Abstract
The present work focused on examining the function of rumen-protected L-arginine (RP-Arg) or N-carbamylglutamate (NCG) in jejunal oxidative resistance, integrity and immune function in the ovine foetal model of intrauterine growth restriction (IUGR). Thirty-two twin-bearing Hu ewes at d 35 of gestation were randomised as 4 treatment groups (n = 8 each): Control (CON), received 100% of the recommended National Research Council (NRC) for pregnancy; Restricted (RES), received 50% of the recommended NRC for pregnancy; RES + ARG, RES ewes added with 20 g/d of RP-Arg; or RES + NCG treatment, RES ewes added with 5 g/d of NCG. Foetal jejunal samples were collected on d 110 of pregnancy and were assayed for biomarkers of oxidative damage, integrity and immune function. The villus height was elevated (p < .05) within the jejunum of the foetuses of RES ewes subjected to dietary NCG or Arg supplementation relative to the RES group. RES + NCG or RES + ARG feeding decreased (p < .05) foetal jejunal tumour necrosis factor-α (TNF-α) and Interleukin (IL)-6 levels and elevated (p < .05) foetal jejunal superoxide dismutase (SOD) activity (p < .05) in relative to RES group. The Arg/NCG supplementation downregulated (p < .05) expression of gene and proteins associated with inflammatory response (TNF-α), upregulated (p < .05) genes and proteins associated with antioxidation (catalase and SOD2) and integrity (claudin-1) relative to those within foetal jejunum of RES group. In conclusion, Arg and NCG supplementation of RES ewes alleviates foetal jejunal oxidative stress, improves integrity, and promotes foetal intestinal development in the ovine foetus with IUGR.
HIGHLIGHTS
NCG or Arg can improve the foetal jejunal barrier function in underfed ewes
NCG or Arg can alleviate foetal jejunal oxidative stress in underfed ewes
NCG or Arg can activate the foetal jejunal NO-dependent pathway
Introduction
Pregnant mothers exposed to undernutrition may cause serious and long-term physical and mental sequelae for foetuses in utero (Xue et al. Citation2019). Optimal maternal nutrition in pregnancy is crucial for the development of the small intestine in the foetus (Meyer and Caton Citation2016). Maternal undernutrition-induced intrauterine growth restriction (IUGR) can be related to abnormal gut development and function among foetuses and neonates in various animal models (Meyer and Caton Citation2016). As the intestinal lumen is in direct contact with oxidants either from microbiota or nutrient intake, it is likely to be affected by oxidative injury induced by the IUGR (Zhang et al. Citation2022). The IUGR foetal gut undergoes several oxidative stress-associated alterations (i.e. higher protein and enzyme levels related to protein degradation and apoptosis, whereas lower protein levels needed to maintain cellular motility and structure, nutrient transport and uptake protein synthesis, and energy metabolism) that may mediate foetal intestinal development, thus inducing the perinatal arrest of intestinal growth, dysfunction and atrophy (Baumgarten et al. Citation2020).
L-arginine (Arg), the essential amino acid in juvenile mammals, particularly those experiencing stress conditions, plays an important role in numerous metabolic pathways (Nüse et al. Citation2023) whereas its precursor, N-carbamylglutamate (NCG), is found to elevate plasma Arg levels together with endogenous Arg production via the activation of intestinal pyrroline-5-carboxylate synthase and carbamylphosphate synthase-1 (Ye et al. Citation2017). Under normal conditions, Arg promotes nitric oxide (NO) production within an identical intestine, thus exerting an important effect on modulating intestinal antioxidant defense (Weckman et al. Citation2019). Recently, energy-restricted ewes bearing multiple foetuses showed an increase in circulating Arg and its metabolites involved in NO production emphasising the importance of the Arg-NO pathway during pregnancy (Berlinguer et al. Citation2019). Also, dietary rumen-protected Arg (RP-Arg) and NCG addition can increase foetal body weight (BW) as well as small intestinal weight in underfed ewes (Zhang et al. Citation2016). These positive changes might be attributed to the enhanced nutrient delivery into the placenta, however, the underlying mechanisms of RP-Arg and NCG addition-induced increase of foetal small intestinal weight are not completely understood. Also, whether maternal dietary Arg or NCG addition in the process for IUGR changes the small intestine redox status, integrity and immune function in foetuses is still unclear.
Considering ethical issues, a pregnant ewe model of maternal undernutrition is utilised for investigating the development and physiology of foetuses (Xue et al. Citation2019). We assumed that maternal Arg or NCG added in diet can promote foetal intestinal growth by reducing oxidative stress (OS) in foetuses exposed to IUGR in underfed ewes. Therefore, the present work aimed to prove this hypothesis and to offer a theoretical basis for using Arg/NCG as the functional component in feeds of pregnant ewes at risk of undernutrition.
Materials and methods
Animal ethics
Every procedure was carried out according to the protocols from the Guide for the Care and Use of Laboratory Animals released by the Ethics Committee of Yang Zhou University (SYXK2013-0057).
Animals, housing, and management
All experiments were performed at the Jiangyan Experimental Station in Taizhou of Jiangsu (China). Altogether 48 multiparous Hu ewes of comparable age (18.5 ± 0.5 months) and BW (40.1 ± 1.2 kg) body condition score (BCS; 2.55 ± 0.18: 0–5 indicating thin-obese; Russel et al. Citation1969) were housed in an indoor barn containing the heating radiators for maintaining environmental temperature under 15.3 °C ± 0.82 °C and automatically-controlled lighting for mimicking normal environmental light cycle (12 L:12D). 0.2 mg/kg antiparasitic ivermectin was added for ewe drenching, followed by synchronisation for oestrus using the intravaginal progestogen sponge protocol (30 mg; Pharmp) for 12 d. On d 2 after the removal of the sponge, ewes were subjected to 3 vasectomised rams (at 08:00 and 16:00) for detecting the oestrus behaviour, and artificial insemination (AI) was performed accordingly (Purdy et al. Citation2020). Briefly, AI was performed with the sheep AI gun (All-2-Mate Insemination Gun, Continental Plastics, Delavan, WI, USA). 0.1 mL fresh semen was deposited for per ewe at the 70–75 × 106 motile sperm/AI insemination dose. Those inseminated ewes (on 0 gestational day) were raised in individual pens (1.05 m × 1.60 m) for a 35-day period. Ewes were fed once a day (at 08:00) with a diet (Supplementary Table 1) matching 100% of their nutrient requirements for gestation according to the National Research Council (NRC, Citation2007) with free access to clean water on 0–35 gestational days. On 35 gestational days, twin pregnancies were diagnosed by ultrasonography (Asonics Microimager 1000 s for scanning instrument; Ausonics) and accordingly 32 ewes carrying twin foetuses were chosen from those initial 48 ewes for the implementation of the ovine model of IUGR in this study.
Experimental design
On 35 gestational days, the 32 ewes that carried twin foetuses were randomly classified as 4 treatment groups (n = 8 per group): Control (CON), received 100% of NRC (Citation2007); Restricted feeding (RES), received 50% of NRC (Citation2007); RES + ARG, RES ewes treated using RP-Arg at 20 g/d; or RES + NCG, RES ewes treated using NCG at 5 g/d. RP-Arg (Beijing Feeding Feed Science Technology Co., Beijing, China) and the NCG (Institute of Subtropical Agriculture, The Chinese Academy of Sciences, Changsha, China) contain 50% Arg and NCG, respectively (Zhang et al. Citation2016). Thus, actual Arg and NCG supplementation were 10 and 2.5 g/d, separately. The RP-Arg is made up of a matrix of glycerides and phospholipids prepared via spray congealing as well as spray drying procedures (Corzo et al. Citation2021). Ruminal protection of Arg is estimated to be ≥ 85%, whereas the intestinal release of Arg is predicted to be ≥ 90% (Chacher et al. Citation2012). We analysed Arg dose according to prior research on pregnant ewes that received parenteral Arg or RP-Arg addition (Zhang et al. Citation2016), and confirmed NCG dose as previously described in piglets (Zeng et al. Citation2012), dairy cows (Chacher et al. Citation2014), together with pregnant sheep (Zhang et al. Citation2016). The restricted feeding regimen was adopted by giving 50% of total diet analysed to satisfy the 100% pregnancy NRC set by the NRC (Citation2007). From 35 gestational days, we determined BW at 10-day intervals, then adjusted feed intake correspondingly. All diets were in the form of pellets included ground forage and the experiment continued until d 110 of gestation.
Foetal intestinal sampling
On 110 gestational days at 08:00 am, every ewe was stunned using a captive bolt gun (Supercash Mark 2; Acceles and Shelvoke), and later euthanasia via exsanguination was completed. Then, the weights of the foetus and the foetal small intestine were measured and recorded and intestinal samples were collected simultaneously by multiple teams (Zhang et al. Citation2016). Approximately 2 cm jejunum was preserved within 4% paraformaldehyde solution to analyse tissue histology while 10 g jejunal samples (washed by pre-chilled PBS) were subjected to immediate freezing within liquid nitrogen prior to preservation under −80 °C till subsequent analyses (Caleb et al. Citation2021).
Foetal jejunal morphological analysis
The 4% paraformaldehyde-fixed jejunal specimens were washed using gradient ethanol and xylene and then exposed to paraffin embedding upon drying. This experiment later prepared five slides (three 5-μm sections each) based on paraffin-embedded jejunal specimens followed by deparaffinization with xylene and rehydration using gradient ethanol dilutions. Each slide was stained using haematoxylin and eosin (H&E) to measure intestinal morphologies using the 20 well-oriented crypts and villi in every section (Optimus software, version 6.5, Media Cybernetics, USA). We later determined the villi to crypt ratio (VCR) and calculated the goblet cell number in each villus (NIS-Elements BR 2.3, Nikon France SAS). Goblet cells within foetal jejunum segments were subjected to periodic acid-Schiff (PAS) staining, later, the PAS+ cell number in each villus was measured and counted via Image-Pro Plus 6.0 software (Media Cybernetics, USA) (Xie et al. Citation2020). Values from 10 villi selected from 20 randomly well-oriented villi in every small intestine segment were determined to take the average value (Zhang et al. Citation2019a). All histology was performed and analysed by the same user.
Insulin, insulin-like growth factor 1 (IGF-1), NO, NO synthase (NOS) and protein levels within the foetal jejunum
Foetal jejunum (about 0.5 g) preserved under −80 °C was washed, followed by homogenisation within a 0.85% cold normal saline for obtaining the 10% (1:10; w/v) homogenate for the measurement of IGF-1, insulin, NO, NOS and protein content following the specific instructions (Zhang et al. Citation2019b). Jejunal protein expression was analysed with a bicinchoninic acid (BCA) protein detection kit (Pierce, Rockford, IL, USA). All samples were measured on an individual plate, with intra-assay CVs being <10%.
Analysis of cytokine in foetal jejunal tissues
We later utilised a 10% foetal jejunum homogenate for analysing cytokine contents. Interleukin-1β (IL-1β) (catalog numbers CB10009-Sp, COIBO BIO, Shanghai, China), IL-6 (catalog numbers CB10013-Sp, COIBO BIO, Shanghai, China), and tumour necrosis factor-α (TNF-α) (catalog numbers CB10066-Sp, COIBO BIO, Shanghai, China) were determined by respective commercially available specific ELISA kits. Absorbance was measured at 450 nm by the BioTek synergy HT microplate reader (BioTek Instruments, Winooski, VT, USA), with detection limits being 2.5 pg/mL, 5 pg/mL, and 6.25 pg/mL for IL-1β, IL-6, together with TNF-α, separately, whereas intra-assay variation coefficients being less than 10%. All samples were analysed on a single plate. All values were presented as ng/g protein in jejunal tissues (Zhang et al. Citation2019b).
Enzyme activity assays in foetal jejunal tissues
Foetal jejunum (about 0.5 g) was subjected to homogenisation and centrifuged within the RIPA lysis buffer at 15,000 × g and 4 °C for a 15-min period. The supernatant was later collected for determining the markers related to the OS as well as the activities of antioxidant enzymes. Jejunal protein levels were later analysed with the BCA protein detection kit (Pierce, Rockford, IL, USA). Maleic dialdehyde (MDA), total antioxidant capacity (T-AOC), glutathione peroxidase (GPX) as well as superoxide dismutase (SOD) activities were explored with the specific kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) by using the spectrophotometer (WFJ 2100; UNIC Instrument Co. Ltd., Shanghai, China) (Gu et al. Citation2023). The detection limits were 0.5 nmol/mL, 0.2 U/mL, 0.3 mg/L, and 0.5 U/mL for MDA, T-AOC, GPX, and SOD, respectively. The intra-assay variation coefficients were less than 10%. All samples were analysed on a single plate.
The mRNA levels assay in foetal jejunal tissues
The total RNA was extracted using grinded tissues immersed in liquid nitrogen (60 mg) with the Trizol reagent (1 mL) (Qiagen, Frankfurt, Germany) based on the specific protocol, and also measured through RT-PCR (Zhang et al. Citation2019b). The total RNA concentration and purity were studied with the NanoDrop ND-1000 spectrophotometer (Nano-drop Technologies, DE), while RNA quality was assessed with agarose gel electrophoresis (Wittmeier and Hummel Citation2022). Thereafter, RNA (1 μg) was utilised for cDNA synthesis using a PrimeScriptTM reverse transcription (RT) reagent Kit with a gDNA Eraser (TaKaRa, Dalian, China) contained within the 20 μL reaction system. RT-PCR was conducted via FastStart Universal SYBR Green Master kit (ROX Reference Dye) (Roche, Mannheim, Germany) using the Step One Plus Real-Time PCR System (Applied Biosystems, CA, USA). Primer 5.0 was employed for primer design (Supplementary Table 2). The reaction mixture (20 μL) included fresh SYBR Premix Ex Taq II (10 μL, Tli RNaseH Plus), cDNA (2 μL), ROX Reference Dye II (0.4 μL, 50×), diethylpyrocarbonate-treated water (6 μL), and each primer (forward and reverse) (0.8 μL; 10 μM). The PCR conditions were: 30-s under 95 °C; 5-s under 95 °C for 40 cycles; then 31-s under 60 °C; 15 s under 95 °C, 1 min under 60 °C, followed by 15 s under 95 °C. Amplification efficiency was determined by the previous method (Zhang et al. Citation2019b). Each correlation coefficient (r) for the standard curve was ≥ 0.99, whereas the amplification efficiency value was 90-110%. Amplification specificity was verified by melting curves, and mRNA levels were confirmed by adopting 2−△△Ct approach (Cappelli et al. Citation2021). GeNorm programme was adopted to analyse four reference genes, namely, β-actin, cyclophilin, 18S ribosomal RNA and glyceraldehyde phosphate dehydrogenase (GAPDH), for determining an appropriate reference gene, thus precisely showing relative levels of those chosen markers (Kumar et al. Citation2022). The stability of gene levels (M-value) was later calculated through measuring the overall stability of those analysed reference genes. As β-actin possessed the optimal M-value, which was chosen to be a reference gene to normalise gene levels. Target gene mRNA expression was determined based on CON group (Zhang et al. Citation2019b).
Western blotting
The total protein was extracted based on approximately 100 mg of foetal jejunum by the 1 mL RIPA buffer containing PMSF (Beyotime Biotechnology, Jiangsu, China) following the specific protocol, which is identical to those mentioned above for protein preparations (Sakuma et al. Citation2022). Afterwards, a BCA protein assay kit (Pierce, Rockford, IL, USA) was used to determine protein content. Thereafter, protein samples collected (typically 30 μg) were subjected to denaturation, separation with 5–10% SDS-PAGE (Bio-Rad, Richmond, CA, USA), and later electroblotting on PVDF membranes (0.45 µm, Millipore, Billerica, MA, USA). Thereafter, 5% defatted milk was added to block membranes within Tris-buffered saline that contained Tween 20 (TBST) (0.1% Tween-20, 150 mM NaCl, 20 mM Tris, pH 7.5) for a 3-h period. After that, they were incubated with primary antibodies. The dilutions of primary antibodies were prepared with 5% defatted milk within TBST and included anti-GPX1 (diluted at 1:2000, Protein Tech, Wuhan, China), anti-catalase (anti-CAT, diluted at 1:2000, Protein Tech, Wuhan, China), anti-haem oxygenase (HO)-1 (diluted at 1:10,000, Protein Tech, Wuhan, China), anti-SOD2 (diluted at 1:5000, Protein Tech, Wuhan, China), anti- nuclear factor erythroid 2-related factor (anti-NRF2, diluted at 1:2000, Protein Tech, Wuhan, China), anti-p65 (diluted at 1:1000, Protein Tech, Wuhan, China), anti-quinone oxidoreductase 1 (anti-NQO1, diluted at 1:1000, Protein Tech, Wuhan, China), anti-IL-1β (diluted at 1:1000, Protein Tech, Wuhan, China), anti-pp65 (diluted at 1:1000, Cell Signalling, Shanghai, China), anti-ZO-1 (diluted at 1:5000, Protein Tech, Wuhan, China), anti-TNF-α (diluted at 1:1000, Protein Tech, Wuhan, China), anti-inducible NO synthase (anti-iNOS, diluted at 1:1000, Protein Tech, Wuhan, China), anti-β-actin (diluted at 1:1500, Protein Tech, Wuhan, China), anti-epithelial NO synthase (anti-eNOS, diluted at 1:800, Cell Signalling, Shanghai, China), and anti-claudin-1 (1:1000, Protein Tech, Wuhan, China). All primary antibodies were raised in rabbits. After overnight membrane incubation under 4 °C, membranes were washed using TBST prior to subsequent incubation using horseradish peroxidase (HRP)-labelled goat anti-rabbit secondary antibody (1:5000, Antgene Biotech, HK, China) for one hour. Signals were measured using ECL kits (ECL-Plus, Thermo, Waltham, MA, USA), followed by scanning to identify the fluorescence intensity with a Bio-Rad gel detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Protein expression levels were determined by quantifying band intensities using ImageJ software (Bio-Rad Laboratories Inc., Hercules, CA, USA) with an identical user responsible for all analyses. Densitometric data were standardised on the basis of β-actin and reported in the form of fold-change in comparison with control (Pillai-Kastoori et al. Citation2020). Every measurement was repeated six times.
Statistical methods
Ewes of four groups sacrificed on 110 gestational days were statistically compared by the one-way ANOVA. Data were represented by least squares means ± SEM. Data analyses among different groups of foetuses were carried out by the one-way ANOVA with PROC GLM procedure of SAS version 9.2 (SAS Institute Inc.Cary, NC, USA). Male to female foetal sex ratio was 9:7 however no significance was found (p > .05) when the sex of the foetus was incorporated into the initial model. Therefore, the eventual model contained the maternal treatment group alone. Inter-group difference was identified using Duncan’s multiple comparison test, and p ≤ .05 stood for statistical significance.
Results
Maternal DMI and BW, foetal BW and small intestinal weight
Maternal DMI and BW in the CON group were increased (p < .05) compared with RES, RES + Arg, and RES + NCG groups (Supplementary Table 3).
Foetal intestinal morphology and density of goblet cells
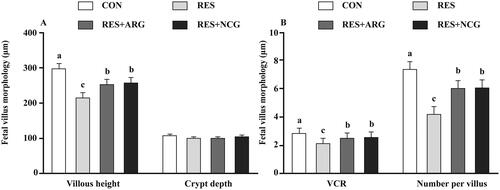
Compared with CON ewes, goblet cell number per villus, villus height, and VCR decreased (p < .05) within the jejunum of the foetuses of the RES group (). In contrast, these variables elevated (p < .05) within the jejunum of the foetuses of RES ewes subjected to dietary NCG or Arg supplementation relative to RES ewes.
Figure 1. Roles of rumen-protected L-arginine (RP-arg) or N-carbamylglutamate (NCG) supplemented in diet in villus morphology of foetal jejunum in underfed Hu ewes at d110 of gestation. Villous height and crypt depth (A), and VCR and number per villus (B) were determined. VCR: villous height: crypt depth ratio; NRC: National Research Council; CON/RES: ewes fed 100%/50% of NRC (Citation2007) recommendations for pregnancy; RES + ARG, ewes fed 50% of NRC (Citation2007) recommendations with supplementation of 20 g/d RP-arg; RES + NCG, ewes fed 50% of NRC (Citation2007) recommendations with supplementation of 5 g/d NCG. Data represent means, and standard errors are shown in vertical bars (n = 8/group for ewes, n = 16/group for the foetus). Labelled means with no identical letters suggest significant differences, p < .05.
Foetal jejunal protein, NO, IGF-1, insulin and NOS contents
In relative to the CON, the RES feeding regimen decreased (p < .05) protein, IGF-1, insulin, NOS, and NO contents in the foetal jejunum (). Relative to the RES group, a marked increase (p < .05) in jejunal contents of these variables was observed in foetuses of NCG- and Arg-supplemented RES ewes (p < .05).
Table 1. Roles of rumen-protected L-arginine (RP-arg) or N-carbamylglutamate (NCG) supplemented in diet in protein, IGF-1, insulin, NO levels, and NOS activities within foetal jejunum of underfed Hu ewes at 110 gestational days.
Foetal jejunal levels of cytokines
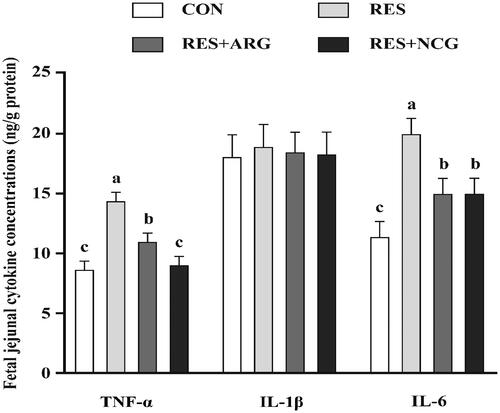
Relative to CON, RES ewes showed an increase (p < .05) with regard to foetal jejunal IL-6 and TNF-α contents (). By contrast, the RES + NCG or RES + ARG feeding decreased (p < .05) foetal jejunal IL-6 and TNF-α contents compared with RES ewes.
Figure 2. Roles of rumen-protected L-arginine (RP-arg) or N-carbamylglutamate (NCG) supplemented in diets in cytokine levels within foetal jejunum of underfed Hu ewes at 110 gestational days. IL, interleukin; TNF-α, tumour necrosis factor α; NRC, National Research Council; CON/RES, ewes fed 100%/50% of NRC (Citation2007) recommendations for pregnancy; RES + ARG, ewes fed 50% of NRC (Citation2007) recommendations with supplementation of 20 g/d RP-arg; RES + NCG, ewes fed 50% of NRC (Citation2007) recommendations with supplementation of 5 g/d NCG. Data represent means, and standard errors are shown in vertical bars (n = 8/group for ewes, n = 16/group for the foetus). Labelled means with no identical letters represent statistical differences, p < .05.
Foetal jejunal antioxidant enzyme activities
Relative to the CON group, the RES feeding regimen decreased (p < .05) foetal jejunal T-AOC activities () which elevated (p < .05) by Arg or NCG treatment in comparison with those of foetuses from the RES group. Also, Arg or NCG treatment reduced (p < .05) foetal jejunal MDA content and elevated (p < .05) foetal jejunal SOD activity relative to RES treatment, respectively.
Table 2. Roles of rumen-protected L-arginine (RP-arg) or N-carbamylglutamate (NCG) supplemented in diet in antioxidant activities within foetal jejunum of underfed Hu ewes at d110 of gestation.
The mRNA level within foetal jejunum
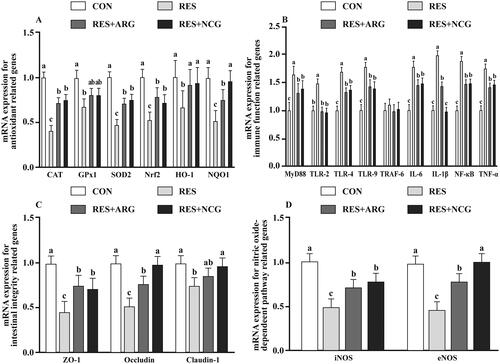
Relative to CON, the MYD88, TLR-2, TLR-4, TLR-9, IL-6, IL-1β, NF-κB and TNF-α mRNA expression was upregulated (p < .05) within foetal jejunum of RES group (). The NCG or Arg treatment downregulated (p < .05) these gene levels compared with the RES group. Compared with the CON, CAT, GPX1, SOD2, NRF2, HO-1, NQO1, ZO-1, occludin, claudin-1, iNOS, and eNOS levels were downregulated (p < .05) within foetal jejunum of RES group. Arg or NCG addition in the RES group upregulated (p < .05) these genes relative to the RES group. Compared with RES + ARG ewes, NQO1, eNOS, and occludin mRNA expression elevated (p < .05), while the IL-1β mRNA level decreased (p < .05), respectively, within foetal jejunum of RES + NCG group.
Figure 3. Roles of rumen-protected L-arginine (RP-arg) or N-carbamylglutamate (NCG) supplemented in diet in gene mRNA levels within foetal jejunum in underfed Hu ewes at 110 gestational days. mRNA expression of anti-oxidation-associated (A), immune-associated (B), intestinal integrity-associated (C), and NO-dependent pathway-associated (D) genes were determined. GPX1: glutathione peroxidase 1; CAT: catalase; SOD2: superoxide dismutase2; HO-1: haem oxygenase-1; NRF2: nuclear factor erythroid 2-related factor 2; MYD88: myeloid differentiation factor 88; NQO1: quinone oxidoreductase 1; TLR: toll-like receptor; TRAF-6: tumour necrosis factor receptor-associated factor 6; TNF-α: tumour necrosis factor α; IL: interleukin; ZO-1: zonula occludens-1; NF-κB: nuclear factor kappa B (p65); NO: nitric oxide; eNOS: epithelial NO synthase; iNOS: inducible NO synthase; NRC: National Research Council; CON/RES: ewes fed 100%/50% of NRC (Citation2007) recommendations for pregnancy; RES + ARG, ewes fed 50% of NRC (Citation2007) recommendations with supplementation of 20 g/d RP-arg; RES + NCG, ewes fed 50% of NRC (Citation2007) recommendations with supplementation of 5 g/day NCG. Data represent means, and standard errors are shown in vertical bars (n = 8/group for ewes, n = 16/group for the foetus). Labelled means with no common letter represent significant differences, p < .05.
Protein expression in foetal jejunum
Relative to CON, RES feeding regimen decreased (p < .05) the CAT, GPX1, NRF2, SOD2, NQO1, HO-1, claudin-1, ZO-1, iNOS and eNOS contents in foetal jejunum (). The NCG or Arg treatment elevated (p < .05) CAT, GPX1, NRF2, SOD2, NQO1, HO-1, claudin-1 ZO-1, iNOS and eNOS protein levels, whereas TNF-α, IL-β, p-p65, and p65 protein levels in foetal jejunum were decreased (p < .05) under Arg or NCG treatment relative to those in the non-supplemented RES ewes (). Compared with the RES + ARG group, CAT and ZO-1 protein expressions were upregulated (p < .05), while the p65 protein level was downregulated (p < .05), respectively, within foetal jejunum of the RES + NCG group.
Figure 4. Roles of rumen-protected L-arginine (RP-arg) or N-carbamylglutamate (NCG) supplemented in diet in antioxidant defence-associated protein levels in foetal jejunum of underfed Hu ewes at 110 gestational days. Typical images showing WB analysis (A) and GPX1, CAT, NRF2, SOD2, NQO-1 and HO-1 levels (B) were measured. GPX1: glutathione peroxidase 1; CAT: catalase; NRF2: nuclear factor erythroid 2-related factor 2; SOD2: superoxide dismutase2; NQO1: quinone oxidoreductase 1; HO-1: haem oxygenase-1; NRC: National Research Council; CON/RES: ewes fed 100%/50% of NRC (Citation2007) recommendations for pregnancy; RES + ARG, ewes fed 50% of NRC (Citation2007) recommendations with supplementation of 20 g/d RP-arg; RES + NCG, ewes fed 50% of NRC (Citation2007) recommendations with supplementation of 5 g/day NCG. Data represent means, and standard errors are shown in vertical bars (n = 8/group for ewes, n = 16/group for the foetus). Labelled means with no identical letters are different, p < .05.
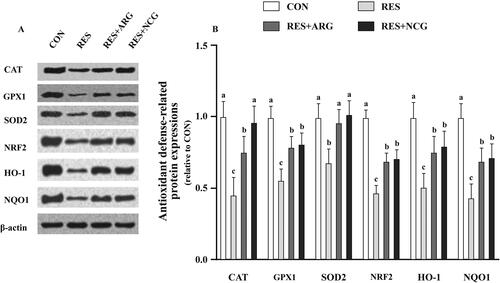
Figure 5. Roles of rumen-protected L-arginine (RP-arg) or N-carbamylglutamate (NCG) supplemented in diet in immune function related protein expression in foetal jejunum in underfed Hu ewes at 110 gestational days. Characteristic images demonstrating the Western blotting assay (A) and p65, p-p65, IL-1β, and TNF-α expression (B) was measured. IL: interleukin; NF-κB: nuclear factor kappa B (p65); TNF-α: tumour necrosis factor α; NRC: National Research Council; CON/RES: ewes fed 100%/50% of NRC (Citation2007) recommendations for pregnancy; RES + ARG, ewes fed 50% of NRC (Citation2007) recommendations with supplementation of 20 g/d RP-arg; RES + NCG, ewes fed 50% of NRC (Citation2007) recommendations with supplementation of 5 g/day NCG. Data represent means, and standard errors are shown in vertical bars (n = 8/group for ewes, n = 16/group for the foetus). Labelled means with on common letter stand for significant differences, p < .05.
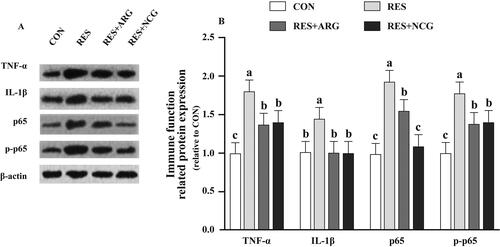
Figure 6. Roles of rumen-protected L-arginine (RP-arg) or N-carbamylglutamate (NCG) supplemented in diet in integrity-related protein levels in foetal jejunum of underfed Hu ewes at 110 gestational days. Typical images showing the Western blotting analysis (A) and claudin-1 and ZO-1 (B) were analysed. ZO-1: zonula occludens-1; NRC: National Research Council; CON/RES: ewes fed 100%/50% of NRC (Citation2007) recommendations for pregnancy; RES + ARG, ewes fed 50% of NRC (Citation2007) recommendations with supplementation of 20 g/day RP-arg; RES + NCG, ewes fed 50% of NRC (Citation2007) recommendations with supplementation of 5 g/day NCG. Data represent means, and standard errors are shown in vertical bars (n = 8/group for ewes, n = 16/group for the foetus). Labelled means with no identical letters are different, p < .05.
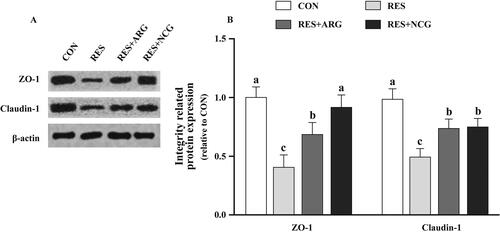
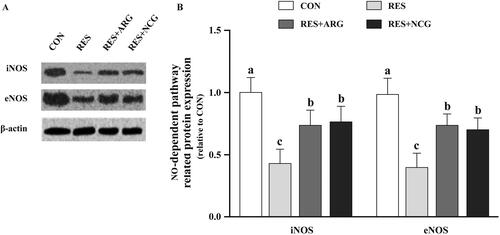
Figure 7. Roles of rumen-protected L-arginine (RP-arg) or N-carbamylglutamate (NCG) supplemented in diet in NO-dependent pathway-associated protein levels in foetal jejunum of underfed Hu ewes at 110 gestational days. Typical images showing the Western blotting analysis (A) and eNOS and iNOS levels (B) were determined. NO: nitric oxide; NOS: inducible NO synthase; eNOS: epithelial NO synthase; NRC: National Research Council; CON/RES: ewes fed 100%/50% of NRC (Citation2007) recommendations for pregnancy; RES + ARG, ewes fed 50% of NRC (Citation2007) recommendations with supplementation of 20 g/d RP-arg; RES + NCG, ewes fed 50% of NRC (Citation2007) recommendations with supplementation of 5 g/d NCG. Data represent means, and standard errors are shown in vertical bars (n = 8/group for ewes, n = 16/group for the foetus). Labelled means with no common letters stand for significant differences, p < .05.
Discussion
Maternal undernutrition has a negative effect on foetal development, particularly for twin pregnancies (Castro-Rodríguez et al. Citation2020). Nutrient insufficiency in conceptus changes the placental development and activity, causing IUGR (Satterfield et al. Citation2021). The IUGR exerts a substantially negative effect on the health of the foetus and newborn (Hoffman et al. Citation2017). Arg or NCG supplemented in diet has a critical effect on ameliorating placental vascular activity while enhancing nutrient supply to the foetus (Wu et al. Citation2022). According to our findings, maternal dietary Arg or NCG supplementation improved foetal weight, foetal small intestinal weight, jejunal morphology, oxidation resistance, immunity and integrity in foetuses suffering from IUGR in underfed ewes. Such improvements in foetal weight, foetal intestinal health and metabolism might be attributed partly to overall nutritional and endocrine improvements induced by Arg metabolites transferred across the placenta. More studies are needed to understand the function of the placenta in improving development, oxidation resistance, and immunity in the foetal intestine.
The development of the intestine is of crucial importance to neonate growth and survival. The foetal intestine develops more rapidly in the last trimester (Zhu et al. Citation2018). The integrity of the small intestine is necessary to maintain appropriate digestive functions (Choksi et al. Citation2018), therefore intestinal health is crucial. In ruminants, the intestine, in particular the ileum, jejunum, ileocaecal junction, as well as the surrounding regions, is a focus of disease pathology (Bayne and Edmondson Citation2021). Therefore, the foetal jejunum, as a representative part of the intestinal tissue was selected for this study. In this study, relative to the CON, jejunal VCR and villus heights were decreased in IUGR foetuses by decreasing claudin-1 and ZO-1 protein and mRNA levels, conforming to the prior results (Su et al. Citation2018). The NCG or Arg could improve IUGR foetal intestinal morphology in RES ewes by promoting enterocyte proliferation and maturation, which might explain the damaged integrity of the intestine in the IUGR ovine foetus and strengthened the effect of Arg or NCG on protection against IUGR foetal intestinal injury.
Arg has been suggested as a strong stimulator of insulin secretion (Kolic et al. Citation2023). Enhanced insulin release strengthens nutrient utilisation by promoting the production of tissue proteins (Rahman et al. Citation2021). Based on our findings, foetal jejunal protein and insulin contents elevated in the RES ewes upon Arg or NCG administration, which are in line with the above-mentioned effects.
The IUGR animals are more susceptible to OS, an effect that is probably associated with the dysregulation between prooxidants and antioxidants. Excess OS affects cell homeostasis and is likely to cause cell injuries (Celik et al. Citation2019), even further resulting in cell death and irreversible oxidative damage if not treated. The OS is also suggested to be a critical factor resulting in bowel injury within animals afflicted by IUGR (Zhang et al. Citation2022). Consequently, the evidently declined jejunal T-AOC levels as well as anti-oxidase activities, together with elevated MDA level in the jejunum of IUGR ovine foetuses indicate the occurrence of oxidative injury under the circumstances of our study. Furthermore, the above changes can lead to bowel-associated diseases by causing oxidative injury of DNA/proteins/lipids, as well as elevating membrane permeability (Yuan et al. Citation2022). Such increased TNF-α, IL-6, and IL-1β contents within jejunal tissues in IUGR ovine foetuses relative to the CON group confirmed that the OS was positively correlated with pro-inflammatory cytokines, while TNF-α serves as the pro-inflammatory cytokine that participates in the systemic inflammation (Liu et al. Citation2016). In addition, it is known that TNF-α increases the intestinal epithelial barrier permeability (Crawford et al. Citation2022). Therefore, the reduction in oxidative stress along with the lower TNF-α and IL-1β protein and mRNA contents within foetal jejunal tissues by Arg and NCG supplementation supports the protective roles of these metabolites.
The intestinal epithelial barrier includes various junctional complexes, and it can guarantee tight epithelial cell binding and suitable maintenance of the epithelial barrier (Zihni et al. Citation2016). Goblet cells are able to generate mucins and trefoil peptides for generating a physical barrier onto the intestinal mucosal surface, whereas tight junction proteins (TJPs) have been recognised to regulate the activity of the paracellular intestinal epithelial barrier (Tokuda and Yu Citation2019). Foetal jejunal TJP contents, like claudin-1 and ZO-1 elevated in the RES group added with Arg or NCG, besides, goblet cell number elevated, thus suggesting that foetal intestine showed a good barrier effect. These results conform to the prior reports that indicated that Arg supplementation boosted intestinal morphology of the weaned as well as the IUGR suckling piglets (Madsen et al. Citation2017), implying that NCG or Arg could enhance the antioxidative defense in IUGR animals by reducing the pro-inflammatory cytokines. After activating nuclear factor erythroid 2-related factor (Nrf2), improving our understanding of the underlying mechanism of the association of Nrf2 with the antioxidant responsive element (ARE)-activated antioxidation and detoxification under the anti-oxidative state is of crucial importance, which suggests that oxidation resistance may serve as the synergistic action regulated by different antioxidative enzymes rather than by the antioxidative reaction alone (Suzuki and Yamamoto Citation2015). As for Arg, it has the key effect of up-regulating Nrf2, the antioxidant transcriptional factor, as well as the phase II metabolising enzymes (like NQO1 or HO-1), together with the anti-oxidases (like CAT, GPX, SOD2) (Zhang et al. Citation2019c). In our study, Arg or NCG treatment notably elevated the mRNA abundance of antioxidant/detoxification genes (HO-1, NQO1, SOD2, CAT, GPX1) by activating Nrf2 that took part in the endogenous defense response of antioxidants. Additionally, the NCG or Arg supplementation positively affected the Nrf2 protein and mRNA levels and elevated the SOD2, CAT, GPX1, and phase II metabolising enzymes (including NQO1 or HO-1) content but reduced MDA level within jejunum in IUGR foetuses, which demonstrated that the above-mentioned compounds had positive effects on the jejunal functions. The present work is the first to discover that Arg or NCG can activate and upregulate ARE-induced antioxidant and detoxification genes controlled by the Nrf2 signalling pathway within the foetal jejunum from animals exposed to IUGR.
Nitric oxide (NO), produced from Arg metabolism, can serve as the essential messenger that plays various roles, such as regulating cell proliferation and survival, immunologic function, as well as cellular redox status (López-Sánchez et al. Citation2020). The NO also has been shown to play a role as a scavenger of free radicals (Martini et al. Citation2020). In accordance with our results, maternal Arg or NCG supplemented in diet acted favourably by promoting the jejunal oxidation resistance of IUGR ovine foetuses, which was ascribed to the fact that Arg exerted a role as the precursor of the NO (Bodis et al. Citation2022). This might be related to the fact that NCG might cross the placental barrier, promote endogenous Arg production within the placenta and produce Arg in the foetus (Wu et al. Citation2022) or produced more Arg in the maternal plasma with more Arg therefore transferred by concentration dependent effects into the foetus (Zhang et al. Citation2016). Its associated mechanism should be further investigated. To study whether the NO pathway was responsible for the Arg or NCG-enhanced foetal antioxidant capacity in the jejunum, we determined both iNOS and eNOS protein and mRNA levels, NO content, and NOS activity. In this study, maternal NCG or Arg treatment significantly increased the jejunal NOS protein and mRNA levels, NO level, or NOS activity in IUGR ovine foetuses. According to Hu et al. (Citation2017), Arg treatment decreased the OS induced by high glucose via the eNOS-independent signalling pathway. The NCG or Arg treatment had a positive effect on the intestinal health of IUGR foetuses in a NO pathway-dependent manner, partly due to the enhanced jejunal oxidation resistance.
Conclusion
In the present work, N-carbamylglutamate or L-arginine supplemented in the diet to underfed ewes can promote the foetal jejunal barrier function and oxidation resistance and activate the nitric oxide-dependent pathway. However, the current results ignore that critical nutrients delivered from the placenta to the foetus is important. Therefore, more investigations are warranted for exploring the effects of the placenta during the above pregnancies on enhancing foetal jejunal oxidation resistance, immunity, and barrier function.
Ethical Approval
The experimental protocol and sample collection were carried out in accordance with the Regulations on the Administration of Laboratory Animals promulgated by the National Science and Technology Commission of the People's Republic of China. And the experiment was approved by Committee of Yang Zhou University (SYXK2013-0057).
Supplementary Tables0522.docx
Download MS Word (28.1 KB)Disclosure statement
We declare that we have no financial and personal relationships with other people or organisations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Data availability statement
Data used in the present work can be obtained from this manuscript and the supplementary materials.
Additional information
Funding
References
- Baumgarten HD, Wright CM, Rossidis AC, Lawrence KM, Kim AG, Mejaddam AY, McGovern PE, Orr MN, Coons BE, Butt Z, et al. 2020. The extrauterine environment for neonatal development supports normal intestinal maturation and development. Cell Mol Gastroenterol Hepatol. 10(3):623–637. doi: 10.1016/j.jcmgh.2020.05.006.
- Bayne JE, Edmondson MA. 2021. Diseases of the gastrointestinal system. In: Pugh DG, editor. Sheep, goat, and cervid medicine. Amsterdam: Elsevier; p. 63–96.
- Berlinguer F, Porcu C, Molle G, Cabiddu A, Dattena M, Gallus M, Pasciu V, Succu S, Sotgiu FD, Paliogiannis P, et al. 2019. Circulating concentrations of key regulators of nitric oxide production in undernourished sheep carrying single and multiple fetuses. Animals. 10(1):65. doi: 10.3390/ani10010065.
- Bodis J, Farkas B, Nagy B, Kovacs K, Sulyok E. 2022. The role of L-arginine-NO system in female reproduction: a narrative review. Int J Mol Sci. 23(23):14908. doi: 10.3390/ijms232314908.
- Caleb I, Erlitz L, Telek V, Vecsernyés M, Sétáló G, Hardi P, Takács I, Jancsó G, Nagy T. 2021. Characterizing autophagy in the cold ischemic injury of small bowel grafts: evidence from rat jejunum. Metabolites. 11(6):396. doi: 10.3390/metabo11060396.
- Cappelli K, Ferlisi F, Mecocci S, Maranesi M, Trabalza-Marinucci M, Zerani M, Dal BA, Acuti G. 2021. Dietary supplementation of olive mill waste water polyphenols in rabbits: evaluation of the potential effects on hepatic apoptosis, inflammation and metabolism through RT-qPCR approach. Animals. 11(10):2932. doi: 10.3390/ani11102932.
- Castro-Rodríguez DC, Rodríguez-González GL, Menjivar M, Zambrano E. 2020. Maternal interventions to prevent adverse fetal programming outcomes due to maternal malnutrition: evidence in animal models. Placenta. 102:49–54. doi: 10.1016/j.placenta.2020.04.002.
- Celik E, Taysi S, Sucu S, Ulusal H, Sevincler E, Celik A. 2019. Urotensin 2 and oxidative stress levels in maternal serum in pregnancies complicated by intrauterine growth restriction. Medicina. 55(7):328. doi: 10.3390/medicina55070328.
- Chacher B, Wang D, Liu H, Liu J. 2012. Degradation of L-arginine and N-carbamoyl glutamate and their effect on rumen fermentation in vitro. Ital J Anim Sci. 11(4):e68. doi: 10.4081/ijas.2012.e68.
- Chacher B, Zhu W, Ye J, Wang D, Liu J. 2014. Effect of dietary N-carbamoylglutamate on milk production and nitrogen utilization in high-yielding dairy cows. J Dairy Sci. 97(4):2338–2345. doi: 10.3168/jds.2013-7330.
- Choksi YA, Reddy VK, Singh K, Barrett CW, Short SP, Parang B, Keating CE, Thompson JJ, Verriere TG, Brown RE, et al. 2018. BVES is required for maintenance of colonic epithelial integrity in experimental colitis by modifying intestinal permeability. Mucosal Immunol. 11(5):1363–1374. doi: 10.1038/s41385-018-0043-2.
- Corzo C, Fuchsbichler A, Savencu I, Afonso Urich J, Zimmer A, Lochmann D, Reyer S, Salar-Behzadi S. 2021. Lipid-microparticles for pulmonary delivery of active pharmaceutical ingredients: impact of lipid crystallization on spray-drying processability. Int J Pharm. 610:121259. doi: 10.1016/j.ijpharm.2021.121259.
- Crawford CK, Lopez Cervantes V, Quilici ML, Armién AG, Questa M, Matloob MS, Huynh LD, Beltran A, Karchemskiy SJ, Crakes KR, et al. 2022. Inflammatory cytokines directly disrupt the bovine intestinal epithelial barrier. Sci Rep. 12(1):14578. doi: 10.1038/s41598-022-18771-y.
- Gu F, Zhu S, Hou J, Tang Y, Liu JX, Xu Q, Sun HZ. 2023. The hindgut microbiome contributes to host oxidative stress in postpartum dairy cows by affecting glutathione synthesis process. Microbiome. 11(1):87. doi: 10.1186/s40168-023-01535-9.
- Hoffman ML, Reed SA, Pillai SM, Jones AK, McFadden KK, Zinn SA, Govoni KE. 2017. Physiology and endocrinology symposium: the effects of poor maternal nutrition during gestation on offspring postnatal growth and metabolism. J Anim Sci. 95(5):2222–2232. doi: 10.2527/jas.2016.1229.
- Hu S, Han M, Rezaei A, Li D, Wu G, Ma X. 2017. L-arginine modulates glucose and lipid metabolism in obesity and diabetes. Curr Protein Pept Sci. 18(6):599–608. doi: 10.2174/1389203717666160627074017.
- Kolic J, Sun WG, Johnson JD, Guess N. 2023. Amino acid-stimulated insulin secretion: a path forward in type 2 diabetes. Amino Acids. 55(12):1857–1866. doi: 10.1007/s00726-023-03352-8.
- Kumar S, Ahmad A, Kushwaha N, Shokeen N, Negi S, Gautam K, Singh A, Tiwari P, Garg R, Agarwal R, et al. 2022. Selection of ideal reference genes for gene expression analysis in COVID-19 and mucormycosis. Microbiol Spectr. 10(6):e01656–e01622. doi: 10.1128/spectrum.01656-22.
- Liu C, Feng X, Li Q, Wang Y, Li Q, Hua M. 2016. Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes: a systematic review and meta-analysis. Cytokine. 86:100–109. doi: 10.1016/j.cyto.2016.06.028.
- López-Sánchez LM, Aranda E, Rodríguez-Ariza A. 2020. Nitric oxide and tumor metabolic reprogramming. Biochem Pharmacol. 176:113769. doi: 10.1016/j.bcp.2019.113769.
- Madsen JG, Pardo C, Kreuzer M, Bee G. 2017. Impact of dietary l-arginine supply during early gestation on myofiber development in newborn pigs exposed to intra-uterine crowding. J Anim Sci Biotechno. 8:1–12.
- Martini S, Austin T, Aceti A, Faldella G, Corvaglia L. 2020. Free radicals and neonatal encephalopathy: mechanisms of injury, biomarkers, and antioxidant treatment perspectives. Pediatr Res. 87(5):823–833. doi: 10.1038/s41390-019-0639-6.
- Meyer AM, Caton JS. 2016. Role of the small intestine in developmental programming: impact of maternal nutrition on the dam and offspring. Adv Nutr. 7(1):169–178. doi: 10.3945/an.115.010405.
- National Research Council. 2007. Nutrient requirements of small ruminants: sheep, goats, cervids and new world camelids. Washington, DC: National Academies Press.
- Nüse B, Holland T, Rauh M, Gerlach RG, Mattner J. 2023. L-arginine metabolism as pivotal interface of mutual host–microbe interactions in the gut. Gut Microbes. 15(1):2222961. doi: 10.1080/19490976.2023.2222961.
- Pillai-Kastoori L, Schutz-Geschwender AR, Harford JA. 2020. A systematic approach to quantitative Western blot analysis. Anal Biochem. 593:113608. doi: 10.1016/j.ab.2020.113608.
- Purdy PH, Spiller SF, McGuire E, McGuire K, Koepke K, Lake S, Blackburn HD. 2020. Critical factors for non-surgical artificial insemination in sheep. Small Ruminant Res. 191:106179. doi: 10.1016/j.smallrumres.2020.106179.
- Rahman MS, Hossain KS, Das S, Kundu S, Adegoke EO, Rahman MA, Hannan MA, Uddin MJ, Pang MG. 2021. Role of insulin in health and disease: an update. Int J Mol Sci. 22(12):6403. doi: 10.3390/ijms22126403.
- Russel A, Doney J, Gunn R. 1969. Subjective assessment of body fat in live sheep. J Agric Sci. 72(3):451–454. doi: 10.1017/S0021859600024874.
- Sakuma C, Nakagawa M, Tomioka Y, Maruyama T, Entzminger K, Fleming JK, Shibata T, Kurosawa Y, Okumura CJ, Arakawa T, et al. 2022. Western blotting of native proteins from agarose gels. Biotechniques. 72(5):207–218. doi: 10.2144/btn-2022-0012.
- Satterfield MC, Edwards AK, Bazer FW, Dunlap KA, Steinhauser CB, Wu G. 2021. Placental adaptation to maternal malnutrition. Reproduction. 162(4):R73–R83. doi: 10.1530/REP-21-0179.
- Su W, Zhang H, Ying Z, Li Y, Zhou L, Wang F, Zhang L, Wang T. 2018. Effects of dietary L-methionine supplementation on intestinal integrity and oxidative status in intrauterine growth-retarded weanling piglets. Eur J Nutr. 57(8):2735–2745. doi: 10.1007/s00394-017-1539-3.
- Suzuki T, Yamamoto M. 2015. Molecular basis of the Keap1–Nrf2 system. Free Radic Biol Med. 88(Pt B):93–100. doi: 10.1016/j.freeradbiomed.2015.06.006.
- Tokuda S, Yu ASL. 2019. Regulation of epithelial cell functions by the osmolality and hydrostatic pressure gradients: a possible role of the tight junction as a sensor. Int J Mol Sci. 20(14):3513. doi: 10.3390/ijms20143513.
- Weckman AM, McDonald CR, Baxter JAB, Fawzi WW, Conroy AL, Kain KC. 2019. Perspective: l -arginine and L-citrulline supplementation in pregnancy: a potential strategy to improve birth outcomes in low-resource settings. Adv Nutr. 10(5):765–777. doi: 10.1093/advances/nmz015.
- Wittmeier P, Hummel S. 2022. Agarose gel electrophoresis to assess PCR product yield: comparison with spectrophotometry, fluorometry and qPCR. Biotechniques. 72(4):155–158. doi: 10.2144/btn-2021-0094.
- Wu G, Bazer FW, Satterfield MC, Gilbreath KR, Posey EA, Sun Y. 2022. L-Arginine nutrition and metabolism in ruminants. In: Cole DA, Haresign W, editors. Recent advances in animal nutrition and metabolism. London: Butterworths; p. 177–206.
- Xie S, Jiang L, Wang M, Sun W, Yu S, Turner JR, Yu Q. 2020. Cadmium ingestion exacerbates Salmonella infection, with a loss of goblet cells through activation of Notch signaling pathways by ROS in the intestine. J Hazard Mater. 391:122262. doi: 10.1016/j.jhazmat.2020.122262.
- Xue Y, Guo C, Hu F, Zhu W, Mao S. 2019. Maternal undernutrition induces fetal hepatic lipid metabolism disorder and affects the development of fetal liver in a sheep model. Faseb J. 33(9):9990–10004. doi: 10.1096/fj.201900406R.
- Ye C, Zeng X, Zhu J, Liu Y, Ye Q, Qiao S, Zeng X. 2017. Dietary N-carbamylglutamate supplementation in a reduced protein diet affects carcass traits and the profile of muscle amino acids and fatty acids in finishing pigs. J Agric Food Chem. 65(28):5751–5758. doi: 10.1021/acs.jafc.7b02301.
- Yuan S, Wang Q, Li J, Xue J-C, Li Y, Meng H, Hou X-T, Nan J-X, Zhang Q-G. 2022. Inflammatory bowel disease: an overview of Chinese herbal medicine formula-based treatment. Chin Med. 17(1):74. doi: 10.1186/s13020-022-00633-4.
- Zeng X, Huang Z, Mao X, Wang J, Wu G, Qiao S. 2012. N-carbamylglutamate enhances pregnancy outcome in rats through activation of the PI3K/PKB/mTOR signaling pathway. PLoS One. 7(7):e41192. doi: 10.1371/journal.pone.0041192.
- Zhang H, Peng A, Guo S, Wang M, Loor JJ, Wang H. 2019a. Dietary N-carbamylglutamate and L-arginine supplementation improve intestinal energy status in intrauterine-growth-retarded suckling lambs. Food Funct. 10(4):1903–1914. doi: 10.1039/c8fo01618f.
- Zhang H, Peng A, Yu Y, Guo S, Wang M, Wang H. 2019c. L-arginine protects ovine intestinal epithelial cells from lipopolysaccharide-induced apoptosis through alleviating oxidative stress. J Agric Food Chem. 67(6):1683–1690. doi: 10.1021/acs.jafc.8b06739.
- Zhang H, Sun H, Peng A, Guo S, Wang M, Loor J, Wang H. 2019b. N-carbamylglutamate and L-arginine promote intestinal function in suckling lambs with intrauterine growth restriction by regulating antioxidant capacity via a nitric oxide–dependent pathway. Food Funct. 10(10):6374–6384. doi: 10.1039/c9fo01752f.
- Zhang H, Sun L, Wang Z, Deng M, Zhang G, Guo R, Ma T, Wang F. 2016. Dietary N-carbamylglutamate and rumen-protected L-arginine supplementation ameliorate fetal growth restriction in undernourished ewes. J Anim Sci. 94(5):2072–2085. doi: 10.2527/jas.2015-9587.
- Zhang H, Zheng Y, Zha X, Ma Y, Liu X, Elsabagh M, Wang H, Wang M. 2022. Dietary L-arginine or N-Carbamylglutamate alleviates colonic barrier injury, oxidative stress, and inflammation by modulation of intestinal microbiota in intrauterine growth-retarded suckling lambs. Antioxidants. 11(11):2251. doi: 10.3390/antiox11112251.
- Zhu L, Luo F, Hu W, Han Y, Wang Y, Zheng H, Guo X, Qin J. 2018. Bacterial communities in the womb during healthy pregnancy. Front Microbiol. 9:2163. doi: 10.3389/fmicb.2018.02163.
- Zihni C, Mills C, Matter K, Balda MS. 2016. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 17(9):564–580. doi: 10.1038/nrm.2016.80.
