?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The study investigated the effects of violin note B7 (Violin), double bass note E1 (Bass) and Mozart’s Sonata for Two Pianos in D Major, K. 448 (Mozart), on laying hens in terms of the production performance, egg quality and physiological characteristics. One hundred and sixty 600-day-old laying hens (ISA Brown) were randomly categorised into four groups with four replicates (10 hens/ replicate), and exposed to specific sound frequency for 12 weeks. The results indicated that Violin group presented significantly higher thick albumin height, Haugh unit and yolk index compared to the control group (p < .05). The treatment groups exhibited higher level of glutathione peroxidase in the jejunum–ileum and serum compared to the control group (p < .05). The Violin and Bass groups displayed lower levels of interleukin (IL)-1β in the jejunum–ileum, while the levels of IL-6 were lower in treatment groups compared to the control group (p < .05). The expression of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) gene was lower in the treatment groups than the control group (p < .05). Bass and Mozart groups exhibited lower messenger RNA (mRNA) level of inducible nitric oxide synthase (iNOS) and tumour necrosis factor-α (TNF-α) compared to those in the control group. The number of ASVs (amplicon sequence variants) in the jejunum–ileum of the Violin group was three times higher than that in the control group. These observations suggest that specific sound frequency stimulation can enhance the egg quality and also aid in regulating oxidative stress of laying hens.
Highlights
Specific sound frequency stimulation has the potential to improve egg quality.
Targeted sound frequency stimulation can assist in the regulation of oxidative stress in laying hens.
Introduction
Eggs are rich in nutritional value and aid in physical development of children and prevent malnutrition. For overweight individuals, eggs provide moderate calories, while inducing a sense of satiety. Compared to other sources of protein, eggs are relatively cost-effective (Puglisi and Fernandez Citation2022). People are exploring ways to improve egg production performance while also considering animal welfare concerns.
Exposure of poultry to intermittent loud noise has been found to decrease egg production rates and growth speed, and an increase in mortality rates (Oh et al. Citation2011). Hamm (Citation1967) showed a decline in poultry egg production during prolonged periods of stress (lasting three days or more) caused by aircraft noise, but remains unaffected by a single brief stress event.
Music can affect the chick embryonic brain before hatching (Alworth and Buerkle Citation2013). In chicks, the development of the auditory system begins as early as the 10th day of incubation. By day 16 of incubation, chicken embryos respond to external sounds below 90 decibels (dB) (Hanafi et al. Citation2023). Music has been widely used in the treatment of various captive species (Snowdon Citation2021). Campo et al. (Citation2005) found that specific noise level of 90 dB had a significant negative impact in terms of stress and fear responses in hens, while classical music at 75 dB, did not affect stress levels, but increased fear responses in hens. However, factors like tempo, rhythm, pitch and tonality of music also play a role in influencing the physiological responses of the animal (Alworth and Buerkle Citation2013).
Mozart’s string quartettes possess rich musical tones, which may induce fear in hens and can reduce stress indices in certain breeds of hens (Dávila et al. Citation2011). Nearly, half of the studies on classical music as a stimulus sound, have utilised compositions by Mozart, with some using his work K. 448 (Snowdon Citation2021). Humans prefer harmonious sounds over discordant ones, and poultry are no different (Chiandetti and Vallortigara Citation2011). For animals, music may simply be relatively less stressful and mask ambient environmental sounds. Nevertheless, non-musical sounds can indeed have beneficial effects on animals (Kriengwatana et al. Citation2022). For the welfare of animals, a moderately quiet environment is also crucial; however, playing music for animals can reduce their stress levels, enhance production efficiency and even attract customers to purchase more animal products (Ciborowska et al. Citation2021).
The effects of different sound frequencies on laying hens are still not well understood. Thus, this study aimed to assess the impact of different frequencies to enhance production performance, egg quality and possibly modulate antioxidative status in egg-laying hens.
Materials and methods
The study protocols and the treatment of animals were approved by the Animal Care and Use Committee, National Chung Hsing University (IACUC: 112-062). The study was conducted from May to July 2023.
Animals and experimental design
Laying hens (n = 160, ISA Brown, 600-day-old) were randomly allocated into four groups, including the control, Violin, Bass and Mozart groups for 12 weeks. Each group involved four replicates of 10 birds, housed in single-tier individual wired cages (43 × 40 × 60 cm), with nipple drinkers provided, and each feeder was shared by two hens. The building was divided into four zones, each maintaining comparable environmental conditions and ensuring no sound interference. Hens in the control group were exposed to ambient noise levels from approximately 65 to 70 dB. The Violin group was exposed to approximately 80 dB of violin note B7 (3969.03 Hz), the Bass group was exposed to approximately 80 dB of double bass note E1 (41.39 Hz), and the Mozart group was exposed to nearly 75–80 dB of Mozart’s Sonata for Two Pianos in D Major, K. 448. The basal diets, as listed in Table , were formulated to meet the nutrient requirement of laying hens (National Research Council Citation1994). Throughout the experiment, the hens had ad libitum access to water and feed. During the laying period, lights (2 Lux intensity) were turned on from 0700 h to 2100 h. Daily, from 0800 h to 1100 h and from 1300 h to 1600 h, three types of experimental sounds were played in cycles. Each day, eggs were collected and the egg-laying rate and average egg weight were recorded. Feed consumption was recorded for each replicate at weekly intervals and calculated as gram/day/bird. For each group, the feed conversion rate (FCR) was calculated on a weekly basis, and expressed as the ratio of feed (kg) consumed to kg of egg produced. Throughout the experimental period, five eggs were randomly collected from each replicate to measure the egg quality. Egg weight, eggshell strength, shell thickness, protein height, Haugh unit, yolk colour score, yolk height, yolk width and yolk index were evaluated with the egg quality tester (DET 6000, NABEL Co., Ltd., Kyoto, Japan). The eggshell strength (kg/cm2) was determined by subjecting each egg to a progressively increasing load applied lengthwise until it broke. Eggshell thickness (mm) was determined as an average of measurements taken at three locations on the eggs (sharp section, blunt section and middle section) using a dial pipe gauge. Haugh unit values were determined using the Haugh unit formula based on the albumen height (measured via an albumen height analyser) and egg weight.
Table 1. Composition and calculated analysis (% as fed) of the diet for laying hens.
Serum biochemical determination
Six chickens were randomly selected at week 12 from each group and blood was collected from the wing vein using a sterilised syringe and needle. The serum was naturally separated and the serum samples were centrifuged at 3000 rpm (1480 × g) for 10 min at 4 °C, and stored at −20 °C until further analysis. Each serum sample (1 mL) was then examined using an automatic biochemical analyser (Hitachi, 7150 auto-analyser, Tokyo, Japan) for various parameters including glucose (GLU), blood urea nitrogen (BUN), creatinine (CREA), urea (UA), serum glutamic-oxaloacetic transaminase (SGOT), total protein (T-P), albumin (ALB), globulin (GLO), alkaline phosphatase (Alk-P), cholesterol (CHOL), triglycerides (TGs), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C).
Total RNA extraction and quantitative real-time polymerase chain reaction (RT-PCR) analysis
The middle 2 cm segment of the jejunum–ileum was washed with phosphate-buffered saline, soaked in RNA shield (Zymo Research Co., Irvine, CA), and stored at −20 °C for later use. Tissues were thawed on ice, and 0.1 g of the tissue was soaked in 1 mL of RNAzol (Molecular Research Center, Cincinnati, OH) for disruption. This was centrifuged at 4 °C (12,000 rpm, 10,400 × g) for 15 min, and 400 µL of the supernatant was collected. RNA was extracted using the commercial RNA extraction kit (Zymo Research Co., Irvine, CA) according to the instructions provided, and quantified using absorbance readings at 260/280 nm wavelengths. Subsequently, reverse transcription was performed using a commercial cDNA synthesis kit, Prime Script™ RT reagent Kit with gDNA Eraser (Applied Biosystems, Waltham, MA). The cDNA was quantified, diluted to 300 ng/µL and subjected to RT-PCR using primers (30 µM) on the StepOnePlus Real-Time PCR system (Thermo Fisher, Waltham, MA). All gene expressions were normalised to β-actin, the housekeeping gene. During RT-PCR, 2X SYBR GREEN PCR Master Mix-ROX (Applied Biosystems, Waltham, MA) was used as a fluorescent marker to detect changes in intensity thresholds (ΔCt) of different samples. Changes in Ct values were recorded and compared to that of the housekeeping gene for relative quantitation. The primer sequences used are listed in Table .
Table 2. Characteristics and performance data of the primers used for qPCR analysis.
Enzyme-linked immunosorbent assay (ELISA) analysis of antioxidant activity and inflammatory cytokine in serum and jejunum–ileum
Antioxidant enzyme activity assays were conducted for serum and jejunum–ileum samples using commercial ELISA kits. The kits used for antioxidant activity analysis (Cayman, Ann Arbor, MI) included malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px). Additionally, cytokine analysis (FineTest, Wuhan, China) included parameters such as tumour necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6 and IL-10, while transcription factors (Cayman, Ann Arbor, MI) such as nuclear factor erythroid 2-related factor 2 (Nrf2) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) were also assessed.
Intestinal sample collection
Intestinal contents from the midsections of the chicken’s jejunum–ileum were collected from the abdominal cavity and preserved on ice for microbial analysis. Additionally, midsections of the jejunum–ileum tissues were washed with phosphate-buffered saline (pH 7.2) and preserved in 10% formalin after removing residual chyme from the intestinal walls, awaiting further analysis. A 2 cm segment of the jejunum–ileum tissue was rapidly frozen in liquid nitrogen for ELISA analysis.
Intestinal morphology
Two centimetres segment of the jejunum–ileum was vertically sectioned at 0.5 cm intervals and embedded in paraffin for H&E staining to produce slide samples. Slide samples were observed using an optical microscope and measurements of villus height and crypt depth were conducted using a Mosaic 2.1 analysis system (Tucsen Photonics Co., Ltd., Fuzhou, China). The ratio of Villus height to Crypt depth (V/C) was calculated, and statistical analysis was performed on 30 image sets per treatment group.
Gut microbiome analysis
Microbial composition in the intestinal contents was examined based on the microbial-specific 16S ribosomal RNA gene (16S rRNA gene). Various methods were used to assess the species composition of intestinal microbiota. The group exhibiting the best performance in previous experiments was selected for further analysis, and the microbial compositions of the jejunum–ileum of the control group and the Violin group were compared. The rRNA genes extracted from intestinal microbiota were amplified using labelled 16S universal primers by PCR, and then sequenced. A series of quality control and corrections were made and high-quality and longer sequencing reads (HiFi reads) were obtained. Subsequently, the obtained sequences were further analysed using DADA2, which involved quality control, dereplication and sequence merging steps, which yielded amplicon sequence variants (ASVs). These ASVs were then matched against databases such as NCBI, GreenGenes, SILVA, eHOMD and UNITE. Subsequently, species information was derived and assessed further for species composition, alpha diversity, statistical testing and functional prediction analysis.
Statistical analyses
The data were statistically analysed through the general linear model (GLM) procedure (SAS software, 2004, Cary, NC) following a random arrangement. The data underwent various normality tests using PROC UNIVARIATE with the normal statement.
The mathematic model used was:
where,
is the observed response of laying hens in the pen; µ is the overall mean;
is the fixed effect of sound frequency treatment; and
is the residual error when the pen was the experimental unit,
The experimental unit was analysed based on four groups. Laying hens were randomly distributed in 12 pens following a completely random design. The mean values between four sound frequency treatments were compared using the analysis of variance with significance at p < .05 if the variables were significantly influenced by the treatments.
Results
Performance
The effects of specific sound frequency on laying performance in hens at week 1–12 are listed in Table . Throughout the experimental period, no mortality was recorded among the hens, and all hens appeared to be in good health. Among the groups, the performance of feed intake and laying rate were similar during entire period (p > .05). The Mozart group exhibited a significantly lower egg mass and a significantly higher FCR than the control group (p < .05).
Table 3. Effects of specific sound frequency on the production performance of laying.
Egg quality
Table presents the impacts of specific sound frequency on egg traits in the laying hens at 1–12 weeks. The Violin group had significantly higher thick ALB height and Haugh unit than those of the control group (p < .05). During the study period, the treatments exhibited significantly higher yolk index compared to that of the control group (p < .05). Nevertheless, no obvious differences were observed between groups in terms of egg weight, eggshell strength, shell thickness, yolk colour score, yolk height and yolk width (p > .05).
Table 4. Effects of specific sound frequency on the egg quality of laying hens.
Blood biochemistry
The specific sound frequency did not exhibit any significant changes in the blood biochemical parameters of laying hens at 12 weeks (Table ), and the results were similar across all groups (p > .05).
Table 5. Effects of specific sound frequency on blood biochemistry of laying hens.
Quantitative real-time PCR analysis
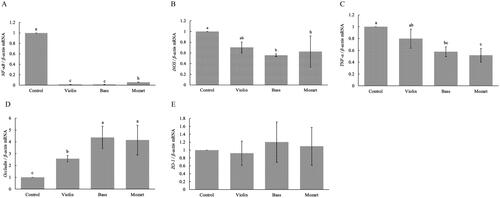
Figure illustrates the effects of specific sound frequency on the expression of pro-inflammatory markers in laying hens at 12 weeks. The levels of NF-κB, inducible nitric oxide synthase (iNOS) and TNF-α had decreased significantly in the Bass and Mozart groups compared to the control group (p < .05). The treatments had considerably higher expression of occludin compared to the control group (Figure ) (p < .05), and the expression levels of zonula occludens-1 did not differ between each group (Figure ) (p > .05).
Figure 1. The effects of specific sound frequency on the mRNA expression levels of antioxidant-regulated genes in laying hens were investigated. The mRNA expression levels of NF-κB (A), iNOS (B), TNF-α (C), Occludin (D) and ZO-1 (E) were examined and exhibited. a–cMeans within the same row with different letters are significantly different (p < .05).
ELISA analysis in serum and intestine
The effects of specific sound frequency on the activities of specific serum antioxidant enzymes from jejunum–ileum and in the laying hens at 12 weeks are displayed in Table . GSH-Px activity of jejunum–ileum and serum had increased significantly in the treatment groups (p < .05). Also, SOD activity of serum in the treatment groups was higher than in the control group; the Violin and Mozart groups had notably higher activity (p < .05). There were no remarkable differences discovered between groups in both MDA and CAT activities in the jejunum–ileum and serum, as well as SOD activities in the jejunum–ileum (p > .05). The effects of specific sound frequency on jejunum–ileum’s immunity of laying hens are presented in Table . The activities of both IL-1β and IL-6 were subsequently diminished in the Violin and Bass groups compared to the control group (p < .05). The activities of TNF-α and IL-10 were similar across all groups (p > .05). The effects of specific sound frequency on the transcription factors, Nrf2 and NF-κB of jejunum–ileum of laying hens between each group did not differ significantly (p > .05) (Table ).
Table 6. Effects of specific sound frequency on jejunum–ileum’s and serum’s antioxidant activity of laying hens.
Table 7. Effects of specific sound frequency on jejunum–ileum’s immunity of laying hens.
Table 8. Effects of specific sound frequency on jejunum–ileum’s transcription factors of laying hens.
Intestinal morphology
The outcomes of the effect of specific sound frequency on the intestinal morphology of the jejunum–ileum in the laying hens at 12 weeks are presented in Figure and Table . Villus height, crypt depth, the ratio of villus height-to-crypt depth, and the muscle layer of the jejunum–ileum did not exhibit substantial differences among the groups (p > .05).
Figure 2. The jejunum–ileum intestinal morphology of laying hens were examined, with presentations of the control group (A), Violin group (B), Bass group (C) and Mozart group (D) jejunum–ileum intestines.
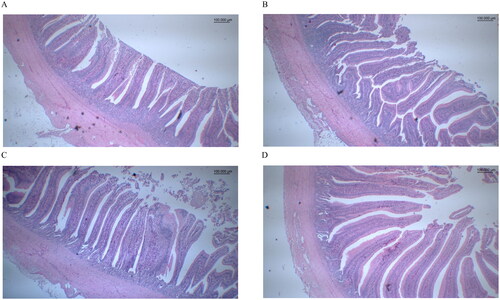
Table 9. Effect of specific sound frequency on jejunum-ileum morphology of laying hens.
Intestinal microbiota
Figure illustrates the influence of specific sound frequency on the microbial composition of jejunum–ileum of laying hens. According to the Venn diagram, only 10.7% of the jejunum–ileum microbiota composition was shared between the control group and the Violin group; in addition, the number of ASVs in the jejunum–ileum of the Violin group was three times greater compared to the control group.
Figure 3. (A) The distribution of amplicon sequence variants (ASVs) across both the control group and the Violin group were analysed. (B) Boxplots illustrating the alpha-diversity indices of both the control group and the Violin group were generated. The Shannon (a) and Simpson (b) indices are used to measure microbial diversity within samples, the observed features (c) represent the actual total species count within samples, the Pielou (d) index estimates species evenness within samples, and the Menhinick (e) and Margalef (f) indices estimate community richness based on the total number of species and total sample size.
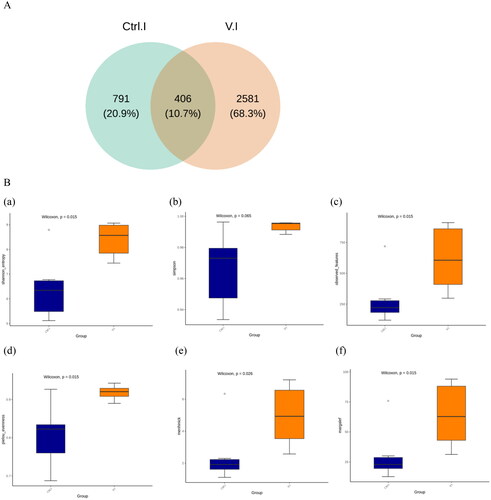
Figure )) presents the results of the differential analysis of alpha diversity, including the Shannon and Simpson indices used to measure microbial diversity within the jejunum–ileum samples. The observed features represent the actual total species count within samples, the Pielou index estimated species evenness within samples, and the Menhinick and Margalef indices estimated community richness based on the total number of species and total sample size. In the findings, except for the Simpson index, all other indices indicated a notable increase in alpha diversity in the Violin group relative to the control group (p < .05).
The relative abundance of top 10 species at levels of family, genus and species in the jejunum–ileum of the control and the Violin groups are presented in Figure )). At the family level, Atopobiaceae was more abundant in the control group than the Violin group. Similarly, at the genus level, higher abundance of Atopobium was observed in the control group compared to the Violin group. At the species level, Atopobium massiliense was also more abundant in the control group than in the Violin group.
Figure 4. (A) Community composition of the gut microbiota among the control group and the Violin group of laying hens were analysed at the family (a), genus (b) and species (c) levels, respectively. (B) Welch’s test indicated significant differences at the genus (a) and species (b) levels. (C) MetagenomeSeq results exhibited significant differences at the genus (a) and species (b) level.
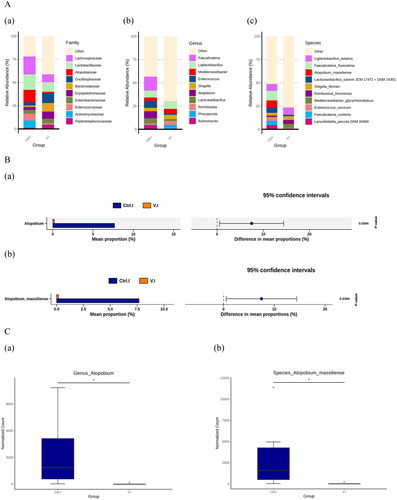
Figure )) presents Welch’s t-test results for the jejunum–ileum at the genus and species levels, and Figure )) presents the results of MetagenomeSeq analysis at the same taxonomic levels. These two findings correspond to the species abundance results presented in Figure )). At the genus level, the abundance of Atopobium in the Violin group was significantly lower compared to the control group (p < .05). Likewise, at the species level, the abundance of Atopobium massiliense in the Violin group was also significantly lower than that in the control group (p < .05).
The functional prediction of jejunum–ileum microbiota in laying hens using PICRUSt2 software is depicted in Figure . The expression level of acetone fermentation pathway (PWY-6588) was significantly higher in the Violin group compared to that in the control group (p < .05). Likewise, the expression of Ribosyn2-pwy pathway, also known as the vitamin B2-synthesis pathway, was significantly higher in the Violin group compared to that in the control group (p < .05).
Discussion
Production performance
Auditory stimuli of livestock animals can impact their production performance. Accordingly, there is still limited research on the effects of single-frequency stimuli on laying hens. Previous studies have shown that playing music during milking sessions can stimulate cows to voluntarily enter the waiting area for milking (Uetake et al. Citation1997; Lemcke et al. Citation2021). Another study reported that exposing young mice to a 432 Hz frequency sound increased the level of ghrelin in their bodies, leading to weight gain (Russo et al. Citation2021). Oh et al. (Citation2011) found that repeated vibration affects the production performance of laying hens, but repeated noise does not affect egg laying rate, egg weight, eggshell thickness, eggshell strength, Haugh unit and xanthophylls. Nevertheless, in the current study, after 12 weeks of the experiment, the Haugh unit in Violin and Mozart groups was significantly higher compared to the control group. This may be related to the duration and type of repeated noise exposure, as the experiments of Oh et al. (Citation2011) lasted only 30 days, while the present study lasted for 84 days (12 weeks).
Natural additives
Plant materials have been utilised to enhance the production and health of laying hens (Lin et al. Citation2017). However, feeding hens with rosemary, oregano and saffron did not notably affect egg production, egg weight, shell thickness, feed conversion ratio and Haugh units (Botsoglou et al. Citation2005). Conversely, Yalçın et al. (Citation2006) found that feeding hens garlic powder led to increase egg weight, with no detrimental effects on egg performance or characteristics. In our previous investigation, we found that dietary garlic scape meal to geese led to a lower feed conversion ratio and did not negatively impact their growth performance (Lin et al. Citation2015). Moreover, Zamanizadeh et al. (Citation2021) observed a marked elevation in the yolk index of quails when fed diets supplemented with Saccharomyces cerevisiae. Hens supplemented with 30% single-cell protein alongside either 50 or 100 mg/kg probiotic showed enhanced egg mass (Dehsahraee et al. Citation2024). In the study, employing sound frequency as a natural supplement resulted in significantly better yolk index compared to that of the control group. Additionally, the violin note B7 and bass note E1 improved egg mass and feed conversion ratio. Furthermore, violin note B7 exhibited significantly higher ALB height and Haugh unit values.
Antioxidant activity and inflammatory cytokine
Redox signalling, in addition to the activation of key transcription factors like Nrf2 and NF-κB, triggers the expression of specific genes related to antioxidant response elements. This leads to the production of crucial antioxidant enzymes such as SOD, GSH-Px, CAT, GSH reductase and GSH transferase, recognised as basis of the antioxidant system regulation. These mechanisms play a vital role in establishing effective antioxidant defence amidst stressful conditions (Surai et al. Citation2017). GSH-Px is a primary antioxidant enzyme responsible for scavenging the super oxygen free radical, superoxide and hydrogen peroxide, while also diminishing or inhibiting the generation of free radicals (Zhao et al. Citation2016). During stressful periods, pro-inflammatory cytokines IL-1β and IL-6 are generated to regulate the acute phase response to stress (Kushner Citation1993). In our previous study, we found that Phyllanthus emblica in broilers significantly reduces MDA levels while the activities of SOD, CAT and GSG-Px were enhanced (Lee et al. Citation2023). The addition of vitamin E and Otostegia persica leaf extract as dietary supplements could augment SOD activity in the thigh meat of broilers (Jafari et al. Citation2021). Gao et al. (Citation2023) revealed that plants as well as music can serve as natural antioxidants; hence, we hypothesise that the violin note B7 potentially enhances antioxidant activity, as evidenced by significantly higher levels of GSH-Px and significantly lower levels of pro-inflammatory cytokines, IL-1β and IL-6, in the jejunum–ileum and the serum the hens in the Violin group compared to those in the control group. These findings suggest that exposure to the violin note B7 may elevate the antioxidant capacity of laying hens.
The transcription factor NF-κB is closely associated with inflammatory responses; and regulates the expression of many pro-inflammatory factors, such as iNOS, IL and TNF-α (Li et al. Citation2021). In the current study, the relative expression of NF-κB, iNOS and TNF-α mRNAs decreased in groups subjected to specific sound frequency stimuli than those in the control group. Thus, specific sound frequency stimuli may reduce the inflammatory response in laying hens.
Intestinal morphology
The intestinal microbiota of laying hens significantly impact better nutrient utilisation, maintain intestinal barrier function, improves production performance and enhance egg quality (Dai et al. Citation2022). Occludin, as a major component of tight junctions, participates in regulating inter-membrane- and paracellular-diffusion of small molecules, playing a crucial role in maintaining intestinal health (Chen et al. Citation2015). Wang et al. (Citation2023) identified more than 1000 microbial species in intestines of chicken. They analysed the faeces of Isa laying hens and identified 2528 bacterial species during the early laying period and 1812 species during the peak laying period. In this study, the Violin group had nearly 3000 bacterial species, indicating a richer intestinal microbiota compared to that of the control group. These results suggest that the violin note B7 may increase the richness of the intestinal microbiota in laying hens. Additionally, the expression of occludin mRNA was relatively significantly increased in groups subjected to specific auditory stimuli, suggesting that specific sound frequency stimuli may aid in maintaining a healthier intestinal tract in laying hens.
The Lachnospiraceae family is recognised for producing short-chain fatty acids (SCFA). These SCFAs contribute to better health, provide nutrients to the host, supply energy to the epithelium of the colon, regulate colon pH and maintain immune balance (Hamid et al. Citation2019). However, the role of Lachnospiraceae in the human gut is controversial. Inflammatory conditions, such as metabolic syndrome, obesity, diabetes, liver diseases, inflammatory bowel disease (IBD) and chronic kidney disease (CKD) are all associated with the Lachnospiraceae family or specific taxa of Lachnospiraceae (Vacca et al. Citation2020). In this study, at the level of family, Lachnospiraceae was relatively abundant in the control as well as Violin groups, and there was no significant difference between these two groups. Therefore, the control and Violin groups are capable of producing SCFA that contribute to better health and maintain a healthy immune balance.
Ligilactobacillus aviarius belongs to the Lactobacillaceae family. Ligilactobacillus aviarius is one of the most abundant microbes in the gut of laying hens; its abundance is associated with weight gain or feed conversion, which may also lead to increased intestinal mucosal permeability in laying hens (Hattab et al. Citation2023). In the present study, although the number of Ligilactobacillus aviarius in the Violin group was slightly higher than that in the control group, the FCR was lower in the Violin group. Therefore, it is speculated that the correlation between Ligilactobacillus aviaries and the FCR in this experiment is not significant.
Throughout reproductive years, Lactobacillus spp. commonly dominate the microbiota in a healthy vagina (Turovskiy et al. Citation2011; de Souza et al. Citation2023). Nonetheless, culture-independent methodologies have revealed that a significant proportion (7–33%) of healthy women exhibit low levels of Lactobacillus species in the vaginal microbiome, which may not necessarily indicate an abnormal condition (Lamont et al. Citation2011). Therefore, the abundance of Lactobacillus spp. alone cannot determine the health status of the vagina. The dominant species in this study were not Lactobacillus either. Collins and Wallbanks (Citation1992) identified the genus Atopobium, while Bordigoni et al. (Citation2020) identified Atopobium massiliense in a vaginal sample from a French woman with vaginosis. When laying hens were stimulated with the violin note B7, in the abundance of Atopobiaceae, Atopobium and Atopobium massiliense decreased significantly, compared to the control group. We propose that exposure to violin note B7 may lead to a healthier uterine environment in hens, reducing the likelihood of vaginal diseases. This is attributed to the fact that previous tests on immunity and antioxidant activity of hens exposed to violin note B7 exhibited enhanced abilities to resist oxidative stress and inflammation.
Conclusions
To conclude, the results indicated that hens exposed to violin note B7 have significantly better Haugh unit and yolk index, significantly higher GSH-Px activity in both jejunum–ileum and serum, and remarkably higher SOD activity in the serum. Furthermore, these hens exhibited substantially lower levels of IL-1β and IL-6, significantly lower NF-κB mRNA expression levels, and significantly higher occludin mRNA expression levels. This study also indicates that these hens may possess a healthier vaginal environment, as evidenced by significantly lower abundance of Atopobiaceae, Atopobium and Atopobium massiliense compared to hens not exposed to the violin note B7. Therefore, violin note B7 can serve as a natural approach for alleviating stress in hens and improving their health.
Author contributions
Conceptualisation, Yi Chen Li; materials analysis, Yi Chen Li, Yung Hao Chen and Shen Chang Chang; methodology, Min Jung Lin; supervision, Li Jen Lin; investigation, Yi Chen Li; writing – original draft preparation, Yi Chen Li; writing – review and editing, Tzu Tai Lee.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data presented in this study are available on request from the corresponding author upon reasonable request.
Additional information
Funding
References
- Alworth LC, Buerkle SC. 2013. The effects of music on animal physiology, behavior and welfare. Lab Anim. 42(2):54–61. doi: 10.1038/laban.162.
- Bordigoni A, Lo CI, Yimagou EK, Nicaise B, Diop K, Raoult D, Desnues C, Fenollar F. 2020. Two new bacteria isolated from vagina of a patient with vaginosis: Atopobium massiliense sp. nov. and Butyricimonas vaginalis sp. nov. New Microbes New Infect. 38:100771. doi: 10.1016/j.nmni.2020.100771.
- Botsoglou N, Florou-Paneri P, Botsoglou E, Dotas V, Giannenas I, Koidis A, Mitrakos P. 2005. The effect of feeding rosemary, oregano, saffron and α-tocopheryl acetate on hen performance and oxidative stability of eggs. S Afr J Anim. 35:143–151. doi: 10.4314/sajas.v35i3.4053.
- Campo JL, Gil MG, Dávila SG. 2005. Effects of specific noise and music stimuli on stress and fear levels of laying hens of several breeds. Appl Anim Behav Sci. 91(1–2):75–84. doi: 10.1016/j.applanim.2004.08.028.
- Chen J, Tellez G, Richards JD, Escobar J. 2015. Identification of potential biomarkers for gut barrier failure in broiler chickens. Front Vet Sci. 2:14. doi: 10.3389/fvets.2015.00014.
- Chiandetti C, Vallortigara G. 2011. Chicks like consonant music. Psychol Sci. 22(10):1270–1273. doi: 10.1177/0956797611418244.
- Ciborowska P, Michalczuk M, Bień D. 2021. The effect of music on livestock: cattle, poultry and pigs. Animals. 11(12):3572. doi: 10.3390/ani11123572.
- Collins MD, Wallbanks S. 1992. Comparative sequence analyses of the 16S rRNA genes of Lactobacillus minutus, Lactobacillus rimae and Streptococcus parvulus: proposal for the creation of a new genus Atopobium. FEMS Microbiol Lett. 74(2–3):235–240. doi: 10.1016/0378-1097(92)90435-q.
- Dai D, Qi GH, Wang J, Zhang HJ, Qiu K, Wu SG. 2022. Intestinal microbiota of layer hens and its association with egg quality and safety. Poult Sci. 101(9):102008. doi: 10.1016/j.psj.2022.102008.
- Dávila S, Campo J, Gil M, Prieto M, Torres O. 2011. Effects of auditory and physical enrichment on 3 measurements of fear and stress (tonic immobility duration, heterophil to lymphocyte ratio, and fluctuating asymmetry) in several breeds of layer chicks. Poult Sci. 90(11):2459–2466. doi: 10.3382/ps.2011-01595.
- de Souza SV, Monteiro PB, de Moura GA, Santos NO, Fontanezi CTB, de Almeida Gomes I, Teixeira CA. 2023. Vaginal microbioma and the presence of Lactobacillus spp. as interferences in female fertility: a review system. JBRA Assist Reprod. 27(3):496–506. doi: 10.5935/1518-0557.20230006.
- Dehsahraee RR, Mahdavi AH, Sedghi M, Saleh H. 2024. Effect of different levels of single cell protein and probiotic microorganisms on performance, immunological responses, and intestinal histology in laying hens. J Anim Physiol Anim Nutr. doi: 10.1111/jpn.13963.
- Gao X, Gong J, Yang B, Liu Y, Xu H, Hao Y, Jing J, Feng Z, Li L. 2023. Effect of classical music on growth performance, stress level, antioxidant index, immune function and meat quality in broilers at different stocking densities. Front Vet Sci. 10:1227654. doi: 10.3389/fvets.2023.1227654.
- Hamid H, Zhang J, Li W, Liu C, Li M, Zhao L, Ji C, Ma Q. 2019. Interactions between the cecal microbiota and non-alcoholic steatohepatitis using laying hens as the model. Poult Sci. 98(6):2509–2521. doi: 10.3382/ps/pey596.
- Hamm D. 1967. Sensory stress effect on layers. Poult Sci. 46:1267.
- Hanafi SA, Zulkifli I, Ramiah SK, Chung ELT, Kamil R, Awad EA. 2023. Prenatal auditory stimulation induces physiological stress responses in developing embryos and newly hatched chicks. Poult Sci. 102(2):102390. doi: 10.1016/j.psj.2022.102390.
- Hattab J, Marruchella G, Sibra A, Tiscar PG, Todisco G. 2023. Canaries’ microbiota: the gut bacterial communities along one female reproductive cycle. Microorganisms. 11(9):2289. doi: 10.3390/microorganisms11092289.
- Jafari S, Saleh H, Mirakzehi MT. 2021. Performance, immune response, and oxidative status in broiler chicken fed oxidized oil and Otostegia persica leaf extract. Ital J Anim Sci. 20(1):878–886. doi: 10.1080/1828051X.2021.1929522.
- Kriengwatana BP, Mott R, Ten Cate C. 2022. Music for animal welfare: a critical review & conceptual framework. Appl Anim Behav Sci. 251:105641. doi: 10.1016/j.applanim.2022.105641.
- Kushner I. 1993. Regulation of the acute phase response by cytokines. Perspect Biol Med. 36(4):611–622. doi: 10.1353/pbm.1993.0004.
- Lamont RF, Sobel JD, Akins RA, Hassan SS, Chaiworapongsa T, Kusanovic JP, Romero R. 2011. The vaginal microbiome: new information about genital tract flora using molecular based techniques. BJOG. 118(5):533–549. doi: 10.1111/j.1471-0528.2010.02840.x.
- Lee TT, Zheng H, Shih C, Chang SC, Lin LJ. 2023. Effects of Phyllanthus emblica leaves and branches mixture on growth performance, oxidative status and intestinal characteristics in broiler chickens. Ital J Anim Sci. 22(1):677–694. doi: 10.1080/1828051X.2023.2235403.
- Lemcke MC, Ebinghaus A, Knierim U. 2021. Impact of music played in an automatic milking system on cows’ milk yield and behavior – a pilot study. Dairy. 2(1):73–78. doi: 10.3390/dairy2010007.
- Li C, Zhang R, Wei H, Wang Y, Chen Y, Zhang H, Li X, Liu H, Li J, Bao J. 2021. Enriched environment housing improved the laying hen’s resistance to transport stress via modulating the heat shock protective response and inflammation. Poult Sci. 100(3):100939. doi: 10.1016/j.psj.2020.12.036.
- Lin MJ, Chang SC, Jea YS, Chen WS, Lee TT. 2015. Effects of dietary garlic scape meal on the growth and meat characteristics of geese. Br Poult Sci. 56(6):716–722. doi: 10.1080/00071668.2015.1096012.
- Lin WC, Lee MT, Chang SC, Chang YL, Shih CH, Yu B, Lee TT. 2017. Effects of mulberry leaves on production performance and the potential modulation of antioxidative status in laying hens. Poult Sci. 96(5):1191–1203. doi: 10.3382/ps/pew350.
- National Research Council, and Subcommittee on Poultry Nutrition. 1994. Nutrient requirements of poultry: 1994. National Academies Press.
- Oh TK, Lee SJ, Chang DI, Jiro C, Hong-Hee C. 2011. The effects of noise and vibration generated by mechanized equipment in laying hen houses on productivity. J Fac Agric Kyushu Univ. 56(2):271–277. doi: 10.5109/20320.
- Puglisi MJ, Fernandez ML. 2022. The health benefits of egg protein. Nutrients. 14(14):2904. doi: 10.3390/nu14142904.
- Russo C, Patanè M, Pellitteri R, Stanzani S, Russo A. 2021. Prenatal music exposure influences weight, ghrelin expression, and morphology of rat hypothalamic neuron cultures. Int J Dev Neurosci. 81(2):151–158. doi: 10.1002/jdn.10084.
- Snowdon CT. 2021. Animal signals, music and emotional well-being. Animals. 11(9):2670. doi: 10.3390/ani11092670.
- Surai P, Kochish I, Fisinin V. 2017. Antioxidant systems in poultry biology: nutritional modulation of vitagenes. Eur J Poult Sci. 81. doi: 10.1399/eps.2017.214.
- Turovskiy Y, Sutyak Noll K, Chikindas ML. 2011. The aetiology of bacterial vaginosis. J Appl Microbiol. 110(5):1105–1128. doi: 10.1111/j.1365-2672.2011.04977.x.
- Uetake K, Hurnik JF, Johnson L. 1997. Effect of music on voluntary approach of dairy cows to an automatic milking system. Appl Anim Behav Sci. 53(3):175–182. doi: 10.1016/S0168-1591(96)01159-8.
- Vacca M, Celano G, Calabrese FM, Portincasa P, Gobbetti M, De Angelis M. 2020. The controversial role of human gut Lachnospiraceae. Microorganisms. 8(4):573. doi: 10.3390/microorganisms8040573.
- Wang XY, Meng JX, Ren WX, Ma H, Liu G, Liu R, Geng HL, Zhao Q, Zhang XX, Ni HB. 2023. Amplicon-based metagenomic association analysis of gut microbiota in relation to egg-laying period and breeds of hens. BMC Microbiol. 23(1):138. doi: 10.1186/s12866-023-02857-2.
- Yalçın S, Onbaşılar EE, Reisli Z, Yalçın S. 2006. Effect of garlic powder on the performance, egg traits and blood parameters of laying hens. J Sci Food Agric. 86(9):1336–1339. doi: 10.1002/jsfa.2515.
- Zamanizadeh A, Mirakzehi MT, Agah MJ, Saleh H, Baranzehi T. 2021. A comparison of two probiotics Aspergillus oryzae and, Saccharomyces cerevisiae on productive performance, egg quality, small intestinal morphology, and gene expression in laying Japanese quail. Ital J Anim Sci. 20(1):232–242. doi: 10.1080/1828051X.2021.1878944.
- Zhao F, Shi B, Sun D, Chen H, Tong M, Zhang P, Guo X, Yan S. 2016. Effects of dietary supplementation of Artemisia argyi aqueous extract on antioxidant indexes of small intestine in broilers. Anim Nutr. 2(3):198–203. doi: 10.1016/j.aninu.2016.06.006.