?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In broiler nutrition, dietary fibre affects the development of the digestive system, nutrient absorption, intestinal flora and growth performance. The objective of this study was to evaluate the effect of orange seed powder (OSP) on growth performance, biochemical parameters, and the caecal bacterial population of broiler chickens. Four hundred and eighty-day-old Cobb 500 broiler chicks were divided into four treatment groups of six replicates. The treatment groups comprised a control group (without OSP supplementation) and three groups with varying levels of OSP supplementation (0.25%, 0.5%, and 0.75%) over 42 days. All groups supplemented with orange seed powder significantly decreased serum cholesterol, triglyceride concentrations, E. coli, and total coliform population as well as feed conversion ratio and feed intake and significantly increased average weight gain and lactobacillus population on day 21 (p < 0.05). Carcase yield did not differ across all the treatment groups (p > 0.05). On day 42, the triglyceride level was significantly superior (p < 0.05) with the supplementation of OSP. The lactobacillus population was not affected on day 42; however, E. coli and the total coliform population remained affected (p < 0.05). Orange seed powder at 0.50% supplementation in poultry feed can optimise the caeca bacterial population and enhance the weight gain and feed conversion ratio.
HIGHLIGHTS
Supplementation of the diet of broiler chickens with orange seed powder is effective in reducing serum cholesterol levels.
Supplementation of orange seed powder in poultry feed increases beneficial bacteria (lactobacillus) and decreases pathogenic bacteria (E. coli).
Supplementation of orange seed powder in the diet of broiler chickens decreases feed conversion ratio.
Introduction
In poultry farming, feed accounts for 70–80% of production costs (Basir and Toghyani Citation2017). A resilient feeding strategy can reduce production costs and make it more efficient while providing consumers with high-quality products (Ebrahimi et al. Citation2013). Additionally, the overuse of antibiotics, both to treat disease and to promote growth in poultry, has led to alarming concerns about antibiotic-resistant bacteria and its residue deposits in poultry products intended for human consumption (Nhung et al. Citation2017). In hot and humid climate regions, climatic problems lead to heat stress and frequent diseases in poultry resulting in a low feed conversion ratio and high mortality in the flock (Kpomasse et al. Citation2023). This has led to the increased use and overuse of synthetic additives in these regions to boost production and prevent diseases in poultry.
In the face of growing concerns over antibiotic resistance and consumer demand for organic poultry products, the use of natural additives, including medicinal plant extracts, has gained significant attention in the research space as potential alternatives to antibiotics (Griggs and Jacob Citation2005; Long et al. Citation2020; SureshKumar et al. Citation2022; Imran Citation2022; Saeed et al. Citation2024). These natural compounds are known to possess antimicrobial, antioxidant, and antimutagenic properties, which may contribute to improved growth performance, gut health, and overall well-being of poultry (Durmic and Blache Citation2012; Long et al. Citation2020; Adjei-Mensah et al. Citation2023). Citrus seeds are important waste generated from citrus processing (Russo et al. Citation2015) because of their antioxidant potential (Carson et al. Citation2002). The seeds contain around 13% oil that is rich in unsaturated fatty acids like oleic, vaccenic, and linolenic acids (Rodrigues et al. Citation2023). Beyond the oil, orange seeds have also been found to be a valuable source of other beneficial compounds, such as tocopherols, carotenoids, and phytosterols (Parry et al. Citation2007; Martínez et al. Citation2010). These components have been shown to possess antioxidant, anti-inflammatory, and cholesterol-lowering properties, making orange seeds a potentially versatile and health-promoting ingredient. Additionally, studies have shown that sweet orange extract can also optimise the gastrointestinal microbiota of broilers (Ebrahimi et al. Citation2015; Oikeh et al. Citation2020). It has been reported that adding phytobiotics or fruit seeds to the rations of animals improves fattening performance. Phytobiotics contain biochemical components that are effective in suppressing pathogenic microorganisms in the intestine with their antioxidant properties and may help regulate hypercholesterolaemia. (Saeed et al. Citation2017; Tufan et al. Citation2023). The present study addresses the paucity of studies on the performance and health status of poultry in hot and humid regions through dietary supplementation of orange seed powder. The majority of research on the possible benefits of plant additives, including citrus by-products has been done in more controlled environments. The dearth of studies conducted in areas with harsh climatic conditions limits the understanding of the effectiveness of nutrition strategies like supplementing citrus seeds in the diets of broiler chickens reared under hot climatic conditions. As such, there is little information about how well these interventions work to mitigate poultry health-related problems and improve gut functioning, particularly in areas where heat stress and diseases are common. Finding solutions to these issues is essential to outlining purposeful strategies to assist poultry producers in such regions.
Furthermore, while studies have demonstrated the antioxidant capacity of citrus seeds and their potential influence on the gut health of broilers (Aranha and JoRGe Citation2013; Ebrahimi et al. Citation2015; Oikeh et al. Citation2020), few studies have been conducted on the various citrus by-products, especially with regard to how well they function to address challenges particular to hot and humid climates.
Highlighting the need to address these challenges is crucial in looking for innovative poultry husbandry practices in regions facing climatic challenges. Thus, it is unclear whether the supplementation of orange seed powder in a hot humid climate may yield similar benefits. In our earlier work, improved intestinal development morphometry and feed efficiency were observed when citrus seeds were supplemented at 0.5% and 0.75% in the diets of broiler chickens (Parobali et al. Citation2024). It is therefore hypothesised that the supplementation of orange seed powder in the diet of broilers will improve microflora function in the gut and enhance feed utilisation. The objective of this study was to evaluate the effect of orange seed powder supplementation on the growth performance, caecal bacterial population, and biochemical profile of broilers reared in hot and humid climates.
Materials and methods
Ethics and study site
This study was conducted at the experimental unit of the Regional Centre of Excellence in Poultry Science, University of Lome (CERSA/UL), with approval from the ethics and scientific committee. The study location is situated in a hot and humid climate with average weather conditions of 28.85 °C ± 0.62 and 71.62% ± 1.75 relative humidity.
Experimental design and birds management
Four hundred and eighty (480) unsexed day-old Cobb 500 chicks with an average weight of 43.5 g were used in this study. During the first 3 days of brooding, the temperature of the house was kept at 33 °C and was gradually reduced to 24 °C on day 21 as the chicks advanced in age. The birds were reared in an open-house concrete floor covered with 5-cm-thick wood shavings with a stocking density of 10 birds/m2. The chicks were assigned to four dietary treatments with 120 chicks per group and 6 replicates using a completely randomised design. The study included one control (without OSP supplementation) and three treatment groups with varying levels of OSP supplementation (0.25%, 0.5%, and 0.75%). The crude protein and metabolisable energy of the starter and finisher diets were respectively 21.17% and 2938.82 kcal/kg and 18.05% and 3067.38 kcal/kg. The birds had free access to feed and water with a lighting program of 12 h of light/day. The average temperature and relative humidity in the pens were 27.8 °C and 83%. The standard Cobb 500 broiler management prophylactic plan was followed in raising the birds.
Orange seed powder preparation
Fresh orange seeds were obtained from the sales point of orange juice producers at the University of Lome campus. The seeds were washed with potable water and then air-dried at 30 °C for 2 weeks. The dried seeds were grounded using an electric mill to a fine powder (2 mm) and stored in bags for later use. The procedure followed in determining the proximate composition of the orange seed powder is outlined in Parobali et al. (Citation2024).
Growth performance
During the entire study period, the body weight of each bird and feed intake (FI) were measured weekly. The feed conversion ratio (FCR) and average daily gain (ADG) were determined during days 0–21, 22–42 and the entire experimental period (0–42 days). The following formulas were used in calculating the FCR and ADG:
Carcase parameters and internal organs measurements
At the end of the experiment, 12 birds (2 birds per replicate) per group were humanely euthanized by cervical dislocation and then eviscerated on day 42. The carcase weight was taken after removing the feathers and blood, and the eviscerated weight was taken after removing the head, feet, and abdominal fat. The carcase weight, breast weight, abdominal fat weight and internal organ weight were expressed as the percentage of the live weight to obtain their respective relative weights as follows:
Determination of caecal bacterial population
On days 21 and 42, 12 birds with similar body weights in each treatment were humanely sacrificed and eviscerated to isolate the caeca. The digesta in the caeca were collected in Petri dishes in an aseptic environment for further microbiological analysis.
The solid medium count method was employed to determine the bacterial population following the procedure of Ebrahimi et al. (Citation2015). All collection tubes were sterilised by autoclave. Man Rogosa Sharpe agar (MRS) was used as the culture medium for culturing lactobacilli, tryptone-bile-glucuronate (TBX) for culturing E. coli, and lactose bile medium with crystal violet and neutral red (VRBL) for culturing total coliforms. For each sample, 1 g of the sample was weighed into a new test tube containing 9 mL of tryptone salt broth and was shaken for half an hour. Then, 1 mL of the prepared suspension was added to 9 mL of the tryptone salt broth in another test tube. Suspensions were prepared using 10−1 dilutions, and serial dilutions were performed (10−2, 10−3, 10−4, 10−5 and 10−6). One hundred (100 μl) was taken from the 10−4, 10−5, and 10−6 dilutions and poured into the Petri dishes already prepared and containing the culture medium. Lactobacillus bacteria was incubated at 37 °C under anaerobic conditions for 72 h. Total coliforms and Escherichia coli were incubated at 37 °C under aerobic conditions for 48 h. Enumeration of bacteria on Petri dishes was performed by counting the number of colonies. The procedures were performed in an aseptic environment equipped with two Bunsen burners.
Analysis of biochemical parameters
On days 21 and 42, blood samples were taken from 12 birds with similar body weights in each treatment. Blood samples were collected in dry tubes and immediately centrifuged at 3000 x g for 15 min to obtain serum. A volume of 2 mL of serum sample was stored in a freezer at 20 °C until analysis of biochemical parameters. The concentrations of glucose, triglycerides, and total cholesterol were evaluated according to a colourimetric enzymatic assay using Cypres Diagnostics kits (Belgium), whereas the Biuret reagent was used for the determination of total proteins through a colourimetric test (Walker et al. Citation1990)
Statistical analysis
The data obtained were subjected to one-way analysis of variance (ANOVA) using R software version 4.3.0 following the model: yij = m + tj + eij, where m is the general mean, t is the treatment effect, and e is random error. The results are presented as mean and pooled standard errors of the means. The means were compared and separated using the Tukey post hoc test at a significance level of 5%. The linear and quadratic treatment effects were determined using orthogonal polynomial contrasts.
Results
Proximate composition of orange seed powder
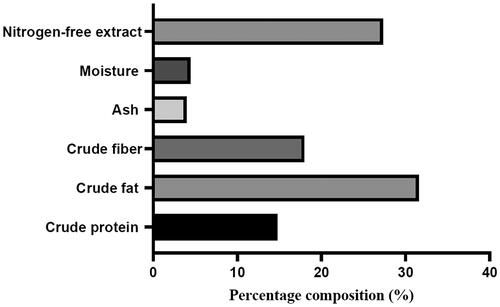
Figure shows the results of the proximate composition of OSP. The analysis of the OSP samples revealed appreciable amounts of fat (31.56%), crude fibre deposit (17.97%) and protein (14.78%).
Growth parameters
The results of the effect of OSP supplementation on growth parameters are summarised in Table . Supplementation of OSP in the diets of the chickens had significant linear (p < 0.0001; p = 0.091; p < 0.0001) and quadratic (p < 0.0001; p = 0.001; p < 0.0001) effects on average daily gain (ADG), feed intake (FI), and feed conversion ratio (FCR). From day 0 to day 21 and from day 22 to day 42, the FI, and FCR of chickens in all groups supplemented with OSP (0.25, 0.5 and 0.75%) were significantly different from those in the control group (p < 0.05). Thus, the FI and FCR of chickens in the 0.25, 0.5, and 0.75 groups were significantly lower than the control group (p < 0.0001; p = 0.003). Average daily gain was significantly higher (p = 0.007) in the 0.50 and 0.75 OSP groups compared to the control group on days 0–21 but on days 22–42, the 0.25 and 0.50 OSP groups were better (p < 0.0001) than the control and 0.75 OSP groups. However, regarding the overall performance (days 0–42), there was no significant effect on ADG and FI (p > 0.05), but FCR and body weight were still affected positively and significantly (p < 0.05) with the supplementation of OSP in the diets of the birds. The supplementation of OSP at 0.50% recorded significantly better (p = 0.023) FCR compared to the control and a higher body weight (p < 0.0001).
Table 1. Effect of orange seeds powder supplementation on growth parameter of broiler chickens during 0–21, 22–42 and 0–42 days of the experiment.
Carcase traits and internal organ weights
Table shows the effect of OSP supplementation on the relative carcase traits and abdominal fat. The supplementation of OSP in the diets of the birds did not significantly (p > 0.05) affect relative carcase, breast and abdominal fat weights at the end of the study. Relative heart weight was significantly higher and quadratically significant in the 0.75 OSP group compared to the 0.25 OSP group (p = 0.010; 0.002). Relative liver weight was significantly superior and linearly significant in the 0.75 OSP group compared to the 0.50 OSP group (p < 0.0001; 0.0009). Besides, relative gizzard weight was significantly increased and quadratically significant in the 0.50 and 0.75 OSP groups compared to the control group (p = 0.003; p = 0.0007).
Table 2. Effect of orange seeds powder supplementation on relative carcase traits and abdominal fat of broiler chickens at 42 days.
Table 3. Effect of orange seeds powder supplementation on biochemical parameters of chickens on days 21 and 42 of the experiment.
Biochemical parameters
On day 21, there were no significant differences in blood glucose and serum total protein (p = 0.648 and p = 0.114, respectively) (Table ). However, the results revealed a significant difference (p < 0.05) in serum cholesterol and triglyceride concentrations between the different treatment groups. Serum cholesterol was significantly lower (p = 0.0009) in the 0.75% OSP-supplemented group than in the control group. A significantly lower concentration of triglycerides was observed in the serum of the 0.75% OSP-supplemented group compared with the other treatment groups (p < 0.0001). Furthermore, there was no significant effect on the blood glucose and serum total protein levels (p = 0.648 and p = 0.114, respectively) of the chicks.
On day 42, a significant difference was observed (p < 0.05) in glucose, triglyceride, cholesterol, and total protein levels between the different groups. A significant linear effect (p < 0.05) on OSP supplementation was observed between the groups in terms of glucose, triglyceride, cholesterol, and total protein levels. The triglyceride level was significantly higher (p < 0.0001) in all groups treated with OSP than in the control group. However, the total protein level was linearly lower (p = 0.012) in the 0.25, 0.50 and 0.75% OSP-supplemented groups than in the control group. The glucose levels of chickens in the 0.25 and 0.5% OSP-supplemented groups were significantly higher than those in the other groups (p < 0.0001). In contrast, cholesterol levels were significantly lower in the 0.25 and 0.50% OSP-supplemented groups compared to the other groups (p = 0.029).
Caecal bacterial population
Table shows the effect of dietary supplementation with OSP on the populations of coliforms, E. coli, and lactobacilli in the caeca of broiler chickens on days 21 and 42. The results showed a significant decrease in the population of total coliforms (p = 0.0027) in all groups treated with OSP. The population of E. coli was significantly lower (p < 0.0001) in the 0.75% OSP group compared to the other OSP groups and the control group. A higher count of lactobacilli was observed in the 0.25% OSP group than in the other OSP groups and the control group (p < 0.0001). The supplementation of OSP (0.25%, 0.5%, and 0.75%) in the diets of the chickens had a significant quadratic effect on the population of total coliforms and E. coli in the caeca of broiler chickens on day 21 (p = 0.0167; p < 0.0001) and day 42 (p < 0.0001; p < 0.0001). The lowest and highest population of total coliforms and E. coli were in 0.75 and control broilers groups, respectively. However, a significant linear effect on the population of total coliforms, E. coli and lactobacilli (p = 0.0124; p = 0.0025; p < 0.0001) was observed exclusively on day 21.
Table 4. Effect of orange seeds powder supplementation on some selected caecal bacterial populations of chickens on days 21 and 42 of the experiment.
Discussion
The proximate composition of OPS yielded 31.56% crude fat, 14.78% crude protein and 17.97% crude fibre. These nutrient values are quite high compared to those reported by Oikeh et al. (Citation2013) who in their study carried out on the proximate composition of C. sinensis fruit wastes, reported 12% crude fat, 6.13% crude protein and 5.3% crude fibre. However, the crude fat content is lower than that reported by Akpata and Akubor (Citation1999) when citrus seed flour was analysed. The differences in these reported values of citrus seeds and waste could be attributed to differences in processing methods and geographical locations (Banerjee and Ramaswamy Citation2017). Dietary fibre is known to influence the development of digestive organs, nutrient absorption, intestinal microbiota and growth performance when added to the diets of broiler chickens (Tejeda and Kim Citation2021).
In this current study, supplementing the broiler diet with OSP increased growth performance parameters such as feed conversion ratio, feed intake, and body weight gain. Our findings are partly consistent with the results of Readh et al. (Citation2023) who assessed the effect of graded levels of dried orange by-products on growth performance, blood profile, and antioxidant capacity of broilers and reported that the FCR and ADG of the birds were improved compared with the control group. On the contrary, Ebrahimi et al. (Citation2013) stated that the effect of different treatments supplemented with dried C. sinensis peel on body weight, and carcase percentage of broilers was not significantly different from the control groups. Furthermore, Abbasi et al. (Citation2015) stated that different levels of dried orange residues in the broiler chicken diet had no significant effect on the FCR. Broiler chicken feed consumption is impacted by a number of factors. Feed intake in broiler chickens is influenced by dietary factors, intestinal microbiota composition, and nutrient-sensing mechanisms (Liu et al. Citation2021; Ataei et al. Citation2022, Parobali et al. Citation2024). The beneficial effect of OSP on chicken performance is related to more efficient use of nutrients, which in turn results in improved FCR (Devriese et al. Citation1993). This efficient use of nutrients should also related to the development of the small intestine of chickens (Parobali et al. Citation2024). The action of C. sinensis seed on crypt depth and villi height was positively effective (Parobali et al. Citation2024).
On day 21, our results revealed a lowering effect of OSP on the concentration of serum triglyceride and cholesterol in chickens. These results are in agreement with those of Readh et al. (Citation2023); Behera et al. (Citation2019) and Abbasi et al. (Citation2015) who concluded that the waste products from C. sinensis juice extraction reduced blood cholesterol and triglycerides. This effect can be attributed to the cholesterol-lowering properties of citrus fruits. Flavonoids contained in Citrus sinensis seeds (Russo et al. Citation2015) have been shown to inhibit cholesterol synthesis in the liver by inhibiting the activity of the liver enzyme 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase (Bok et al. Citation1999, Gilani et al. Citation2018). In contrast to day 21, on day 42; plasma triglyceride concentration in the OSP-fed groups was significantly higher than that in the control group. This increase may be attributed to enhanced hepatic lipogenesis (Herzberg and Rogerson Citation1988; Boumezrag et al. Citation2018). Furthermore, the detailed mechanism of triglyceride-lowering and increasing by OSP is worth further study.
Carcase, abdominal fat, and relative breast weights were not influenced by OSP supplementation. However, relatively heavier organ weights (heart, liver and gizzard) were observed in the 0.50 and 0.75% OSP groups. The findings of this study suggest that while orange seed powder supplementation did not significantly impact the overall carcase traits, it did have specific effects on the weights of certain organs, such as the heart, liver, and gizzard. These results are consistent with previous research that has shown the potential of phytogenic feed additives, like orange seed powder, to influence the development and function of various organs in poultry (Sukhanova et al. Citation2019). This result is partly in agreement with the study of Abbasi et al. (Citation2015) who observed that the use of dried orange residues significantly decreased the abdominal fat of broilers, while the mean carcase percentage between treatments was not significantly affected. Further investigation is needed to fully understand the mechanisms behind these observed effects and their potential implications for poultry production and health.
Regarding the microbiological parameters in the caecal cavity, the results showed that the caecal bacterial population was significantly affected. Supplementation with OSP at 0.5% and 0.75% significantly reduced the caecal population of E. coli and total coliforms and significantly increased that of lactobacilli. The results obtained in this study are similar to those of Pourhossein et al. (Citation2012) who observed a significant reduction in the population of E. coli and coliforms and an increase in the number of lactobacilli when the peel extract of C. sinensis was supplemented in the diets of broilers. On the other hand, Ebrahimi et al. (Citation2015) showed that the supplementation of dried peels of C. sinensis in the diet of broiler chickens improved the population of E. coli in the caecum and had no significant effect on the coliforms and lactobacilli. The action of orange seeds can be attributed to the bioactive molecules they contain, such as phenols, aldehydes, and ketones (Jing et al. Citation2014). A thorough phytochemical analysis of the by-products of processed citrus fruits from industries showed the presence of flavonoids, limonoids, and dietary fibres with higher bioactive compounds in the orange seeds (48 g/kg) but the limonoid content was only found in the orange seeds (Tokusoglu and Hall Citation2011; Russo et al. Citation2015). This implies that the antibacterial activity of the OSP was effective in reducing the population of pathogenic bacteria and proliferating that of beneficial ones. It is established that the essential oils in citrus fruits can act as natural antimicrobials (Kim et al. Citation1995; Fisher and Phillips Citation2006). Citrus essential oils exert their bactericidal effects at the membrane level (Dušan et al. Citation2006) where they increase the permeability of the cell membrane (Gill and Holley Citation2006). Among these citrus essential oils, citrulline and limonene exert greater antimicrobial activity (Di Pasqua et al. Citation2007). For instance, the antimicrobial activity of such essential oils has been effective against strains of E. coli (Dušan et al. Citation2006), some Salmonella spp. (Kim et al. Citation1995), and foodborne pathogenic bacteria (Fisher and Phillips Citation2006). Interestingly, a study has shown that the antioxidant activity of citrus compounds can inhibit or reduce the production of virulence factors in bacteria (Yu et al. Citation2005). Additionally, orange seeds contain a high concentration of hesperidin (Russo et al. Citation2015), the most represented bioactive molecules in orange seeds (40.5 g/kg), and have several biological properties to mediate gut functioning. The effect of hesperidin on gut microbiota and gut-associated lymphoid tissue in healthy rats resulted in an altered composition of the microbiota, such as a higher proportion of lactobacilli. Moreover, the components of fermented dietary fibres can modulate the digestive microflora, ensuring competitive exclusion against digestive pathogenic bacteria and promoting the production of metabolites that promote good digestive health (Huyghebaert et al. Citation2011). Dietary fibre promotes the growth of lactobacilli and bifidobacteria, a role mainly attributed to fructooligosaccharides, and these beneficial bacteria would in turn compete with pathogenic bacteria for attachment sites in the intestine as well as for the availability of nutrients. The growth of beneficial bacteria would further lead to the stimulation of the immune system to better fight pathogenic bacteria (Huyghebaert et al. Citation2011).
The relative weight of the gizzard increased as the OSP inclusion levels increased in the diets. In addition to controlling feed flow, it is well known that the gizzard responds quickly to changes in the coarseness of the meal, which helps with digestion by lowering nutrient particle size and chemical degradation (Svihus Citation2011). There is an alternative perspective that suggests introducing larger grain particles and cellulose can lead to a significant increase in the structural components of the gizzard (Amerah et al. Citation2009; Svihus Citation2011). Furthermore, according to Starck (Citation1999), there was a sharp rise in the size of the quail gizzard following a 14-day high-fibre diet, suggesting that the gizzard muscle’s stimulative grinding effect was responsible for the increase in size.
Despite the limitations of this study regarding the phytochemical composition and mechanism of actions of the bioactive components of OSP, the implications of the findings are noteworthy. The observed enhancements in caecal microbiota composition present a potential role for OSP in promoting gut health and mitigating gastrointestinal infections in broiler chickens, which in turn have a positive impact on overall performance, offering a sustainable alternative to antibiotic use even as keeping animal welfare and production standards. Additionally, OSP supplementation may align with consumers’ demand for antibiotic-free and organic poultry products, supporting the poultry industry’s efforts to meet evolving market demands. Furthermore, this study underscores the broader potential of citrus by-products like OSP as a functional feed additive in promoting the growth and health of broiler chickens.
Conclusion
The inclusion of orange seed powder in the diet of broiler chickens resulted in an increase in lactobacilli and a decrease in the E. coli population in their gut. Furthermore, supplementing the diet of the broiler chickens with orange seed powder was found to effectively reduce serum cholesterol and triglyceride levels during the first 21 days of growth. As a result, the feed conversion ratio and weight gain significantly improved with the addition of 0.50% orange seed powder. The specific mechanism by which triglyceride levels decrease on day 21 and increase on day 42 due to orange seed powder supplementation warrants further investigation.
Acknowledgements
The authors would like to express their indebtedness to staff and technicians at the University of Lomé, Togo for their enormous support.
Disclosure statement
The authors of this article, T. Parobali, B. Adjei-Mensah, T. Yarkoa, T. Songuine, S. D. Karou, and K. Eklu-Gadegbeku, hereby certify that there is no conflict of interest pertaining to this work, either personally or professionally.
Data availability statement
All data generated or analysed in the present study are available upon reasonable request.
Additional information
Funding
References
- Abbasi H, Seidavi A, Liu W, Asadpour L. 2015. Investigation on the effect of different levels of dried sweet orange (Citrus sinensis) pulp on performance, carcass characteristics and physiological and biochemical parameters in broiler chicken. Saudi J Biol Sci. 22(2):139–146. doi: 10.1016/j.sjbs.2014.09.006.
- Adjei-Mensah B, Koranteng AAA, Hamidu JA, Tona K. 2023. Antibacterial activities of garlic (Allium sativum) in broiler and laying hens production. Worlds Poult Sci J. 79(1):155–176. doi: 10.1080/00439339.2023.2164236.
- Akpata MI, Akubor PI. 1999. Chemical composition and selected functional properties of sweet orange (Citrus sinensis) seed flour. Plant Foods Hum Nutr. 54(4):353–362. doi: 10.1023/A:1008153228280.
- Amerah AM, Ravindran V, Lentle RG. 2009. Influence of insoluble fibre and whole wheat inclusion on the performance, digestive tract development and ileal microbiota profile of broiler chickens. Br Poult Sci. 50(3):366–375. doi: 10.1080/00071660902865901.
- Aranha CPM, JoRGe N. 2013. Physico-chemical characterization of seed oils extracted from oranges (Citrus sinensis). FSTR. 19(3):409–415. doi: 10.3136/fstr.19.409.
- Ataei AH, Moheghi MM, Fazel Y. 2022. Effect of grower dietary energy level on feed intake and performance of modern broiler chickens. J Poult Res. 19(1):1–6. doi: 10.34233/jpr.1111291.
- Banerjee S, Ramaswamy S. 2017. Dynamic process model and economic analysis of microalgae cultivation in open raceway ponds. Algal Res. 26:330–340. doi: 10.1016/j.algal.2017.08.011.
- Basir R, Toghyani M. 2017. Effect of dietary graded levels of dried lemon (Citrus aurantifulia) pulp on performance, intestinal morphology, and humoral immunity in broiler chickens. Int J Recycl Org Waste Agricult. 6(2):125–132. doi: 10.1007/s40093-017-0159-5.
- Behera DP, Sethi APS, Singh C, Singh U, Wadhwa M. 2019. Effect of citrus waste on blood parameters of broiler birds with and without cocktail of enzymes. Vet World. 12(4):483–488. doi: 10.14202/vetworld.2019.483-488.
- Bok SH, Lee SH, Park YB, Bae KH, Son KH, Jeong TS, Choi MS. 1999. Plasma and hepatic cholesterol and hepatic activities of 3-hydroxy-3-methyl-glutaryl-CoA reductase and acyl CoA: cholesterol transferase are lower in rats fed citrus peel extract or a mixture of citrus bioflavonoids1. J Nutr. 129(6):1182–1185. doi: 10.1093/jn/129.6.1182.
- Boumezrag A, Khiati B, Benaraba R, Boukraa L, Hammoudi SM, Chicoteau P, Benarbia MEA. 2018. Modulation of broilers’ productivity and blood biochemical parameters by Citruselements dietary supplementation. Veterinaria. 67:129–137.
- Carson CF, Mee BJ, Riley TV. 2002. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob Agents Chemother. 46(6):1914–1920. doi: 10.1128/AAC.46.6.1914-1920.2002.
- Devriese LA, Daube G, Hommez J, Haesebrouck F. 1993. In vitro susceptibility of Clostridium perfringens isolated from farm animals to growth‐enhancing antibiotics. J Appl Bacteriol. 75(1):55–57. doi: 10.1111/j.1365-2672.1993.tb03407.x.
- Di Pasqua R, Betts G, Hoskins N, Edwards M, Ercolini D, Mauriello G. 2007. Membrane toxicity of antimicrobial compounds from essential oils. J Agric Food Chem. 55(12):4863–4870. doi: 10.1021/jf0636465.
- Durmic Z, Blache D. 2012. Bioactive plants and plant products: effects on animal function, health and welfare. Anim Feed Sci Technol. 176(1–4):150–162. doi: 10.1016/j.anifeedsci.2012.07.018.
- Dušan F, Marián S, Katarína D, Dobroslava B. 2006. Essential oils—their antimicrobial activity against Escherichia coli and effect on intestinal cell viability. Toxicol In Vitro. 20(8):1435–1445. doi: 10.1016/j.tiv.2006.06.012.
- Ebrahimi A, Qotbi AAA, Seidavi A, Laudadio V, Tufarelli V. 2013. Effect of different levels of dried sweet orange (Citrus sinensis) peel on broiler chickens growth performance Abbas. Arch Anim Breed. 56(1):11–17. doi: 10.7482/0003-9438-56-002.
- Ebrahimi A, Qotbi AAA, Seidavi A. 2013a. The effects of different levels of dried Citrus sinensis peel on broiler carcass quality. Acta Sci Vet. 14:713–717.
- Ebrahimi A, Santini A, Alise M, Pourhossein Z, Miraalami N, Seidavi A. 2015. Effect of dried Citrus sinensis peel on gastrointestinal microbiota and immune system traits of broiler chickens. Ital J Anim Sci. 14(4):4194. doi: 10.4081/ijas.2015.4194.
- Fisher K, Phillips CA. 2006. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J Appl Microbiol. 101(6):1232–1240. doi: 10.1111/j.1365-2672.2006.03035.x.
- Gilani SMH, Zehra S, Ul-Hassan F, Galani S, Ashraf A. 2018. Effect of natural growth promoters on immunity, and biochemical and haematological parameters of broiler chickens. Trop J Pharm Res. 17(4):627–633. doi: 10.4314/tjpr.v17i4.9.
- Gill AO, Holley RA. 2006. Disruption of Escherichia coli, Listeria monocytogenes and Lactobacillus sakei cellular membranes by plant oil aromatics. Int J Food Microbiol. 108(1):1–9. doi: 10.1016/j.ijfoodmicro.2005.10.009.
- Griggs J, Jacob J. 2005. Alternatives to antibiotics for organic poultry production. Elsevier BV. 14(4):750–756. doi: 10.1093/japr/14.4.750.
- Herzberg GR, Rogerson M. 1988. Hepatic fatty acid synthesis and triglyceride secretion in rats fed fructose- or glucose-based diets containing corn oil, tallow or marine oil12. J Nutr. 118(9):1061–1067. doi: 10.1093/jn/118.9.1061.
- Huyghebaert G, Ducatelle R, Van Immerseel F. 2011. An update on alternatives to antimicrobial growth promoters for broilers. Vet J. 187(2):182–188. doi: 10.1016/j.tvjl.2010.03.003.
- Imran A. 2022. Anticoccidial efficacy of citrus sinensis essential oil in broiler chicken. PVJ. 42(04):461–466. doi: 10.29261/pakvetj/2022.082.
- Jing L, Lei Z, Li L, Xie R, Xi W, Guan Y, Sumner LW, Zhou Z. 2014. Antifungal activity of citrus essential oils. J Agric Food Chem. 62(14):3011–3033. doi: 10.1021/jf5006148.
- Kim J, Marshall MR, Wei C. 1995. Antibacterial activity of some essential oil components against five foodborne pathogens. J Agric Food Chem. 43(11):2839–2845. doi: 10.1021/jf00059a013.
- Kpomasse CC, Kouame YAE, N’nanle O, Houndonougbo FM, Tona K, Oke OE. 2023. The productivity and resilience of the indigenous chickens in the tropical environments: improvement and future perspectives. J Appl Anim Res. 51(1):456–469. doi: 10.1080/09712119.2023.2228374.
- Liu J, Stewart SN, Robinson K, Yang Q, Lyu W, Whitmore MA, Zhang G. 2021. Linkage between the intestinal microbiota and residual feed intake in broiler chickens. J Anim Sci Biotechnol. 12(1):22. doi: 10.1186/s40104-020-00542-2.
- Long L, Kang B, Jiang Q, Chen J. 2020. Effects of dietary Lycium barbarum polysaccharides on growth performance, digestive enzyme activities, antioxidant status, and immunity of broiler chickens. Poult Sci. 99(2):744–751. doi: 10.1016/j.psj.2019.10.043.
- Martínez ML, Labuckas DO, Lamarque AL, Maestri DM. 2010. Walnut (Juglans regia L.): genetic resources, chemistry, by‐products. J Sci Food Agric. 90(12):1959–1967. doi: 10.1002/jsfa.4059.
- Nhung NT, Chansiripornchai N, Carrique-Mas JJ. 2017. Antimicrobial resistance in bacterial poultry pathogens: a review. Front Vet Sci. 4:126. doi: 10.3389/fvets.2017.00126.
- Oikeh EI, Ayevbuomwan M, Irabor F, Oikeh AO, Oviasogie FE, Omoregie ES. 2020. Evaluation of the phenolic content, antioxidant and antimicrobial activities of oil and non-oil extracts of Citrus sinensis (L.) Osbeck seeds. Prev Nutr Food Sci. 25(3):280–285. doi: 10.3746/pnf.2020.25.3.280.
- Oikeh EI, Oriakhi K, Omoregie ES. 2013. Proximate analysis and phytochemical screening of citrus sinensis fruit wastes. Biosci J. 1:164–170.
- Parobali T, Adjei-Mensah B, Songuine T, Yarkoa T, Karou SD, Eklu-Gadegbeku K. 2024. Influence of Citrus sinensis seed powder on growth performance, morphological and histological development of the small intestine of broiler chickens. J Appl Poult Res. 33(2):100395. doi: 10.1016/j.japr.2023.100395.
- Parry J, Zhou K, Luther M, Yu L. 2007. Phytochemical compositions and free radical scavenging capacities of selected cold-pressed edible seed oils. Ame Chem Soc. 956:255–267. doi: 10.1021/bk-2007-0956.ch018.
- Pourhossein Z, Qotbi AAA, Seidavi AR. 2012. Investigation on the effects of different levels of Citrus sinensis peel extract on gastrointestinal microbial population in commercial broilers. Afr J Microbiol Res. 6(34):6370–6378. doi: 10.5897/AJMR12.828.
- Readh CEA, Miloud L, Abdelkarim L, Kaddour B, Chaima B. 2023. Effect of graded levels of dried orange (Citrus sinensis) byproducts on production efficiency, blood parameters and antioxidant status of broiler chickens. AJDFR. 42:314–319. doi: 10.18805/ajdfr.DRF-307.
- Rodrigues C, Paula CDD, Lahbouki S, Meddich A, Outzourhit A, Rashad M, Pari L, Coelhoso IM, Fernando A, Souza VGL. 2023. Opuntia spp.: an overview of the bioactive profile and food applications of this versatile crop adapted to arid lands. Foods. 12(7):1465–1465. doi: 10.3390/foods12071465.
- Russo M, Bonaccorsi I, Inferrera V, Dugo P, Mondello L. 2015. Underestimated sources of flavonoids, limonoids and dietary fiber: availability in orange’s by-products. J Funct Foods. 12:150–157. doi: 10.1016/j.jff.2014.11.008.
- Saeed M, El-Hack MEA, Arif M, El-Hindawy MM, Attia AI, Mahrose KM, Bashir I, Siyal FA, Arain MA, Fazlani SA, et al. 2017. Impacts of distiller’s dried grains with solubles as replacement of soybean meal plus vitamin E supplementation on production, egg quality and blood chemistry of laying hens. Ann Anim Sci. 17(3):849–862. doi: 10.1515/aoas-2016-0091.
- Saeed M, Kamboh AA, Huayou C. 2024. Promising future of citrus waste into fermented high-quality bio-feed in the poultry nutrition and safe environment. Poult Sci. 103(4):103549. doi: 10.1016/j.psj.2024.103549.
- Starck JM. 1999. Phenotypic flexibility of the avian gizzard: rapid, reversible and repeated changes of organ size in response to changes in dietary fibre content. J Exp Biol. 202 Pt 22(22):3171–3179. doi: 10.1242/jeb.202.22.3171.
- Sukhanova SF, Pozdnyakova NA, Marshaniya IV. 2019. Effects of bio-sorb-selenium on productive and biological indicators of gosling broilers. IOP Conf Ser: Earth Environ Sci. 341(1):012048–012048. doi: 10.1088/1755-1315/341/1/012048.
- Sureshkumar S, Park JH, Sampath V, Kim IH. 2022. Silymarin seed extract supplementation enhances the growth performance, meat quality, and nutrients digestibility, and reduces gas emission in broilers. Asian-Austra. Assoc. Anim. Prod. Soc. 35(8):1215–1222. https://doi.org/10.5713/ab.21.0539
- Svihus B. 2011. The gizzard: function, influence of diet structure and effects on nutrient availability. World’s Poult Sci J. 67(2):207–224. doi: 10.1017/S0043933911000249.
- Tejeda OK, Kim W. 2021. Role of dietary fiber in poultry nutrition. Animals. 11(2):461. doi: 10.3390/ani11020461.
- Tufan T, Bolacali M, İrak K, Arslan C, Özcan C, Kaplan O, Irmak M. 2023. Dietary fig seeds improve growth performance and antioxidant capacity of quail. South Afr J Anim Sci. 53:302–314.
- Tokusoglu Ö, Hall III CA. 2011. Fruit and cereal bioactives: sources, chemistry, and applications. 1st ed. CRC Press. https://doi.org/10.1201/b10786.
- Walker HK, Hall WD, Hurst JW. 1990. Clinical methods: the history, physical, and laboratory examinations. 3rd ed. Butterworths, Boston: Butterworth Hennemann, Elsevier.
- Yu HB, Zhang YL, Lau YL, Yao F, Vilches S, Merino S, Tomas JM, Howard SP, Leung KY. 2005. Identification and characterization of putative virulence genes and gene clusters in Aeromonas hydrophila PPD134/91. Appl Environ Microbiol. 71(8):4469–4477. doi: 10.1128/AEM.71.8.4469-4477.2005.