, Huang S, Wei J. 2022. Effects of magnolol and honokiol blend on performance, egg quality, hepatic lipid metabolism, and intestinal morphology of hens at late laying cycle. Animal. 16(6):100532. doi: 10.1016/j.animal.2022.100532.
- Cui Y, Wang Q, Liu S, Sun R, Zhou Y, Li Y. 2017. Age-related variations in intestinal microflora of free-range and caged hens. Front Microbiol. 8:1310. doi: 10.3389/fmicb.2017.01310.
- Dai D, Wang J, Zhang H, Wu S, Qi G. 2023. Uterine microbial communities and their potential role in the regulation of epithelium cell cycle and apoptosis in aged hens. Microbiome. 11(1):251. doi: 10.1186/s40168-023-01707-7.
- Dai H, Lv Z, Huang Z, Ye N, Li S, Jiang J, Cheng Y, Shi F. 2021. Dietary hawthorn-leaves flavonoids improves ovarian function and liver lipid metabolism in aged breeder hens. Poultry Sci. 100(12):101499. doi: 10.1016/j.psj.2021.101499.
- Dansou DM, Zhang H, Yu Y, Wang H, Tang C, Zhao Q, Qin Y, Zhang J. 2023. Carotenoid enrichment in eggs: from biochemistry perspective. Anim Nutr. 14:315–333. doi: 10.1016/j.aninu.2023.05.012.
- E X, Shao D, Li M, Shi S, Xiao Y. 2023. Supplemental dietary genistein improves the laying performance and antioxidant capacity of Hy-Line brown hens during the late laying period. Poult Sci. 102(5):102573. doi: 10.1016/j.psj.2023.102573.
- Gao S, Li R, Heng N, Chen Y, Wang L, Li Z, Guo Y, Sheng X, Wang X, Xing K, et al. 2020. Effects of dietary supplementation of natural astaxanthin from Haematococcus pluvialis on antioxidant capacity, lipid metabolism, and accumulation in the egg yolk of laying hens. Poultry Sci. 99(11):5874–5882. doi: 10.1016/j.psj.2020.08.029.
- Guo S, Lei J, Liu L, Qu X, Li P, Liu X, Guo Y, Gao Q, Lan F, Xiao B, et al. 2021. Effects of Macleaya cordata extract on laying performance, egg quality, and serum indices in Xuefeng black-bone chicken. Poultry Sci. 100(4):101031. doi: 10.1016/j.psj.2021.101031.
- Huang Z, Lv Z, Dai H, Li S, Jiang J, Ye N, Zhu S, Wei Q, Shi F. 2022. Dietary mulberry-leaf flavonoids supplementation improves liver lipid metabolism and ovarian function of aged breeder hens. J Anim Physiol Anim Nutr. 106(6):1321–1332. doi: 10.1111/jpn.13658.
- Janmohammadi H, Hosseintabar-Ghasemabad B, Oliyai M, Alijani S, Gorlov IF, Slozhenkina MI, Mosolov AA, Suarez RL, Seidavi A, Laudadio V, et al. 2023. Effect of dietary amaranth (Amaranthus hybridus chlorostachys) supplemented with enzyme blend on egg quality, serum biochemistry and antioxidant status in laying hens. Antioxidants. 12(2):456. doi: 10.3390/antiox12020456.
- Jeon Y, Kang S, Moon K, Lee J, Kim D, Kim W, Kim J, Ahn B, Jin J. 2018. The effect of Aronia Berry on type 1 diabetes in vivo and in vitro. J Med Food. 21(3):244–253. doi: 10.1089/jmf.2017.3939.
- Jing B, Xiao H, Yin H, Wei Y, Wu H, Zhang D, Tsopmejio ISN, Shang H, Jin Z, Song H. 2022. Feed supplemented with Aronia melanocarpa (AM) relieves the oxidative stress caused by ovulation in peak laying hens and increases the content of yolk precursors. Animals. 12(24):3574. doi: 10.3390/ani12243574.
- Jurendić T, Ščetar M. 2021. Aronia melanocarpa products and by-products for health and nutrition: a review. Antioxidants. 10(7):1052. doi: 10.3390/antiox10071052.
- Kabir MA, Rabbane MG, Hernandez MR, Shaikh M, Moniruzzaman M, Chang X. 2023. Impaired intestinal immunity and microbial diversity in common carp exposed to cadmium. Comp Biochem Physiol C Toxicol Pharmacol. 276:109800. doi: 10.1016/j.cbpc.2023.109800.
- Kang S, Jeon Y, Moon K, Lee J, Kim D, Kim W, Myung H, Kim J, Kim H, Bang K, et al. 2017. Aronia Berry extract ameliorates the severity of dextran sodium sulfate-induced ulcerative colitis in mice. J Med Food. 20(7):667–675. doi: 10.1089/jmf.2016.3822.
- Krysiak K, Konkol D, Korczyński M. 2021. Overview of the use of probiotics in poultry production. Animals. 11(6):1620. doi: 10.3390/ani11061620.
- Li J, Zhou D, Li H, Luo Q, Wang X, Qin J, Xu Y, Lu Q, Tian X. 2022. Effect of purple corn extract on performance, antioxidant activity, egg quality, egg amino acid, and fatty acid profiles of laying hen. Front Vet Sci. 9:1083842. doi: 10.3389/fvets.2022.1083842.
- Liu X, Lin X, Mi Y, Li J, Zhang C. 2018. Grape seed proanthocyanidin extract prevents ovarian aging by inhibiting oxidative stress in the hens. Oxid Med Cell Longev. 2018:9390810. doi: 10.1155/2018/9390810.
- Liu Y, Wang X, Podio NS, Wang X, Xu S, Jiang S, Wei X, Han Y, Cai Y, Chen X, et al. 2023. Research progress on the regulation of oxidative stress by phenolics: the role of gut microbiota and Nrf2 signaling pathway. J Sci Food Agr.104(4):1861–1873. doi: 10.1002/jsfa.13062.
- Ma Y, Shi Y, Wu Q, Ma W. 2021. Dietary arsenic supplementation induces oxidative stress by suppressing nuclear factor erythroid 2-related factor 2 in the livers and kidneys of laying hens. Poult Sci. 100(2):982–992. doi: 10.1016/j.psj.2020.11.061.
- Mahfuz S, Piao XS. 2019. Application of Moringa (Moringa oleifera) as natural feed supplement in poultry diets. Animals. 9(7):431. doi: 10.3390/ani9070431.
- Miao S, Li Y, Mu T, Wang X, Zhao W, Li R, Dong X, Zou X. 2023. Dietary coated sodium butyrate ameliorates hepatic lipid accumulation and inflammation via enhancing antioxidative function in post-peaking laying hens. Metabolites. 13(5):650. doi: 10.3390/metabo13050650.
- Mu K, Kitts DD. 2023. Intestinal polyphenol antioxidant activity involves redox signaling mechanisms facilitated by aquaporin activity. Redox Biol. 68:102948. doi: 10.1016/j.redox.2023.102948.
- Negreanu-Pirjol B, Oprea OC, Negreanu-Pirjol T, Roncea FN, Prelipcean A, Craciunescu O, Iosageanu A, Artem V, Ranca A, Motelica L, et al. 2023. Health benefits of antioxidant bioactive compounds in the fruits and leaves of Lonicera caerulea L. and Aronia melanocarpa (Michx.) Elliot. Antioxidants. 12(4):951. doi: 10.3390/antiox12040951.
- Olechno E, Puścion-Jakubik A, Zujko ME. 2022. Chokeberry (A. melanocarpa (Michx.) Elliott)—a natural product for metabolic disorders? Nutrients. 14(13):2688. doi: 10.3390/nu14132688.
- Radak Z, Pan L, Zhou L, Mozaffaritabar S, Gu Y, A. Pinho R, Zheng X, Ba X, Boldogh I. 2024. Epigenetic and “redoxogenetic” adaptation to physical exercise. Free Radical Bio Med. 210:65–74. doi: 10.1016/j.freeradbiomed.2023.11.005.
- Saberifar T, Samadi F, Dastar B, Hasani S, Kazemifard M, Ganji F. 2021. Enhancement of productive performance, bone physical characteristics, and mineralization of laying hens during the post-peak period by genistein. Arch Razi Inst. 76(2):359–369. doi: 10.22092/ari.2020.342143.1454.
- Sajadi MA, Afrashteh A, Ma W, Xia L, Valilo M, Fakheri H. 2023. The nuclear factor erythroid 2-related factor 2/p53 axis in breast cancer. Biochem Med. 33(3):030504. doi: 10.11613/BM.2023.030504.
- Seidavi A, Tavakoli M, Asroosh F, Scanes CG, Abd El-Hack ME, Naiel MAE, Taha AE, Aleya L, El-Tarabily KA, Swelum AA. 2022. Antioxidant and antimicrobial activities of phytonutrients as antibiotic substitutes in poultry feed. Environ Sci Pollut Res. 29(4):5006–5031. doi: 10.1007/s11356-021-17401-w.
- Selim S, Abdel-Megeid NS, Alhotan RA, Ebrahim A, Hussein E. 2023. Grape pomace: agrifood by-product with potential to enhance performance, yolk quality, antioxidant capacity, and eggshell ultrastructure in laying hens. Vet Sci. 10(7):461. doi: 10.3390/vetsci10070461.
- Sha J, Zhou Y, Yang J, Leng J, Li J, Hu J, Liu W, Jiang S, Wang Y, Chen C, et al. 2019. Maltol (3-hydroxy-2-methyl-4-pyrone) Slowsd-galactose-induced brain aging process by damping the Nrf2/HO-1-mediated oxidative stress in mice. J Agric Food Chem. 67(37):10342–10351. doi: 10.1021/acs.jafc.9b04614.
- Sharma MK, Liu G, White DL, Kim WK. 2023. Graded levels of Eimeria infection linearly reduced the growth performance, altered the intestinal health, and delayed the onset of egg production of Hy-Line W-36 laying hens when infected at the prelay stage. Poult Sci. 103(1):103174. doi: 10.1016/j.psj.2023.103174.
- Shi H, Deng X, Ji X, Liu N, Cai H. 2023. Sources, dynamics in vivo, and application of astaxanthin and lutein in laying hens: a review. Anim Nutr. 13:324–333. doi: 10.1016/j.aninu.2023.02.008.
- Sidor A, Gramza-Michałowska A (2019) Black Chokeberry Aronia melanocarpa L. –a qualitative composition, phenolic profile and antioxidant potential. Molecules. 24(20):3710 doi: 10.3390/molecules24203710.
- Silveira RF, Roque-Borda CA, Vicente EF. 2021. Antimicrobial peptides as a feed additive alternative to animal production, food safety and public health implications: an overview. Anim Nutr. 7(3):896–904. doi: 10.1016/j.aninu.2021.01.004.
- Wan Y, Ma R, Qi R, Lu J, Wang Z, Ma Q, Liu W, Li J, Li Y, Zhan K. 2022. Effects of dietary fermented peony seed dreg on the laying performance, albumen quality, antioxidant capacity, and n-3 PUFA-enriching property of laying hens. Front Vet Sci. 9:1109869. doi: 10.3389/fvets.2022.1109869.
- Wang J, Jia R, Celi P, Ding X, Bai S, Zeng Q, Mao X, Xu S, Zhang K. 2020. Green tea polyphenol epigallocatechin-3-gallate improves the antioxidant capacity of eggs. Food Funct. 11(1):534–543. doi: 10.1039/C9FO02157D.
- Wang JP, He KR, Ding XM, Bai SP, Zeng QF, Zhang KY. 2017. Effect of feeding and withdrawal of vanadium and vitamin C on egg quality and vanadium residual over time in laying hens. Biol Trace Elem Res. 177(2):367–375. doi: 10.1007/s12011-016-0887-9.
- Wen J, Livingston KA, Persia ME. 2019. Effect of high concentrations of dietary vitamin D3 on pullet and laying hen performance, skeleton health, eggshell quality, and yolk vitamin D3 content when fed to W36 laying hens from day of hatch until 68 wk of age. Poultry Sci. 98(12):6713–6720. doi: 10.3382/ps/pez386.
- Wójtowicz A, Combrzyński M, Biernacka B, Różyło R, Bąkowski M, Wojtunik-Kulesza K, Mołdoch J, Kowalska I. 2023. Fresh Chokeberry (Aronia melanocarpa) fruits as valuable additive in extruded snack pellets: selected nutritional and physiochemical properties. Plants. 12(18):3276. doi: 10.3390/plants12183276.
- Wu H, Yuan J, Yin H, Jing B, Sun C, Nguepi TI, Jin Z, Song H. 2023. Flammulina velutipes stem regulates oxidative damage and synthesis of yolk precursors in aging laying hens by regulating the liver-blood-ovary axis. Poult Sci. 102(1):102261. doi: 10.1016/j.psj.2022.102261.
- Xing C, Wang Y, Dai X, Yang F, Luo J, Liu P, Zhang C, Cao H, Hu G. 2020. The protective effects of resveratrol on antioxidant function and the mRNA expression of inflammatory cytokines in the ovaries of hens with fatty liver hemorrhagic syndrome. Poultry Sci. 99(2):1019–1027. doi: 10.1016/j.psj.2019.10.009.
- Yang F, Smith MJ. 2023. Metal profiling in coronary ischemia-reperfusion injury: implications for KEAP1/NRF2 regulated redox signaling. Free Radic Biol Med. 210:158–171. doi: 10.1016/j.freeradbiomed.2023.11.013.
- Yang JX, Chaudhry MT, Yao JY, Wang SN, Zhou B, Wang M, Han CY, You Y, Li Y. 2018. Effects of phyto-oestrogen quercetin on productive performance, hormones, reproductive organs and apoptotic genes in laying hens. J Anim Physiol Anim Nutr. 102(2):505–513. doi: 10.1111/jpn.12778.
- Zhu Y, Cai PJ, Dai HC, Xiao YH, Jia CL, Sun AD. 2023. Black chokeberry (Aronia melanocarpa L.) polyphenols attenuate obesity-induced colonic inflammation by regulating gut microbiota and the TLR4/NF-kappaB signaling pathway in high fat diet-fed rats. Food Funct. 14(22):10014–10030. doi: 10.1039/d3fo02177g.
- Zou L, Yu X, Cai K, Xu B, Chen C, Xiao G. 2023. Identification of antioxidant peptides targeting Keap1 − Nrf2 − ARE pathway from in vitro digestion of pork sausage with partial substitution of NaCl by KCl. Food Res Int. 174(Pt 1):113585. doi: 10.1016/j.foodres.2023.113585.